Abstract
Recent studies have revealed that YKL-40 is involved in the pathogenesis of asthma. However, its specific mechanism remains unclear. The present study aims to investigate the effect of adenovirus vector-mediated YKL-40 short hairpin RNA (shRNA) on regulation of airway inflammation in a murine asthmatic model. Mice were assessed for airway hyperresponsiveness (AHR), total leukocytes and the percentage of eosinophil cells in bronchoalveolar lavage fluid (BALF). YKL-40 mRNA and protein expression levels were detected using quantitative real-time PCR and western blot assays. Enzyme-linked immunosorbent assay (ELISA) was used to detect YKL-40 and eosinophil-related chemokine expression levels in BALF and serum. Lung histology analyses were performed to evaluate the degree of inflammatory cell infiltration around the airway and airway mucus secretion.YKL-40 shRNA significantly inhibited the YKL-40 gene expression in asthmatic mice. In addition, YKL-40 shRNA alleviated eosinophilic airway inflammation, AHR, airway mucus secretion and decreased the levels of YKL-40 in BALF and serum in a murine asthmatic model. The levels and mRNA expression of IL-5, IL-13 in asthmatic mice lung tissues, eotaxin, and GM-CSF in BALF and serum significantly decreased. Bone marrow signaling molecules including IL-5, eotaxin, and GM-CSF were correlated with decreased levels of YKL-40. The study reveals that YKL-40 could be involved in asthma inflammation by altering bone marrow signaling molecules. YKL-40 gene RNA interference could provide new therapeutic strategies for asthma.
Similar content being viewed by others
Introduction
Asthma is a chronic inflammatory disease of the airways. It is characterized by infiltration of inflammatory cells, AHR, mucus hypersecretion, and airway remodeling [1]. Eosinophils are the key effector cells in the pathogenesis of asthmatic airway inflammation. Activated eosinophils facilitate airway inflammation by releasing inflammatory mediators, such as platelet-activating factor and leukotrienes that subsequently induce airway smooth muscle contraction and increase microvascular permeability. In addition, eosinophils secrete eosinophil cationic protein, which is one of the major basic proteins and eosinophil derived neurotoxin that can cause airway epithelial damage. Loss of airway epithelial integrity leads to exposure of sensory nerves, and AHR [2,3,4]. Eosinophilic airway inflammation plays a key role in asthma. Therefore, preventing eosinophilic airway inflammation could be a potential treatment strategy for asthma.
Eosinophils originate from CD34+ pluripotent hematopoietic stem cells. Moreover, certain cytokines such as IL-5 and granulocyte macrophage-colony stimulating factor (GM-CSF) influence release of eosinophils [5, 6]. Several cytokines such as stem cell factor, IL-6, IL-11, IL-12, IL-3, IL-4, IL-5, GM-CSF, and eotaxin have been reported to be involved in regulating proliferation and differentiation of multipotent hematopoietic stem cells into Eos/B progenitors.
Glucocorticoids are widely used as anti-inflammatory drugs for asthma. Asthma symptoms vary; symptoms can be effectively managed in some patients with asthma whereas in other patients, asthma treatments are unable to effectively relieve symptoms due to steroid insensitiveness or resistance [7]. Therefore, effective management of asthma symptoms in patients with severe asthma remains a challenge.
YKL-40, also called human cartilage gp-39 or chitinase-3 like 1 protein (CHI3L1), is a chitinase-like glycoprotein that is expressed and secreted by numerous cell types, including articular chondrocytes, synoviocytes, osteoblasts, macrophages, neutrophils, and epithelial cells [8,9,10,11,12,13]. In asthma, YKL-40 expression is considerably upregulated in the airway epithelium and alveolar macrophages, which in turn increases its concentration in the serum [14,15,16]. Previous studies have revealed that the YKL-40 expression level is positively correlated with asthma severity, thickness of the subepithelial basement membrane, and pulmonary function [17,18,19,20,21]. Anti-CHI3L1 treatment markedly ameliorates eosinophilic airway inflammation; however, the mechanisms are poorly understood [22]. In our previous study, we reported that ovalbumin (OVA) enhances the expression of YKL-40, IL-5, eotaxin, and GM-CSF in mouse tracheal epithelial cells in vitro [23]. Furthermore, the level of YKL-40 expression was positively correlated with the expression levels of IL-5, eotaxin, and GM-CSF [23]. Therefore, we hypothesized that YKL-40 may regulate eosinophilic airway inflammation in asthma by modulating bone marrow signaling molecules.
To test the hypothesis, we performed RNAi mediated silencing of YKL-40 expression using adenovirus-mediated shRNA vector. The silencing reduced eosinophilia and Th2-mediated airway inflammation in a murine asthmatic model. Furthermore, YKL-40 altered the expression of IL-5, eotaxin, and GM-CSF.
Materials and Methods
Construction of YKL-40 shRNA using adenovirus vectors
GeneChem Co. Ltd. (Shanghai, China) constructed the recombinant adenoviral shuttle vector hU6-MCS-CMV-EGFP (GV119) carrying CHI3L1 (YKL-40) (GeneBank; NM_007695) shRNA (YKL-40 shRNA). The optimal shRNA sequence, (YKL-40:5’-CCACATCATCTACAGCTTT-3’) was selected for knockdown. No significant homology to any known mouse gene sequence was observed in the GeneBank database. Scrambled sequences were used as negative controls (YKL-40:5’-TTCTCCGAACGTGTCACGT-3’). The success of CHI3L1 silencing in 293 T cells was assessed by immunoblot analysis. Helper plasmid and the target gene recombinant vector were co-transfected into HEK-293 cells. Viruses were then collected and concentrated.
Animals
Specific pathogen free male C57BL/6 mice (6–8 week, 18-20 g) were purchased from SLRC Animal Laboratory (Shanghai, China) and housed under specific pathogen free conditions. All experimental protocols involving animals were approved by the Laboratory Animal Ethics Committee of Shanghai General Hospital (Approval Protocol Number: 2018KY201). All surgeries were performed under anesthesia, and efforts made to reduce suffering.
Antigen sensitization, challenge and interventions
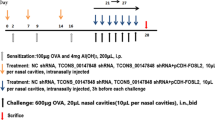
According to random number table, 32 mice were divided into 4 groups: saline sensitized and challenged group (saline group), OVA-sensitized and challenged group (OVA group), OVA-sensitized and challenged + adenovirus vector-mediated YKL-40 shRNA treated group (OVA + YKL-40 shRNA group), and OVA-sensitized and challenged + negative virus treated group (OVA + negative group). Immunization and challenging of the mice were performed as previously described [24]. An intraperitoneal injection of 200 µl containing 50 µg OVA (Grade V, Sigma-Aldrich, St. Louis, MO, USA) emulsified in alum (Pierce, Rockford, IL, USA) was administered on day 0,7 and 14, and the mice subsequently exposed to aerosolized 5% OVA in saline or saline only for 40 min on days 21–27 using a nebulizer (OMRON NE-C900 Model, Kyoto, Japan) [24]. YKL-40 shRNA adenovirus or negative viruses were intratracheally injected with 1 × 108 plaque-forming units (pfu) per mouse diluted in 50 µl saline on day 20.
Airway hyperresponsiveness
Mice were anesthetized and lung resistance (RL) measured using a plethysmograph (EMMS, Hants, UK), 24 h after performing the final challenge [25]. Lung resistance was measured after at different concentrations of the aerosol acetylcholine challenge (0 mg/ml, 4 mg/ml, 8 mg/ml, 16 mg/ml, 32 mg/ml, 64 mg/ml, 128 mg/ml, and 256 mg/ml).
BALF and blood collection, and cell counting
Mice were euthanized using pentobarbital sodium after measurement of airway resistance. Blood was withdrawn from the left ventricles of the mice using a 1 ml syringe, centrifuged for 15 min at 5000 rpm and 4 °C, and the serum stored at −80°C for subsequent analyses. Mice lungs were lavaged three times with 0.4 ml phosphate buffered saline (PBS) through a tracheal cannula, and bronchoalveolar lavage fluid (BALF) subsequently collected. The BALF was centrifuged for 10 min at 4 °C 1000 rpm. The supernatant was stored at −80°C for subsequent analyses. The sediments were resuspended to facilitate counting of total leukocytes, and the remaining cell suspensions were smeared on glass slides and stained with Wright-Giemsa. Eosinophils were counted according to a previously described protocol [26].
ELISA analysis for cytokines in BALF and serum
The levels of IL-5, IL-13, and GM-CSF in BALF or serum were determined using enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (eBioscience San Diego, CA, USA). ELISA Kits (R&D Systems, Minneapolis, MN, USA) were used to determine eotaxin concentration according to the manufacturer’s instructions. YKL-40 concentration was determined using ELISA Kits (Wuhan Boster Biological Technology Co. Ltd, China).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA was quantified and subsequently reverse transcribed into cDNA using a standard protocol. Transcript levels were measured using SYBR Green on an ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The sequences of murine primers in Table 1 were purchased from Sangon Biotech Co. Ltd (Shanghai, China). GAPDH was used as an endogenous reference and the relative amounts of mRNA were calculated using the ΔΔCT method.
Western blot analysis
Lung tissues were washed three times with cold PBS and weighed. Subsequently, tissue lysates and enzyme inhibitor were mixed in a ratio of 50:1 proportion. Protein lysates were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies at 4 °C overnight against YKL-40 (MAB2649; 1:500; R&D System, Minneapolis, MN, USA) after blocking with 5% skimmed milk powder for 2 h at room temperature. Afterwards, the membranes were washed three times with TBST and incubated with a secondary antibody (HAF005; 1:1000; R&D System, Minneapolis, MN, USA) for 2 h at room temperature. The protein bands were detected using enhanced chemiluminescence ECL kit (Epizyme Biotech, Shanghai, China) and Bio-Rad ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc, CA, USA). β-actin was used as an internal reference and protein bands were quantified using Image J software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Histological analysis of lung tissues
BALF samples were collected from the lungs of mice, after which the left lung lobes were removed and fixed in 4% paraformaldehyde and embedded in paraffin. The paraffin-embedded sections (4 µm) were stained with hematoxylin-eosin (H&E) and Periodic Acid-Schiff (PAS). A semi-quantitative scoring system was applied to the histological findings to grade the degree of lung infiltration around the bronchioles, and goblet cells in airway epithelium were measured double-blind using a numerical scoring system [27].
Statistical analysis
Data were presented as mean ± standard deviation (SD). GraphPad Prism version 5.0 (GraphPad Software Inc, San Diego, CA, USA) was used to perform statistical analysis. Two-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test was used to compare percentage changes in lung resistance between individual groups. One-way ANOVA with Bonferroni’s post-hoc test was used to compare multiple groups. A P value < 0.05 was considered statistically significant.
Results
Interference effect of YKL-40 shRNA
Adenovirus-mediated silencing resulted in increased expression of YKL-40 in the OVA group. However, treatment of mice with YKL-40 shRNA markedly reduced YKL-40 protein expression (Fig. 1a, c). Quantitative real-time PCR analyses revealed that OVA sensitization resulted in increased expression of YKL-40 mRNA (Fig. 1b), and mice treated with YKL-40 shRNA-containing adenovirus exhibited low levels of YKL-40 expression. The negative control that contained a fragment with no homology to any mouse gene sequence exhibited a slight decrease in YKL-40 mRNA expression.
The expression of YKL-40 proteins were detected by western blot analysis (a, c). RT-PCR analysis of mRNA expression of YKL-40 in lung tissues of mice (b). mRNA expression normalized to GAPDH expression in the saline group. β-actin expression was used as control. Data are expressed as means ± SD, n = 8; *P < 0.05; **P < 0.01; ***P < 0.001, One-way ANOVA with Bonferroni’s post-hoc test.
YKL-40 shRNA alters total cell and eosinophil count in BALF
BALF was collected 24 h after performing the last OVA challenge. The total number of cells and eosinophils significantly increased in the OVA group relative to the saline group (Fig. 2a, b). Similar results were observed in the OVA + negative group (Fig. 2a, b). A significant decrease in total cells, especially eosinophil count in BALF was observed in YKL-40 shRNA-treated mice compared to the OVA group (Fig. 2a, b).
YKL-40 shRNA decreased the total number of inflammatory cells (a) and eosinophil percentage (b) in BALF compared with the OVA and OVA + negative groups. Data are expressed as means ± SD from three independent experiments, n = 8; **P < 0.01; ***P < 0.001, One-way ANOVA with Bonferroni’s post-hoc test.
YKL-40 shRNA alleviates airway hyperresponsiveness
No significant difference was observed in baseline lung resistance values after saline challenge in the four experimental groups. However, the OVA-sensitized and challenged mice (OVA group) demonstrated a leftward shift of the concentration responsive to acetylcholine, indicating an increase in bronchial responsiveness to acetylcholine when compared with the saline control mice (Fig. 3). Airway resistance analysis curve revealed that YKL-40 shRNA down-regulated AHR in the OVA group. No significant difference was observed in AHR between the OVA and OVA + negative groups (Fig. 3).
Results are expressed for each acetylcholine concentration and defined as the percentage of RL after PBS nebulization of three independent experiments. Data are expressed as means ± SD, n = 8; **P < 0.01; *** P < 0.001(saline group vs OVA group); #P < 0.05; ###P < 0.001(OVA group vs OVA + YKL-40 shRNA group); &&P < 0.01; &&&P < 0.001(saline group vs OVA + negative group), Two-way ANOVA with Bonferroni’s post-hoc test was used to compare percentage (%) change in RL between individual groups.
YKL-40 shRNA alters cytokine levels in BALF and serum
BALF and serum levels of YKL-40 were significantly decreased in YKL-40 shRNA-treated mice exposed to OVA compared to the OVA and OVA + negative groups that did not exhibit any changes (Fig. 4a, e). IL-5 is one of the eosinophil-specific chemoattractants. Eotaxin induces recruitment of eosinophils, basophils, and Th2 lymphocytes in the lungs. Evaluation of the levels of Th2 inflammation cytokines such as IL-5 and IL-13 revealed that their levels in BALF were significantly increased in the OVA group when compared with the saline group (Fig. 4c, g). However, eotaxin and GM-CSF concentrations in BALF significantly decreased in the OVA + YKL-40 shRNA group when compared with the OVA and OVA + negative groups (Fig. 4b, d). Similarly, serum eotaxin levels also decreased in the OVA + YKL-40 shRNA group when compared with the OVA group (Fig. 4f). Therefore, YKL-40 was associated with IL-5, eotaxin, and GM-CSF.
YKL-40 shRNA decreased the mRNA expression of gene
The IL-5, eotaxin, and GM-CSF gene expressions in lung tissues were evaluated to investigate the mechanism of YKL-40 shRNA in inhibiting airway inflammation and AHR in asthmatic mice (Fig. 5a, b, c). The findings revealed that mRNA expression of IL-5, eotaxin, and GM-CSF increased in the OVA group. In addition, treatment with YKL-40 shRNA adenovirus significantly decreased the average levels of IL-5, eotaxin and GM-CSF in OVA + YKL-40 shRNA group when compared with the OVA and OVA + negative groups.
Expression of IL-5 (a), eotaxin (b) and GM-CSF mRNA (c) in lung tissues of mice detected by quantitative real-time PCR. mRNA expression normalized to GAPDH expression in the saline group. Data are expressed as means ± SD, n = 8; *P < 0.05; **P < 0.01; ***P < 0.001, One-way ANOVA with Bonferroni’s post-hoc test.
YKL-40 shRNA protein expression decreases airway inflammation and airway mucus secretion
Pathological changes of lung sections in the asthma mouse model revealed a higher degree of inflammatory cell infiltration around airways than those in saline controls. YKL-40 shRNA-treated mice exposed to OVA exhibited decreased eosinophilic infiltration when compared with the OVA and OVA + negative groups (Fig. 6a, b). Histological evaluation of mice lungs exposed to OVA revealed a significant bronchial mucus production and goblet cell hyperplasia compared with saline controls (Fig. 6a, c). Treatment with YKL-40 shRNA significantly reduced bronchial mucus secretion and goblet cell hyperplasia, and similar results were observed in saline controls (Fig. 6a, c).
Lungs of different groups of mice excised after BALF collection and fixed with 4% Paraformaldehyde. Airway and lung sections were stained with H&E (×400) (a) and PAS (×400) (a) for inflammatory cells around the airway and airway mucus secretion. Mean values of airway inflammatory cell infiltration score (b) and airway mucus secretion score (c) in four independent experimental groups. Data are expressed as means ± SD, n = 8; ***P < 0.001, One-way ANOVA with Bonferroni’s post-hoc test. Bars correspond to 100 µm.
Discussion
Numerous studies have revealed that YKL-40 is an inflammatory glycoprotein associated with several diseases, such as cardiovascular and autoimmune diseases, tumor and diabetes [13, 28,29,30,31,32,33,34]. A recent study has revealed that CHI3L1 expression is significantly upregulated in eosinophilic chronic sinusitis (ECRS) with Clara cell 10-kD protein (CC-10) gene knockout in an allergic mouse model. CC-10 can inhibit eosinophilic airway inflammation in ECRS by regulating the expression of CHI3L1 (YKL-40) [35]. Asthma is a chronic allergic airway inflammatory disease associated with eosinophilic airway inflammation in Th2 type inflammation. Clinical and experimental studies have revealed a strong correlation between YKL-40 and asthma [15, 22, 36,37,38]. YKL-40 levels are increased in asthmatic patients, and the levels correlate with disease severity and degree of airway remodeling, which plays a vital role in the initiation of effector phase of Th2 inflammation in mice [21, 39, 40]. Moreover, YKL-40 regulates Th2 cytokine effector functions by stimulating production and activation of dendritic cells (DCs) [41]. Lai et al. established that YKL-40 serum levels in asthmatic patients significantly decreased after appropriate treatment, and the levels were correlated with forced expiratory volume in one second as percentage of predicted volume (FEV1%) and asthma control test (ACT) [17].
In the present study, we used a mouse model of eosinophilic asthma and overexpressed YKL-40 in BALF and lung tissues as previously described by Lee and Xu et al. [39, 41]. Th2 cytokine levels including IL-5 and IL-13 were elevated in BALF of the asthma group. In addition, we investigated the function of a knockdown-expressing YKL-40 adenovirus vector administered intratracheally in a mouse model of asthma. YKL-40 shRNA adenovirus vector significantly inhibited the expression of YKL-40 in BALF, serum and lung tissues, and reduced the levels of IL-5, eotaxin, and GM-CSF in vivo. Furthermore, the down-regulation of YKL-40 expression attenuates eosinophilic infiltration, AHR, and airway mucus secretion. However, the negative adenovirus group did not exhibit similar changes.
IL-5 is an important cytokine that stimulates maturation and differentiation of bone marrow CD34+ hemopoietic progenitor cells into eosinophils [42]. Eotaxin (CCL11) is a member of the CC chemokine subfamily, generated from eosinophils, lymphocytes, macrophages, smooth muscle cells, and epithelial cells. Our previous study suggested that eotaxin induced migration of bone marrow CD34+ progenitor cells to the airways, and differentiation into eosinophils [43]. Therefore, eotaxin is an eosinophil-specific attractant that activates eosinophils through a highly selective receptor, CCR3 [43, 44]. GM-CSF regulates the growth and differentiation of hematopoietic progenitor cells, and its levels have been reported to decrease in BALF [45]. GM-CSF influences eosinophil maturation, survival, and function.
The mentioned mediators are potent chemotactic factors contributing to airway inflammation and recruitment of inflammatory progenitor cells into inflammation sites [46, 47]. Activated antigen-specific Th2 cells are an primary sources of eotaxin, GM-CSF, and IL-5 [48, 49]. DCs can activate CD4+ naive T cells differentiation into Th2 cells [50]. YKL-40 is a regulatory cytokine that stimulates DCs to promote Th2 cell polarization [40]. Recent studies have revealed that YKL-40 is closely associated with IL-18 [51,52,53]. IL-18 can be secreted by a variety of cells, including mast cells, basophils, DCs, and lymphocytes [54]. DCs secrete IL-18 and other cytokines when allergic asthma occurs. Previous studies have revealed that IL-18 can stimulate eosinophils and lymphocytes to secrete IL-5, GM-CSF, and induce release of IL-13 and eotaxin from lung tissues of transgenic mice that have been sensitized and stimulated with OVA [54, 55]. IL-18 can directly initiate polarization of naive CD4 + T cells to Th2 through an NF-κB-dependent pathway, promote development of Th2 cells, also contribute to the enhancement of GM-CSF activity [56]. Moreover, YKL-40 can inhibit Fas-mediated inflammatory cell apoptosis, including T cell, macrophages, eosinophil apoptosis through PKB/AKT and Faim3 signaling pathways, and increases Th2 inflammation [40]. Therefore, YKL-40 can regulate the expression of cytokines by participating in the above-mentioned multiple signaling pathways, regulating eosinophil-mediated airway inflammation and contributing to pathogenesis of asthma. YKL-40 can be a potential therapeutic target in asthma treatment.
Presently, adenovirus vector is widely used for treating diseases in gene therapy field. Numerous studies have revealed that gene transfer using viral vectors is relatively safe for humans and animals [57]. In relation to the associated safety concerns of YKL-40 shRNA intervention, several studies have revealed that intratracheal administration of adenovirus vector is a relatively safe method in asthma models. A recent study has revealed that intranasal instillation with 100 µl solution containing 4.1 × 109 pfu per mouse of adenoviral vector does not induce a detrimental inflammatory response in treated cotton rats [58]. Furthermore, all organs from treated cotton rats except the lungs tested negative for viral DNA [58]. A study by Zhang et al. revealed that injection of four groups of BALB/c mice with recombinant P53 adenovirus, Ad5CMV-P53 at doses ranging from 107 to 1010 pfu per mouse that the viral injections proved safe [59]. In addition, mild inflammation was observed when adenoviral vector was injected at doses of 109 and 1010 pfu per mouse, whereas the lung tissues of treated groups appeared similar to those of control groups at doses of 107 and 108 pfu per mouse [59]. According to previous studies, no evident safety issues were observed in C57BL/6 J mice intratracheally injected with 50 µl solution containing 1×108 pfu per mouse of adenoviral vector [60]. In relation to the immunogenicity of adenovirus, the present study has preliminarily explored the safe dose of adenovirus injection. First, different doses of adenovirus were used to treat asthmatic mice, and to explore the appropriate dose of adenovirus vector. The results revealed that intratracheal administration of a high dose of adenovirus vectors (2 × 109 pfu per mouse) exacerbated lung inflammation in asthmatic mice. However, injection of asthmatic mice with adenoviral vector at doses of 1 × 108 pfu per mouse did not exacerbate inflammation (data not shown in the text). The dosage used in the present study was less harmful to mice in asthma models than the dosage used in previous studies [58, 59]. Generally, YKL-40 shRNA intervention could be potentially safe for use in asthma models.
Previous studies have revealed that YKL-40 plays a crucial role in both antigen sensitization and during the effector phases of allergic inflammation and remodeling. The present study has demonstrated that down-regulation of YKL-40 expression attenuates eosinophilic infiltration, AHR, and airway mucus secretion. Studies conducted over the last few years have reported contrasting findings. A study reported that serum YKL-40 levels in non-eosinophilic asthma were significantly elevated compared with eosinophilic asthma, and increased YKL-40 was associated with IL-6, IL-1β, and IL-8 [61]. High serum YKL-40 clusters are characterized by airway neutrophils that are not associated with Th2 inflammation [51]. The results suggest that YKL-40 could be a non-Th2 asthma biomarker. Therefore, YKL-40 could be involved in the pathogenesis of non-Th2 asthma through an unknown signaling pathway. Future studies should be conducted to explore the mechanisms of YKL-40 in the pathogenesis of asthma.
In summary, the present study reveals that YKL-40 silencing attenuates eosinophilic infiltration that could be associated with reduced expressions of IL-5, eotaxin, and GM-CSF. Therefore, YKL-40 can be a potential therapeutic target in asthma treatment.
References
Komi DE, Kazemi T, Bussink AP. New insights into the relationship between chitinase-3-like-1 and asthma. Curr Allergy Asthma Rep. 2016;16:57.
Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570.
Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126:61–9.e3.
Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376:835–43.
Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8.
Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O’Byrne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–6.
Luhadia SK. Steroid resistant asthma. J Assoc Physicians India. 2014;62:38–40.
Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10.
Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5.
Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270:13076–83.
Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 2006;281:21082–95.
Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26.
Rusak A, Jablonska K, Dziegiel P. The role of YKL-40 in a cancerous process. Postepy Hig Med Dosw (Online). 2016;70:1286–99.
Hansen JW, Thomsen SF, Porsbjerg C, Rasmussen LM, Harmsen L, Johansen JS, et al. YKL-40 and genetic status of CHI3L1 in a large group of asthmatics. Eur Clin Respir J. 2015;2:25117.
Usemann J, Frey U, Mack I, Schmidt A, Gorlanova O, Roosli M, et al. CHI3L1 polymorphisms, cord blood YKL-40 levels and later asthma development. BMC Pulm Med. 2016;16:81.
Hartl D, Lee CG, Da Silva CA, Chupp GL, Elias JA. Novel biomarkers in asthma: chemokines and chitinase-like proteins. Curr Opin Allergy Clin Immunol. 2009;9:60–6.
Lai T, Chen M, Deng Z, Wu LY, Li D., et al. YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment. BMC Pulm Med. 2015;15:1.
Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501.
Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–91.
Tang H, Shi Z, Xiu Q, Li B, Sun Y. YKL-40-mediated interleukin 8 production may be closely associated with remodeling of bronchial smooth muscle cells. Am J Respir Crit Care Med. 2012;186:386. author reply -7
Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–27.
Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2:20–7.
Ben SQ, Qiu YL, Zhou J, Zhou XY, Zhang S, Wu Y, et al. Ovalbumin enhances YKL-40, IL-5, GM-CSF, and eotaxin expression simultaneously in primarily cultured mouse tracheal epithelial cells. In Vitro Cell Dev Biol Anim. 2014;50:243–50.
Khanna K, Chaudhuri R, Aich J, Pattnaik B, Panda L, Prakash YS, et al. Secretory inositol polyphosphate 4-phosphatase protects against airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2019;60:399–412.
Bao W, Zhang Y, Zhang M, Bao A, Fei X, Zhang X, et al. Effects of ozone repeated short exposures on the airway/lung inflammation, airway hyperresponsiveness and mucus production in a mouse model of ovalbumin-induced asthma. Biomed Pharmacother. 2018;101:293–303.
Fei X, Bao W, Zhang P, Zhang X, Zhang G, Zhang Y, et al. Inhalation of progesterone inhibits chronic airway inflammation of mice exposed to ozone. Mol Immunol. 2017;85:174–84.
McMillan SJ, Xanthou G, Lloyd CM. Therapeutic administration of Budesonide ameliorates allergen-induced airway remodelling. Clin Exp Allergy. 2005;35:388–96.
Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143:35–42.
Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, et al. High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J. 2009;30:1066–72.
Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–8.
Bonneh-Barkay D, Wang G, Laframboise WA, Wiley CA, Bissel SJ. Exacerbation of experimental autoimmune encephalomyelitis in the absence of breast regression protein 39/chitinase 3-like 1. J Neuropathol Exp Neurol. 2012;71:948–58.
Kazakova M, Batalov A, Deneva T, Mateva N, Kolarov Z, Sarafian V. Relationship between sonographic parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol Int. 2013;33:341–6.
Sekine T, Masuko-Hongo K, Matsui T, Asahara H, Takigawa M, Nishioka K, et al. Recognition of YKL-39, a human cartilage related protein, as a target antigen in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:49–54.
Suzuki H, Boki H, Kamijo H, Nakajima R, Oka T, Shishido-Takahashi N, et al. YKL-40 promotes proliferation of cutaneous T-cell lymphoma tumor cells through extracellular signal-regulated kinase pathways. J Invest Dermatol. 2020;140:860–8.
Wang H, Long XB, Cao PP, Wang N, Liu Y, Cui YH, et al. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2010;181:908–16.
Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438–46.
Ortega H, Prazma C, Suruki RY, Li H, Anderson WH. Association of CHI3L1 in African-Americans with prior history of asthma exacerbations and stress. J Asthma. 2013;50:7–13.
Naglot S, Aggarwal P, Dey S, Dalal K. Estimation of serum YKL-40 by real-time surface plasmon resonance technology in North-Indian asthma patients. J Clin Lab Anal. 2017;31:e22028. https://doi.org/10.1002/jcla.22028.
Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med. 2012;185:692–4.
Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–66.
Xu Q, Chai SJ, Qian YY, Zhang M, Wang K. Breast regression protein-39 (BRP-39) promotes dendritic cell maturation in vitro and enhances Th2 inflammation in murine model of asthma. Acta Pharmacol Sin. 2012;33:1525–32.
Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201:1891–7.
Ben S, Li X, Xu F, Xu W, Li W, Wu Z, et al. Treatment with anti-CC chemokine receptor 3 monoclonal antibody or dexamethasone inhibits the migration and differentiation of bone marrow CD34 progenitor cells in an allergic mouse model. Allergy. 2008;63:1164–76.
Salter BM, Sehmi R. Hematopoietic processes in eosinophilic asthma. Chest. 2017;152:410–6.
Nobs SP, Kayhan M, Kopf M. GM-CSF intrinsically controls eosinophil accumulation in the setting of allergic airway inflammation. J Allergy Clin Immunol. 2019;143:1513–24.e2.
Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8.
Allakhverdi Z, Delespesse G. Hematopoietic progenitor cells are innate Th2 cytokine-producing cells. Allergy. 2012;67:4–9.
Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, et al. Modeling T(H) 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017;278:20–40.
Kumar RK, Thomas PS, Seetoo DQ, Herbert C, McKenzie AN, Foster PS, et al. Eotaxin expression by epithelial cells and plasma cells in chronic asthma. Lab Invest. 2002;82:495–504.
Han M, Hu R, Ma J, Zhang B, Chen C, Li H, et al. Fas signaling in dendritic cells mediates Th2 polarization in HDM-induced allergic pulmonary inflammation. Front Immunol. 2018;9:3045.
Gomez JL, Yan X, Holm CT, Grant N, Liu Q, Cohn L, et al. Characterisation of asthma subgroups associated with circulating YKL-40 levels. Eur Respir J. 2017;50:1700800. https://doi.org/10.1183/13993003.00800-2017.
Li TM, Liu SC, Huang YH, Huang CC, Hsu CJ, Tsai CH, et al. YKL-40-induced inhibition of miR-590-3p promotes interleukin-18 expression and angiogenesis of endothelial progenitor cells. Int J Mol Sci. 2017;18:920. https://doi.org/10.3390/ijms18050920.
Kang MJ, Yoon CM, Nam M, Kim DH, Choi JM, Lee CG, et al. Role of chitinase 3-like-1 in interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; alveolar destruction; and airway fibrosis in the murine lung. Am J Respir Cell Mol Biol. 2015;53:863–71.
Kandikattu HK, Upparahalli Venkateshaiah S, Mishra A. Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98.
Sawada M, Kawayama T, Imaoka H, Sakazaki Y, Oda H, Takenaka S, et al. IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13. PLoS One. 2013;8:e54623.
Xu MH, Yuan FL, Wang SJ, Xu HY, Li CW, Tong X. Association of interleukin-18 and asthma. Inflammation. 2017;40:324–7.
Leggiero E, Labruna G, Iaffaldano L, Lombardo B, Greco A, Fiorenza D, et al. Helper-dependent adenovirus-mediated gene transfer of a secreted LDL receptor/transferrin chimeric protein reduces aortic atherosclerosis in LDL receptor-deficient mice. Gene Ther. 2019;26:121–30.
Zabner J, Petersen DM, Puga AP, Graham SM, Couture LA, Keyes LD, et al. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994;6:75–83.
Zhang WW, Alemany R, Wang J, Koch PE, Ordonez NG, Roth JA. Safety evaluation of Ad5CMV-p53 in vitro and in vivo. Hum Gene Ther. 1995;6:155–64.
Chen L, Zheng J, Xue Q, Zhao Y. YKL-40 promotes the progress of atherosclerosis independent of lipid metabolism in apolipoprotein E(-/-) mice fed a high-fat diet. Heart Vessels. 2019;34:1874–81.
Liu L, Zhang X, Liu Y, Zhang L, Zheng J, Wang J, et al. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir Res. 2019;20:95.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No: 81570018 and No: 81970022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Bao, A., Zheng, Y. et al. Adenovirus vector-mediated YKL-40 shRNA attenuates eosinophil airway inflammation in a murine asthmatic model. Gene Ther 28, 177–185 (2021). https://doi.org/10.1038/s41434-020-00202-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00202-0
- Springer Nature Limited