Abstract
Recent researches have reported that long noncoding RNA (lncRNA) five prime to Xist (FTX) plays a crucial role in the initiation and progression of cancers. In the current study, the clinical significance and functional roles of lncRNA FTX in colorectal cancer (CRC) progression were investigated. A significant increase of lncRNA FTX expression in CRC tissue and cell lines was observed. Overexpression of lncRNA FTX was significantly associated with the bigger tumor diameter, the advanced TNM stage, the lymph node, and distant metastasis, and also predicted poor prognosis of patients with CRC. Functional analyses demonstrated that knockdown of lncRNA FTX markedly inhibited CRC cell proliferation, migration, and invasion in vitro. Mechanistically, FTX directly interacted with miR-215 and suppressed miR-215 expression. FTX also bind to vimentin and reduced its phosphorylation level on Ser83 in CRC cells. Finally, using siRNAs against lncRNA FTX could dramatically inhibit CRC growth and distant metastasis in vivo. Taken together, our data demonstrated an oncogenic role of lncRNA FTX in CRC tumorigenesis and progression via interaction with miR-215 and vimentin. Then, a promising therapeutic target for CRC was provided.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers. Although the mortality rate of CRC has declined for several decades, CRC is still the third leading cause of cancer death in both men and women in the United States [1]. Even though the advancement of CRC treatment has been achieved, the 5-year overall survival rate of CRC patients is still low [2]. Hence, revealing the underlying molecular mechanisms in the pathogenesis of CRC is vital for the development of effective treatment.
Long noncoding RNAs (lncRNAs) are defined as transcripts larger than 200 nucleotides in length without or with limited protein coding potential [3]. lncRNAs take in the modulation of gene expression as microRNA sponges or scaffolds in some physiological processes, including cell proliferation, migration, invasion, autophagy, and differentiation [4,5,6]. It has been found that lncRNAs are abnormally expressed in cancers. For instance, the expression of lncRNA SNHG3 is dramatically increased in CRC tissue, and is associated with shorter survival time of patients with CRC. Mechanistically, SNHG3 promotes the growth and metastasis of CRC by functioning as the competing endogenous RNA (ceRNA) of oncoprotein c-Myc [7]. lncRNA five prime to Xist (FTX) is a kind of evolutionarily conserved lncRNA and locates within the X-inactivation center [8]. The function of FTX in different cancers is paradoxical. In hepatocellular carcinoma (HCC), lncRNA FTX inhibits proliferation and invasion by associating with MCM2 and miR-374a [9]. Conversely, FTX facilitates the growth and metastasis in glioma cells through sponging miR-342-3p [10]. However, to date, the functional roles and molecular mechanisms of FTX in CRC progression remain unclear. Here, the expression pattern and functional roles of FTX in the malignant development of CRC were explored. Our data demonstrated the upregulation of lncRNA FTX in CRC tissue and cell lines. Further investigation revealed the underlying mechanism of FTX in CRC progression via targeting miR-215 and vimentin.
Results
lncRNA FTX is upregulated in CRC tissue and predicts poor prognosis
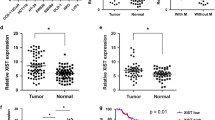
Firstly, FTX expression in 80 paired CRC tumors and corresponding adjacent non-tumor tissue was detected. The results of qRT-PCR exhibited a significant increase of lncRNA FTX expression in CRC tumor tissue compared with that in non-tumor tissue (Fig. 1a). Next, lncRNA FTX levels in five CRC cell lines and a normal colon epithelial cell line were examined. CRC cells expressed a higher level of FTX than FHC cells did (Fig. 1b). To reveal the association between lncRNA FTX expression and clinicopathological features of CRC patients, patients were divided into two groups as the median of CRC tissue’s FTX expression: the high FTX expression group and the low FTX expression group. As shown in Table 1, high FTX expression was more frequent to be detected in tumors with the bigger tumor diameter, the advanced TNM stage, lymph node metastasis, and distant metastasis. However, lncRNA FTX expression was not correlated with age, gender, histological differentiation, and primary tumor sites.
lncRNA FTX is upregualted in CRC and is associated with poor prognosis. a lncRNA FTX expression was measured by qRT-PCR in 80 pairs of CRC and the adjacent non-tumor tissues. b lncRNA FTX expression was measured by qRT-PCR in different cell lines. c Kaplan–Meier survival analysis reveals overall survival rate in the two crowds of CRC patients
To determine the relationship between lncRNA FTX expression and CRC patients’ prognosis, the Kaplan–Meier analysis and the log-rank test were performed. The results showed that patients with higher FTX levels had shorter overall survival time than those with lower FTX levels (Fig. 1c). These data clarified that upregulated FTX might be involved in CRC progression.
Knockdown of lncRNA FTX suppresses CRC cell proliferation
It was probed that lncRNA FTX expression was relatively higher in SW480 and HCT116 cell lines than that in HT29, HCT8, and LS513 cells (Fig. 1b). Thus, SW480 and HCT116 cell lines were selected for the following loss-of-function studies. To further investigate the role of FTX in CRC cells, the lentiviral particles that expressed FTX shRNA were transfected into SW480 and HCT116 cells. As shown in Fig. 2a, cells that transfected shFTX obviously reduced the FTX RNA expression level compared with the control cells. CCK-8 assays were conducted to determine the effect of FTX on the proliferation of CRC cells, and the results exhibited that silence of FTX apparently suppressed the proliferative ability of both SW480 and HCT116 cells (Fig. 2b). Similarly, the results of the colony formation assay indicated that CRC cells that expressed FTX shRNA dramatically suppressed the colony numbers (Fig. 2c). Then, flow cytometry analysis was made to investigate whether FTX is involved in CRC cell apoptosis. Knockdown of FTX significantly increased the apoptotic rate of the CRC cell (Fig. 2d). These data showed that FTX facilitates proliferation and inhibits apoptosis in CRC.
Knockdown of FTX suppressed proliferation of CRC cells. a One shRNA targeting lncRNA FTX were tested for its knockdown efficiency by qRT-PCR. *p < 0.05. b Cell proliferation was analyzed using Cell Counting Kit-8 (CCK-8). Knockdown of FTX inhibits cell proliferation. c Cell colony formation was analyzed. FTX-silencing cells have a decreased capacity of colony formation compared with the parallel control cells. *p < 0.05. d Cell apoptosis was analyzed by flow cytometry analysis. Compared with the control cells, knockdown of FTX significantly increased the CRC cell apoptotic rate. *p < 0.05
lncRNA FTX knockdown inhibits cell migration and invasion in CRC
To examine the effect of FTX on cell migration and invasion, transwell assays were conducted in SW480 cells and HCT116 cells. Downregulation of FTX dramatically attenuated cell migratory capability of SW480 and HCT116 cells compared with the control group (Fig. 3a). Moreover, matrigel transwell invasion assay was performed to explore the effect of FTX on the invasiveness of CRC cells. The result showed that silence of FTX significantly inhibited cell invasion (Fig. 3b). To sum up, these data suggest that lncRNA FTX plays a crucial role in CRC migration and invasion.
Silencing of FTX abrogated migration and invasion of CRC cells. a Cell migration was analyzed using transwell chamber without matrigel. FTX downregulation suppresses cell migration. *p < 0.05. b Cell invasion was analyzed using a transwell chamber with matrigel. FTX downregulation suppresses cell invasion. *p < 0.05
lncRNA FTX physically associates with miR-215
lncRNAs function as microRNA sponges to suppress the targets of microRNAs. The TargetScan software predicted that miR-215, a known tumor suppressor microRNA [11,12,13], may be a target of lncRNA FTX. It was speculated that lncRNA FTX may act as a molecular sponge of miR-215. qRT-PCR showed that silence of FTX markedly upregulated the expression of miR-215 (Fig. 4a). Transfection of miR-215a caused a significant decrease in luciferase activities of the wild-type FTX, but not the mutant-type FTX reporter vector (Fig. 4b). An anti-AGO2 RIP assay was performed to examine whether FTX and miR-215 are in the same RNA-induced silencing complex (RISC). Both FTX and miR-215 could be significantly enriched by anti-AGO2 antibody (Fig. 4c). Furthermore, the MS2-RIP was carried out to pull down endogenous microRNAs associated with FTX, and demonstrated that the FTX RIP in SW480 and HCT116 cells was significantly enriched for miR-215 compared with the empty vector (MS2), IgG and mutant-type FTX (Fig. 4d). The direct interaction of miR-215 and FTX was further confirmed by the RNA pull-down assay (Fig. 4e).
lncRNA FTX is physically associated with the miR-215. a The effect of FTX knockdown on miR-215 expression was analyzed by qRT-PCR. *p < 0.05. b Luciferase activity in SW480 cells cotransfected with miR-215 and luciferase reporters containing nothing, lncRNA FTX or mutant transcript. *p < 0.05. c Anti-AGO2 RIP was performed in CRC cells. *p < 0.05. d MS2-RIP followed by microRNA qRT-PCR to detect miR-215 endogenously associated with FTX. *p < 0.05. e CRC cell lysates were incubated with biotin-labeled FTX; after pull-down, miR-215 was extracted and assessed by qRT-PCR. *p < 0.05. f miR-215 expression was measured by qRT-PCR in 50 pairs of CRC and the adjacent non-tumor tissues. g The correlation between miR-215 and lncRNA FTX expression in CRC tissues
Next, the qRT-PCR assay was conducted to examine the miR-215 expression in 50 pairs of CRC tumors and corresponding non-tumor tissue. CRC tissue expressed lower miR-215 levels than non-tumor tissue (Fig. 4f). The Spearman correlation analysis demonstrated an inverse correlation between miR-215 and FTX expression in CRC tissue (r = −0.4758, p = 0.0001, Fig. 4g). These data suggest that lncRNA FTX directly interacts with miR-215 and suppresses its expression.
The function of lncRNA FTX in CRC cells is partially dependent on miR-215
To further determine whether the effects of FTX in CRC cells are dependent on miR-215, rescue experiments were performed. The suppression of cellular proliferation mediated by FTX knockdown was abolished by co-transfection with the miR-215 inhibitor (Fig. 5a). However, the FTX-silencing-induced migration and invasion inhibition were partially rescued by miR-215 knockdown (Fig. 5b), indicating that other mechanisms were involved in FTX-mediated migration and invasion.
lncRNA FTX reverses the miR-215-mediated suppression of target mRNAs
The targets of miR-215 include ZEB2, HOXB9, NOB1 and YY1, which are critical for cancer initiation and progression [12,13,14,15]. Then, it was speculated that lncRNA FTX may modulate these kinds of gene expression through suppression of miR-215. The depletion of FTX decreased ZEB2, HOXB9, NOB1, and YY1 mRNA levels, while miR-215 inhibitor abolished these effects (Fig. 6a). In contrast, overexpression of FTX promoted ZEB2, HOXB9, NOB1, and YY1 expression, whereas miR-215 mimics attenuated this upregulation (Fig. 6b). These data reveal that lncRNA FTX regulated miR-215-targeting mRNA expression.
lncRNA FTX suppresses the phosphorylation of vimentin
To investigate other mechanisms, by which FTX enhanced CRC migration and invasion, we performed the RNA pull-down assay with biotin-labeled FTX and mass spectral analysis to search the endogenous proteins associated with FTX. Vimentin was identified to bind lncRNA FTX in CRC cells, when it is strongly associated with tumor metastasis. The interaction of lncRNA FTX with vimentin was validated by the RIP assay. A significant association of FTX with vimentin was observed (Fig. 7a). Then, a series of FTX truncations was constructed to map its binding fragment with vimentin, and it was identified that the 5′-end fragment of FTX (0–172) was essential to bind vimentin (Fig. 7b). However, FTX knockdown failed to affect the expression levels of vimentin (Fig. 7c), which suggested that FTX could not influence the stability of vimentin. Previous study reported that lncRNA regulated the phosphorylation of its interacting proteins [16]. The function of vimentin was also regulated by its phosphorylation modification. Therefore, it was speculated that FTX may affect the phosphorylation of vimentin. Two antibodies against different phosphorylation sites (Ser83 and Ser39) of vimentin were employed. Interestingly, it was found that depletion of lncRNA FTX upregulated the phosphorylation level of vimentin on Ser83 (Fig. 7c). However, the Ser39 phosphorylation of vimentin on was not changed after FTX knockdown. Above all, FTX inhibits the phosphorylation of vimentin on Ser83 in CRC cells.
FTX interacts with vimentin and suppresses its phosphorylation level. a lncRNA FTX levels in immunoprecipitates were determined by qRT-PCR. lncRNA FTX RNA expression levels are presented as fold enrichment values relative to IgG immunoprecipitates. b Deletion mapping of vimentin-binding domain in FAL1. (Up) The schematic diagram of full-length and deleted fragments of lncRNA FTX; (down) western blot of vimentin in protein samples pulled down by different lncRNA FTX fragments. c Western blotting shows the protein expression of vimentin and its phosphorylation level on Ser83 or Ser39
Knockdown of lncRNA FTX by siRNA delivery inhibits growth and metastasis of CRC cells in vivo
Finally, the therapeutic potential of siRNA targeting lncRNA FTX was determined. SW480 cells were injected subcutaneously into one flank and the tail vein of nude mice, respectively. After two weeks, mice were randomly classified into two groups to receive either the cholesterol-conjugated control scramble or FTX siRNAs via intraperitoneal injection for 30 days. It was found that xenograft tumors with injection of FTX siRNA had smaller mean volumes and weights than tumors grown with the injection of control siRNA (Fig. 8a, b). Besides, the FTX siRNAs significantly decreased the pulmonary metastasis compared with the control group (Fig. 8c). Moreover, xenografts in nude mice injected SW480 cells with FTX siRNAs expressed higher miR-215 and vimentin phosphorylation than that in the control group (Fig. 8d). These results suggest that lncRNA FTX can be adopted as an effective target for CRC treatment.
Knockdown of lncRNA FTX by siRNA delivery inhibits growth and metastasis of CRC cells in vivo. a Tumor growth of control and FTX siRNA-treated mice. b Tumor weight of control and FTX siRNA-treated mice. c The number of pulmonary metastasis in control and FTX siRNA-treated mice. d The miR-215 expression in xenografts tumors treated with control and FTX siRNA. e The vimentin phosphorylation level on Ser83 in xenografts tumors treated with control and FTX siRNA. *p < 0.05
Discussion
In the present study, the main findings showed that lncRNA FTX was overexpressed in CRC tissue and CRC cell lines. Depletion of FTX exerted suppressive effects on malignant phenotypes of CRC cells, such as proliferation, migration, and invasion. Further mechanistic investigations highlighted the important roles of FTX-miR-215 and FTX-vimentin association in CRC progression.
lncRNA FTX was first identified in the HCC, and the functional roles of lncRNA FTX were demonstrated in some cancers. FTX was found to inhibit HCC growth and metastasis through binding MCM2 and miR-374a [9]. However, in other cancers, lncRNA FTX functions as an oncogene. For example, lncRNA FTX was upregulated and promoted proliferation, migration, and invasion in renal cell carcinomas [17]. lncRNA FTX also acted as a microRNA sponge to suppress miR-342-3p activities, which subsequently enhanced the proliferative and invasive abilities of glioma cells [10]. The present study is the first to systematically evaluate the roles of lncRNA FTX in CRC progression. The upregulation of lncRNA FTX in CRC tissue and the relationship between lncRNA FTX expression and clinicopathological features of CRC patients were demonstrated. Loss-of-functional assays showed that knockdown of lncRNA FTX suppressed CRC cell proliferation, migration, and invasion, suggesting that lncRNA FTX works as an oncogene in CRC.
Recently, researches revealed a new regulatory mechanism between lncRNAs and miRNAs. lncRNAs acted as a ceRNA to interact with miRNAs. Here, the bioinformatics analysis revealed the potential interaction between lncRNA FTX and miR-215, which was confirmed by the RIP, luciferase reporters and RNA pull-down assays. Knockdown of lncRNA FTX increased the expression of miR-215 in CRC cells, and the miR-215 inhibitor rescued the effect of lncRNA FTX in CRC cells, suggesting that FTX promotes CRC progression via acting as a ceRNA of miR-215, which is a known tumor suppressor in some cancers. For example, miR-215 inhibited tumor metastasis via directly targeting ZEB2 in human pancreatic cancer [18]. miR-215 targeted HOXB9 to inhibit proliferation and migration of CRC cells and lead to cell cycle arrest [11]. miR-215 also suppresses epithelial ovarian cancer growth and metastasis [13]. In CRC, miR-215 significantly inhibited cell proliferation, migration and invasion via targeting YY1 [15]. These reports demonstrated a critical role of miR-215 in inhibiting tumor progression. Our results showed that overexpression of lncRNA FTX upregulated ZEB2, HOXB9, NOB1, and YY1 transcripts, whereas miR-215 mimics abolished this effect. An inverse correlation between lncRNA FTX and miR-215 expression in CRC tissue samples was also observed, and it strongly indicated that lncRNA FTX acts as a ceRNA for miR-215 in CRC.
Intriguingly, rescue experiments showed that depletion of miR-215 could not completely rescue the migration and invasion decreased by FTX knockdown, indicating that other mechanisms were involved in FTX-mediated migration and invasion. Vimentin is closely associated with tumor metastasis. The phosphorylation level of vimentin is critical for its function, such as Ser83 and Ser39 [19]. Here, it was showed that FTX interacted with vimentin and downregulated the Ser83 phosphorylation level of vimentin. The exact mechanisms, by which lncRNA FTX inhibits the vimentin phosphorylation need further investigation.
In conclusion, our results revealed the oncogenic role of lncRNA FTX in CRC progression via associating with miR-215 and vimentin. Our finding provides new insight into the function of lncRNA FTX in CRC tumorigenesis through miR-215 and vimentin.
Materials and methods
Tissue samples
All CRC tissue samples and their paired nontumor tissues were collected in Second Hospital of Xi’an Jiaotong University from Oct 2014 to Dec 2016. All CRC tissues were verified by histopathological dectection. Informed consent had been received from all patients from Second Hospital of Xi’an Jiaotong University before tissue collection. This study was approved by the Research Ethics Committee of the Second Hospital of Xi’an Jiaotong University.
Cell culture
Five CRC cell lines, including HT29, LS513, SW480, HCT8, and HCT116, and normal colon epithelial FHC cell line were obtained from American Type Culture Collection (ATCC, USA) and cultured in DMEM supplemented with 10% fetal bovine serum.
Tranfection
The negative control and miR-215 mimic were purchased from Ribobio (Guangzhou, China). Cell transfections were performed by using Turbofect (Thermo) as the manufacturer’s instructions.
Cell proliferation assays
To detect the effect of FTX on cell proliferation, the CCK-8 assay and the colony formation assay were performed as previous study described [10].
Migration and invasion assays
To detect cell migration and invasion, transwell assays were performed as previously described [10].
Lentiviral production and transduction
shRNA targeting lncRNA FTX or scrambled oligonucleotides were ligated into the pLKO.1 vector. The target sequences of shRNAs were provided as follow: shFTX: CTGCTACGACACTGAATTC. HEK293T cells were cotransfected with lentiviral packaging vectors and the lentiviral vectors using Turbofect (Thermo). After 48 h, lentiviral particles in the supernatant were harvested. Cells were then transfected with above lentivirus, respectively. Stable cells were selected with puromycin (1 μg/mL) for one week.
RNA isolation and quantitative real-time PCR (qRT-PCR)
RNAs from CRC tissue and cells were isolated using Trizol reagent (Invitrogen, Carlsbad, Calif, USA) as the standard protocol. Subsequently, cDNA was synthesized by Reverse Transcription System Kit (Invitrogen, Carlsbad, CA, USA). The qRT-PCR analysis was performed on ABI StepOne Plus. GAPDH mRNA was used as the endogenous control. The primer sequences for target genes were shown as follow: FTX-F: GTGTCTCTCTCTCTCTCTCTCTT, FTX-R: CCTCTTCAGCAGTAGCATAGTT; ZEB2-F: CGCCACGAGAAGAATGAAGA, ZEB2-R: GATTACCTGCTCCTTGGGTTAG; HOXB9-F: GGGACGCTTAGCAGCTATTAT, HOXB9-R: GTACTGGCCAGAAGGAAACT; NOB1-F: GGAGGAGGAGGAGGAAGAAA, NOB1-R: CTGCTGGATCTGCTTGATGT; YY1-F: GGATAACTCGGCCATGAGAAA, YY1-R: GAAAGGGCTTCTCTCCAGTATG. The relative expression levels were calculated using 2–ΔΔCt method.
RNA immunoprecipitation (RIP) assay
RIP assay was performed by using a Magna RNA-binding protein immunoprecipitation kit (Millipore, Bedford, MA) according to the manufacturer’s instructions. Cell lysates were incubated with anti-AGO2 (Abcam, ab57113), anti-GFP (Abcam,ab290) antibody, or negative control IgG (CST, 2729).
Xenograft model in vivo
Six to eight week old male nude mice were used for the xenograft assays. 1 × 106 SW480 cells were injected subcutaneously into one flank of the mice. Two weeks later, tumor size was measured and calculated by the formula V = ½ (L × W2). The pulmonary metastasis was detected by H&E staining. The animal study protocol was approved by the Research Ethics Committee of the Second Hospital of Xi’an Jiaotong University.
Statistical analyses
All experiments were repeated three times, and the results were presented as the mean ± SD. The difference was analyzed by student’s t-test or multi-way classification ANOVA tests by using the SPSS software (version 19.0, SPSS Inc.). The Kaplan–Meier method and log-rank test were used to determine the relationship between lncRNA FTX expression and survival of CRC patients. P value less than 0.05 was considered statistically significant.
References
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
Day LW, Velayos F. Colorectal cancer screening and surveillance in the elderly: updates and controversies. Gut Liver. 2015;9:143–51.
Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–61.
Chen S, Zhu J, Wang F, Guan Z, Ge Y, Yang X, et al. LncRNAs and their role in cancer stem cells. Oncotarget. 2017;8:110685–92.
Wangyang Z, Daolin J, Yi X, Zhenglong L, Lining H, Yunfu C, et al. NcRNAs and Cholangiocarcinoma. J Cancer. 2018;9:100–7.
Slaby O, Laga R, Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochem J. 2017;474:4219–51.
Huang W, Tian Y, Dong S, Cha Y, Li J, Guo X, et al. The long non-coding RNA SNHG3 functions as a competing endogenous RNA to promote malignant development of colorectal cancer. Oncol Rep. 2017;38:1402–10.
Chureau C, Chantalat S, Romito A, Galvani A, Duret L, Avner P, et al. Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet. 2011;20:705–18.
Liu F, Yuan JH, Huang JF, Yang F, Wang TT, Ma JZ, et al. Long noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374a. Oncogene. 2016;35:5422–34.
Zhang W, Bi Y, Li J, Peng F, Li H, Li C, et al. Long noncoding RNA FTX is upregulated in gliomas and promotes proliferation and invasion of glioma cells by negatively regulating miR-342-3p. Lab Investig; a J Tech Methods Pathol. 2017;97:447–57.
Vychytilova-Faltejskova P, Merhautova J, Machackova T, Gutierrez-Garcia I, Garcia-Solano J, Radova L, et al. MiR-215-5p is a tumor suppressor in colorectal cancer targeting EGFR ligand epiregulin and its transcriptional inducer HOXB9. Oncogenesis. 2017;6:399.
Chen DL, Lu YX, Zhang JX, Wei XL, Wang F, Zeng ZL, et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–49.
Lin Y, Jin Y, Xu T, Zhou S, Cui M. MicroRNA-215 targets NOB1 and inhibits growth and invasion of epithelial ovarian cancer. Am J Transl Res. 2017;9:466–77.
Zang Y, Wang T, Pan J, Gao F. miR-215 promotes cell migration and invasion of gastric cancer cell lines by targeting FOXO1. Neoplasma. 2017;64:579–87.
Chen Z, Han S, Huang W, Wu J, Liu Y, Cai S, et al. MicroRNA-215 suppresses cell proliferation, migration and invasion of colon cancer by repressing Yin-Yang 1. Biochem Biophys Res Commun. 2016;479:482–8.
Yang Y, Jiang C, Yang Y, Guo L, Huang J, Liu X et al. Silencing of LncRNA-HOTAIR decreases drug resistance of non-small cell lung cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem Biophys Res Commun. 2018.
He X, Sun F, Guo F, Wang K, Gao Y, Feng Y, et al. Knockdown of Long Noncoding RNA FTX inhibits proliferation, migration, and invasion in renal cell carcinoma cells. Oncol Res. 2017;25:157–66.
Li QW, Zhou T, Wang F, Jiang M, Liu CB, Zhang KR, et al. MicroRNA-215 functions as a tumor suppressor and directly targets ZEB2 in human pancreatic cancer. Genet Mol Res: GMR. 2015;14:16133–45.
Dave JM, Bayless KJ. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation. 2014;21:333–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, Y., Zhang, J., Chen, X. et al. LncRNA FTX sponges miR-215 and inhibits phosphorylation of vimentin for promoting colorectal cancer progression. Gene Ther 25, 321–330 (2018). https://doi.org/10.1038/s41434-018-0026-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-018-0026-7
- Springer Nature Limited
This article is cited by
-
LncRNA RPLP0P2 Promotes Colorectal Cancer Proliferation and Invasion via the miR-129-5p/Zinc Finger and BTB Domain-Containing 20 Axis
Biochemical Genetics (2024)
-
Emerging roles of long non-coding RNA FTX in human disorders
Clinical and Translational Oncology (2023)
-
Prognostic significance of long non-coding RNA five prime to XIST in various cancers
BMC Cancer (2022)
-
Non-coding RNAs and epithelial mesenchymal transition in cancer: molecular mechanisms and clinical implications
Journal of Experimental & Clinical Cancer Research (2022)
-
Long noncoding RNA Ftx regulates the protein expression profile in HCT116 human colon cancer cells
Proteome Science (2022)