Abstract
The uveal vascular bed is the largest vascular system in the eye and has a role in supplying almost every tissue in the eyeball. This makes it the most important ocular vascular system. This is an up-to-date review of the literature of the entire uveal vascular bed in health based on detailed anatomy of the posterior ciliary arteries (PCAs), anterior ciliary arteries, cilioretinal arteries, and vortex veins. Although postmortem injection cast preparations gave us useful information on the morphology of the choroidal vascular bed; in vivo studies showed that they misled us for centuries about the in vivo situation. According to the postmortem cast studies, the uveal vascular bed has no segmental distribution, the uveal vessels anastomose freely with one another, there are inter-arterial and arteriovenous anastomoses in the choroid, and the choriocapillaris form a freely communicating and an uninterrupted vascular bed in the entire choroid.
Similar content being viewed by others
“Who does not know that every scientific accomplishment dislodges some deeply rooted error and that behind it is usually concealed injured pride, if not enraged interest?” Ramón y Cajal (1923) [1].
Introduction
The eyeball contains two sets of vascular beds: (i) retinal and (2) uveal vascular beds. With the advent of ophthalmoscopy in 1851, the retinal vascular bed has always been primarily the centre of the interest clinically, with little interest in the background uveal vascular bed since it is not visible on ophthalmoscopy. So, there is a massive amount of literature on the retinal vascular bed, having been the primary focus of interest all along. As regards the uveal vascular beds, however, apart from bits and pieces reported over the years, there is no full composite review published in any scientific ophthalmic journal based on the latest scientific advances. The objective of this review is to provide a comprehensive account of: (A) the anatomy of the posterior ciliary arteries (PCAs), anterior ciliary arteries (ACAs), cilioretinal arteries, and vortex veins (VVs), and (B) lesions produced by their occlusion and acute uveal ischemic lesions seen clinically.
Before the advent of fluorescein fundus angiography (FFA) in 1961, our understanding of the uveal (ciliary) vascular bed since 1700 was based primarily on postmortem cast studies. In 1964, some enigmatic observations inspired me to use FFA to explore this vascular bed comprehensively.
1. In my FFA studies on central retinal artery occlusion in the early 1960s, I noticed that the optic nerve head (ONH) showed vascular filling but no filling of the retinal vasculature (Fig. 1); [2, 3] this finding contradicted the then prevalent concept that the ONH was supplied by the central retinal artery. Thus, this new finding showed for the first time that the ONH was supplied by the PCA circulation and not by the central retinal artery. And FFA also unveiled the in vivo filling pattern of the choroid.
2. There were reports in the literature in which ophthalmoscopically seen choroidal infarcts (initially whitish, and then resolving to chorioretinal pigmented lesions) were erroneously diagnosed as retinal infarcts due to branch retinal artery occlusion.
3. Duke-Elder [4] in 1961 rightly commented: “The tendency for inflammatory and degenerative diseases of the choroid to show a considerable degree of selective localization, despite the fact that anatomically (postmortem casts) the vessels would appear to form a continuous network, has given rise to speculations regarding the anatomical isolation of specific choroidal areas”. As discussed below, fluorescein fundus angiographic studies provided the answer and confirmed Duke-Elder’s views.
4. It was observed that the findings of postmortem casts are not always supported by the in vivo studies of the uveal vascular bed. As discussed below, FFA studies amply showed that.
Uveal vascular system is the largest and most important vascular system in the eye, and has a role in supplying almost every tissue in the eyeball. Following is a comprehensive, but very abbreviated, summary of the uveal vascular bed in health and disease, based on (A) the anatomy of the PCAs, ACAs, cilioretinal arteries, and VVs, (After all, most importantly, basic sciences are the foundation of Medicine), and (B) the lesions produced by their occlusion and acute uveal ischemic lesions seen clinically.
Method of literature search
This review is based on bibliographies of my 36 research studies on the subject, published in peer review ophthalmic journals (mainly British and American, and a few European) since 1962; and an update of the literature by using the following “PubMed” search strategy.
[“Optic Disk”[Mesh] AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh])) OR ((“Uvea”[Mesh]) AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh]))) OR ((“ciliary processes”) AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh]))) OR ((“Choroid”[Mesh]) AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh]))) OR ((“Iris”[Mesh]) AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh]))) OR ((“Ciliary Body”[Mesh]) AND ((“Blood Circulation”[Mesh]) OR (“Blood Vessels”[Mesh]))) AND (“ocular circulation”) OR (“uveal circulation”) Filters: from 1950 – 2022.]
The uveal (ciliary) vascular bed anatomy
ANATOMY OF THE POSTERIOR CILIARY ARTERIES (PCAs)
The PCAs are the main source of blood supply to the choroid up to the equator and the ONH, the retinal pigment epithelium (RPE), the outer 130 µ of retina (and, when a cilioretinal artery is present, the entire thickness of the retina in that region), the ciliary body and the iris. That makes the PCA circulation the most important component of the ocular and ONH circulation.
The ophthalmic artery
PCAs are branches of the ophthalmic artery. The ophthalmic artery is the first major branch of the internal carotid artery. In a study [5] of 170 human specimens, in 164 the ophthalmic artery arose from the internal carotid artery. In four of the remaining 6, although it arose from the internal carotid artery as usual, the main contribution of blood to the orbit and the eyeball came from the middle meningeal artery. In the remaining two specimens, the trunk of origin of the ophthalmic artery from the internal carotid artery was either absent or obliterated in its intracranial and intracanalicular course, so that the middle meningeal artery was the only source of the blood supply to the orbit and the eyeball, instead of the ophthalmic artery. This shows that, contrary to the universal belief, rarely the blood supply to the orbit and eyeball does not come from the internal carotid artery.
Posterior ciliary arteries
There is widespread confusion about the following aspects of the PCAs in man: their nomenclature, number, origin, and distribution. This is because information in the literature is primarily based on reports containing only a few specimens.
The number, and mode, and site of origin of PCAs is discussed at length elsewhere [6].
Site of origin from the ophthalmic artery
According to some authors, PCAs arise from the first part of the ophthalmic artery, or the second part superior to the nerve, or medial to the nerve; others state that they arose on both side of the optic nerve. Meyer [7] in 1887, based on a study of 20 specimens, described the medial PCA and the central retinal artery arising together first, followed by the lateral PCA; in eleven of his seventeen specimens, in which the ophthalmic artery crossed over the optic nerve, the medial PCA with the central retinal artery arose before the lateral PCA; in the remaining six the lateral PCA (alone or with other branches such as the central retinal artery or muscular arteries) arose before the medial PCA. In the three specimens in whom the ophthalmic artery crossed under the optic nerve, the lateral PCA always arose first, followed by the central retinal artery and then the medial PCA in common with the muscular arteries.
In a comprehensive study [6] of 59 human specimens, there was a wide variation in the sites of origin of the PCAs from the ophthalmic artery. The medial and lateral PCAs arose from the first part of the ophthalmic artery in 25; from the angle between the first and second parts in 39; and 48 medial and 37 lateral PCAs arose distal to the angle. Where the origin was distal to the angle, the PCAs arose not only from the second part but even from the bend between the second and third parts and the initial portion of the third part, especially the medial PCA. In nine specimens, all the PCAs arose distal to the angle, and in all of them the ophthalmic artery crossed over the optic nerve. In the remaining specimens, one or both medial and lateral PCAs arose before the angle. There was no relationship of any kind between the number of PCAs present in a specimen and their site of origin. All these points contradict the views expressed by Sudakevitch [8]. PCAs may arise in common with the central retinal artery—this has clinical importance (discussed later). The PCAs and their branches are usually markedly tortuous.
The following description is based on that comprehensive study [6] of PCAs.
Terminology of PCAs and its Implications
In the literature, there has been confusion in the nomenclature applied to the PCAs. Most investigators describing their findings have used the term “PCA” loosely and as a generic term, covering all types of PCAs and their branches. Others have erroneously designated the “main PCAs” arising from the ophthalmic artery as the “long PCAs” right from their point of origin from the ophthalmic artery as. This comprehensive study [6] showed that the old nomenclature is misleading.
Main PCAs
In the literature since 1781, the number of PCAs arising from ophthalmic artery in man have been described highly variably. The Main PCAs are the ones which arise directly from the ophthalmic artery. There are one to five of them (one in 3%, two in 48%, three in 39%, four in 8% and five in 2%) [6]. Usually, there are 2 or 3. The main PCAs usually lie medial and lateral to the optic nerve (Fig. 2); hence they are called “medial PCA” (one in 71% and two in 29%), and “lateral PCA” (one in 75%, two in 20%, three in 2%, none in 3%) PCAs; small and inconstant “superior PCA” in 9% (one in 7% and two in 2%).
Branches of the main pcas
The main PCAs run forward, then divide into multiple branches before entering the eyeball near the optic nerve (Fig. 2) [9]. At that point, they are of two types:
1. Short PCAs (SPCAs)
These may be 10 to 20 in number, depending upon the intraorbital subdivisions of the PCAs before they reach the sclera. They are further subdivided into two subgroups (Fig. 2C):
(a) Paraoptic SPCAs: These are only a few small SPCAs that enter the sclera close to the optic nerve. These arteries are also described by other authors [10]. Available evidence suggests that they mostly supply the ONH.
(b) Distal SPCAs: These constitute the majority of the SPCAs, and enter the sclera midway between the paraoptic SPCAs and the Long PCAs (Fig. 2) on the medial and lateral sides, and run radially toward the equator. On the temporal side, they enter the eyeball in the region of the macula and run forwards radially (Fig. 3). The distal SPCAs mainly supply the choroid.
From this account it is evident that there may be 1 to 5 Main PCAs and then 3 sets of branches of the Main PCAs supplying three different areas.
It is essential to have a clear understanding of the various types of PCAs to avoid confusion because their areas of distribution are different. It must be stressed very strongly that unless this terminology is clearly understood and adhered to, confusion will follow.
2. Long PCAs (LPCAs)
These are two in number - one medial and one lateral (Fig. 2). The long and short PCAs have frequently been confused with the Main PCAs - they are in fact the branches of the Main PCAs and do not arise directly from the ophthalmic artery.
LPCAs enter the eyeball in the horizontal plane on the medial and lateral sides, some distance away from the distal SPCAs, and run radially in the horizontal meridian forward to the iris [11]. Siam et al. [12], in a study of 12 cadaver eyes, found that the lateral LPCA enters the sclera 3 mm temporal to the optic nerve sheath and runs intrasclerally, being visible for 4 mm. Its lateral end is just overlapped by the nasal end of the inferior oblique muscle insertion.
LPCAs supply a small sector of the choroid posterior to the equator on the nasal and temporal sides, in addition to the corresponding segments of the anterior uvea [11].
Ducournau [10, 13] found that (1) the PCAs and their branches were arranged into two independent groups: (a) paraoptic and (b) distal PCA, (2) there was no specific macular SPCA, and (3) observed no anastomoses between the SPCAs.
Distribution patterns of the PCAs
Postmortem cast studies
Frederick Ruysch [14] in about 1700 AD, was the first to describe the vascular pattern of the PCAs and their branches based mainly on the cast studies. Since then, extensive anatomical studies have been conducted, using the postmortem choroidal casts, and those have formed the basis of the classical textbook anatomical description of the choroidal vasculature. According to most of these descriptions: (i) PCAs have no segmental distribution, (ii) they anastomose freely with one another as well as with the ACAs, (iii) there are inter-arterial and arteriovenous anastomoses in the choroid, and (iv) choriocapillaris form a freely communicating and an uninterrupted vascular bed in the entire choroid [15, 16]. From these anatomical descriptions, it was generally concluded that occlusion of PCA or one of its branches should not produce an ischemic lesion.
Classical textbook description of the choroidal vasculature
This was well summed up by Hogan et al. [17] when they wrote that, in the choroid, “Extensive anastomoses exist between the various branches of all short ciliary arteries, so that occlusion of one vessel ordinarily does not produce infarction of the choroid”. However, it is also well known that clinically, inflammatory, ischemic, metastatic, and degenerative choroidal lesions are usually localized. Duke-Elder (1961) [4] commented: “The tendency for inflammatory and degenerative diseases of the choroid to show a considerable degree of selective localization, despite the fact that anatomically the vessels would appear to form a continuous network, has given rise to speculations regarding the anatomical isolation of specific choroidal areas”.
In view of this important discrepancy, over the years, I investigated comprehensively in vivo the choroidal vascular bed, clinically in the human and experimentally in rhesus monkeys.
In vivo studies on pattern of the PCAs and their branches
Experimental studies
FFA studies in rhesus monkeys, on occlusion of the various PCAs and their branches, for the first time, showed that the PCAs, their branches and choriocapillaris in vivo have segmental distributions in the choroid [18, 19].
That contradicted the findings of most of the previous postmortem choroidal cast studies. However, Wybar [15], Ring & Fujino [20], and Uyama et al. [21] from their cast studies, also concluded that these arteries were segmentally arranged and that each branch supplied a localized zone of the choroid. Olver [22], in microvascular casts study, described that distal and para-optic SPCAs supply wedge-shaped areas of choroid, and the choriocapillaris show regional variations and the lobules—densely packed at the posterior pole with a high capillary to inter-capillary ratio.
Clinical studies
PCA occlusion caused by thrombosis is well-documented in patients with giant cell arteritis (GCA), by various histopathological studies [23]. That provided a model of PCA occlusion in man, to evaluate the in vivo distribution and anastomoses of the PCAs in the choroid and ONH by FFA [24]. These GCA studies showed that each PCA has a segmental distribution in the choroid and ONH, and the various PCAs DO NOT anastomose either with the adjacent PCAs or with the ACAs [19].
Similarly, with ACA occlusion (by recession and resection of the various recti in rhesus monkeys as well as in patients with strabismus), the PCAs did not fill the area of the anterior uvea supplied by the occluded ACA [25].
The PCAs not only supply the choroid but also play an important role in the blood supply of the ONH [26, 27].
Distribution patterns of the two main PCAs
The FFA studies showed that the lateral and medial PCAs supply the corresponding parts of the choroid [18]. However, there is a marked inter-individual variation in the area supplied by the two PCAs in man [27]. The medial PCA may supply the entire nasal choroid up to the level of the fovea, including the ONH (Fig. 4b), or its supply may stop short nasal to the nasal peripapillary choroid (Fig. 5), so that it may take no part in the blood supply of the ONH. The medial PCA supplies the area of the choroid not supplied by the lateral PCA or vice versa (Fig. 6). There may be any variations between these two extremes. When there are more than one medial or lateral PCAs, the area supplied by each of them may be one quadrant or only a sector. When the superior PCA is present, it would accordingly supply a superior sector (Fig. 7). Given the marked inter-individual variation in number and distribution by the various PCAs, there can be an extremely variable pattern of distribution by them in the choroid and ONH. Clinical and experimental studies have shown that the various PCAs do not anastomose with one another, and each behaves like an end-artery [19]. All this has tremendous clinical importance.
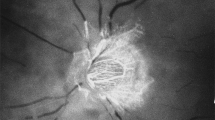
Fundus photograph (a) and fluorescein fundus angiogram (b), of left eye of a GCA patient with arteritic AION, and a cilioretinal artery occlusion. a Fundus photograph shows a classical appearance of arteritic AION, i.e., chalky white optic disc oedema with some hyperaemia. b Fluorescein fundus angiogram shows normal filling of the area supplied by the lateral PCA, but no filling of the area supplied by the medial PCA (including the entire optic disc, with no perception of light).
Distribution patterns of the short PCAs
Experimental occlusion of one or another SPCA in 87eyes of rhesus monkeys [19] resulted in a segmental choroidal filling defect in the area of distribution of the occluded artery (Fig. 8). There are no anastomoses between the adjacent SPCAs. As described above, the SPCAs are of two types (Fig. 2):
(a) Paraoptic SPCAs: Branches from these go to the corresponding parts of the ONH, peripapillary choroid, the circle of Haller and Zinn (CHZ) (when present), and recurrent branches to the retrolaminar ONH pial vascular plexus. Thus, available evidence indicates that all these branches of the paraoptic SPCAs constitute the main source of blood supply to the ONH. The CHZ is discussed at length below.
(b) Distal SPCAs: Each supplies a sector of the choroid, usually extending from the posterior pole to the equator [19]. Each sector varies markedly in shape, size, and location, and has irregular margins (Fig. 8). The various sectors fit together like pieces of a jigsaw puzzle. Further subdivisions of the SPCAs supply correspondingly smaller segments of irregular shape and size, so that the blood supply by the various choroidal arteries has a geographic pattern—the smaller the artery, the smaller the size of the geographic area. Finally, each terminal choroidal arteriole supplies a lobule of the choriocapillaris (Fig. 9) [28].
A The early arterial filling phase of the choriocapillaris, showing each lobule of the choriocapillaris (supplied by the terminal choroidal arteriole) forming a big fluorescent spot. Each spot is surrounded by a polygonal unfilled zone, producing a mosaic pattern in the choriocapillaris. B Peak arterial filling phase. Note the extraordinarily well-defined mosaic pattern, with each unit of the mosaic an independent entity, and the isolated nonfilling or slow filling of some of the lobules is clearly visible. It suggests that there is no communication between adjacent lobules. C Venous phase of the choriocapillaris filling, showing a honeycomb pattern; the fluorescent pattern is reverse of that seen in A, i.e., the fluorescent areas are nonfluorescent and vice versa.
In addition to that, the SPCAs give off branches during their intraorbital course to the pial plexus on the optic nerve, at their site of penetration into the scleral and episcleral arterial plexus and during their course in the sclera to the arterial CHZ (Fig. 10).
Distribution patterns of the long PCAs [11]
The textbook description of the LPCAs
This has been virtually unchanged since the early accounts of Leber [9] in 1903 and other authors at the beginning of twentieth century. The arteries pierce the sclera nasal and temporal to the optic nerve (the former 3.6 and the latter 3.9 mm from the optic nerve [29, 30]), and run horizontally at first in a scleral canal (3–7 mm long [9]; and 3–5 mm long [30]), and then in the suprachoroidal space, as far forward as the posterior part of the ciliary body, where each artery divides into two main division - superior and inferior. The superior and inferior divisions of the two LPCAs unite to form the “major arterial circle of the iris” in the anterior part of the ciliary body behind the root of the iris. Of the ACAs, some join the greater arterial circle of the iris and others the LPCAs or their branches [17]. The LPCA, according to all these descriptions, gives no branches throughout its entire course until it divides into its two main divisions in the ciliary muscle. The greater arterial circle of the iris, the ACAs, and the terminal part of the LPCAs give recurrent choroidal branches, which supply the anterior part of the choroid up to the equator. No anastomoses between the SPCAs and the recurrent choroidal arteries at the equator have been found [9, 31]. Thus, according to all the available descriptions, the LPCAs supply the choroid only in front of the equator via the recurrent choroidal arteries, with no supply to the choroid behind the equator.
in vivo distribution of the LPCA in in the choroid
My study [11] investigated the in vivo distribution of the temporal LPCA in in the choroid in 22 rhesus monkey eyes by FFA, after the following experimental procedures:
Group 1: The temporal LPCA was cauterized outside the eyeball near its site of penetration into the sclera in nine eyes. The accompanying ciliary nerves were carefully separated from the artery and left intact. The rest of the ocular circulation was left undisturbed. None of the rectus muscles was cut, to prevent interference with the ACA circulation.
Group 2: All the temporal SPCAs were cauterized outside the sclera close to their site of penetration into the sclera in 13 eyes. The temporal LPCA was left intact. As in Group 1, the rest of the ocular circulation was also left intact.
Contrary to the earlier classical concept, an in vivo study [11] in rhesus monkeys revealed that the artery invariably supplies an area of the choroid posterior to the equator, starting almost immediately from the point where it joins the choroid and extending forward (Fig. 11).
A Diagrammatic representation of the distribution by the various ciliary arteries in the choroid and watershed zones between them. The choroid posterior to the equator is supplied by the medial and lateral PCAs. In the area supplied by the lateral PCA are shown the segments supplied by the various short PCAs and the one by the long PCA, with the watershed zones between them (dotted circle in this area indicates the macular region). Recurrent choroidal arteries from the ACAs and the greater arterial circle of the iris supply in front of the equator. The watershed zone between the anterior and posterior choroidal arteries lies in the equatorial region. B Composite fluorescein fundus angiograms of rhesus monkey eye, after cutting the temporal long PCA, showing no filling of the choroid in the extreme temporal periphery, temporal to the macular region.
The fundus lesions produced by experimental occlusion of small branches of the PCAs are sectoral distribution because the distribution by the small choroidal arteries is segmental, with no direct anastomosis with the adjacent choroidal arteries. Occurrence of fundus lesions on experimental occlusion of small branches of the PCAs is discussed elsewhere [32].
In patients, chorioretinal lesions are known to occur due to occlusion of the LPCA. I found a whitish lesion in the distribution of the LPCA three days after the onset of severe posterior scleritis (Fig. 12A). This resolved through pigmentary degeneration and left a sector-shaped pigmentary degenerative lesion (Fig. 12B). This lesion resembled in appearance to those produced on experimental occlusion of the PCAs [33]. Those are also called “Amalric’s triangular syndrome”. In posterior scleritis the LPCAs would be much more vulnerable to occlusion (because of their long, oblique course through the scleral canal) than the SPCAs, which have a straight, extremely short course through the sclera. The scleral canal for the LPCA and the accompanying nerves is 3–7 mm long in man [34], divided into two compartments (for artery and nerves) by a fibrous septum, almost parallel to the outer surface of the sclera in the posterior part, and steeper and narrow in the anterior part. Thus, the canal forms a flat bow with its concavity inwards [34, 35]. Scleral oedema and swelling in this region would occlude the canal and consequently the LPCA.
A Whitish choroidal ischemic lesion in the distribution of the temporal long PCA three days after the onset of severe posterior scleritis. B A triangular chorioretinal lesions situated in the area corresponding to the distribution of the resolved ischemic lesion of LPCA. (Reproduced by kind courtesy of late Dr. Pierre Amalric).
The temporal LPCA is intimately related to the insertion of the inferior oblique muscle; the artery pierces the sclera at the nasal margin of the attachment of the tendon and the scleral canal of the artery lies along the line of attachment of the tendon. This fact may be important to bear in mind in surgical intervention on the inferior oblique muscle. It is not stressed adequately in the literature.
Each LPCA extends radially in the horizontal meridian - one on the medial and the other on the lateral side (Fig. 2). On the temporal side, the LPCA supplies a sector of the choroid temporal to the macular region, with its apex posteriorly (Fig. 11). A detailed account of the blood supply by the LPCAs is given elsewhere [11]. Like the SPCAs, their smaller subdivisions supply smaller geographic segments. In addition to supplying a sector of the peripheral choroid, each artery also supplies a small sector of the ciliary body and iris on the medial and lateral sides.
Contrary to the prevalent old classical concept, in vivo study in rhesus monkeys revealed that the LPCA invariably supplies an area of the choroid posterior to the equator. The temporal LPCA joins the choroid 5 mm temporal to the optic disc and the area of the choroid supplied by the artery starts 4.6 to 9 mm (average 6.2 ± 1.0 mm) from the disc. The distance of the equator and ora serrata on the temporal side from the optic disc in rhesus monkeys is 13 mm and 16.5 mm respectively. This shows that the supply by the LPCA to the choroid extends well posterior to the equator. The distribution is sectoral, with the apex of the sector pointing backwards (Fig. 11).
Shimizu and Ujiie [16] in their study of vascular casts of rhesus monkey eyes found that the LPCA runs a long, oblique intrascleral course till it makes a sharp bend to enter the choroid temporal to the fovea on the temporal side, and that it supplies a triangular-shaped sector of the choroid by several local branches. In the temporal fundus beyond the equator, recurrent arteriolar branches from the LPCA supply adjacent small choroidal areas. Thus, these cast studies show the same choroid distribution by the LPCA as seen in my angiographic study. Song et al. [36]. in the human found that the LPCA formed several branches before entering the iris root, and those branches formed the major arterial circle of the iris with diverse diameters, in the vicinity of the iris root and the ciliary process. The watershed zone in the equatorial region shown in Fig. 11 was also demonstrated on wide-angle indocyanine green angiography using a scanning laser ophthalmoscope in the human [37].
The occurrence of chorioretinal lesions due to occlusion of the LPCA in patients (Fig. 12) confirms the above findings in the rhesus monkeys.
Peripapillary choroid
This part of the choroid is supplied by branches from the SPCAs. As in the rest of the choroid, postmortem cast studies have consistently shown free anastomoses in the peripapillary choroidal arteries to form a continuous vascular network [12]. Shimizu and Ujiie [16] in their choroidal casts, found that branches of the SPCAs run centripetally while undergoing a series of bifurcations; an incomplete arterial ring was found along the margins of the ONH, formed by the numerous arteries entering the arterial ring, and the latter was regarded by the authors as an interarterial anastomosis. They concluded that the peripapillary choroidal arterioles therefore cannot be regarded as end arteries.
The peripapillary choroid is a very important part of the choroidal vasculature since it is the main source of blood supply to the prelaminar and retrolaminar parts of the optic nerve (Fig. 13) [27], and NOT by the choriocapillaris, which stop short at the disc margin (Fig. 14). There are some serious misunderstandings prevailing on the subject. It is not uncommon to find ophthalmologists asking: if the peripapillary choroid is so very important in the blood supply of the anterior part of the optic nerve, then why does the optic disc remain normal in eyes with peripapillary atrophy? The branches supplying the optic nerve arise essentially are from the main arteries in the peripapillary choroid (Fig. 15) and NOT from the choriocapillaris; the choriocapillaris stops abruptly at the disc margin (Fig. 14). In almost all the ophthalmoscopically visible peripapillary atrophy, the loss is limited to the choriocapillaris, fine vessels and the overlying RPE, with the large vessels still normal and intact.
Abbreviations used: A arachnoid, C choroid, CRA central retinal artery, Col. Br. Collateral branches, CRV central retinal vein, CZ circle of Zinn and Haller; D dura, LC lamina cribrosa, OD optic disc, ON optic nerve, PCA posterior ciliary artery, PR prelaminar region, R retina, S sclera, SAS subarachnoid space.
My in vivo FFA experimental [27] and clinical studies on anterior ischemic optic neuropathy (AION), glaucomatous optic neuropathy and other ischemic disorders of the ONH have revealed that in vivo the peripapillary choroid has a segmental pattern (Fig. 5); [27], this in turn is responsible for the segmental pattern of blood supply in the ONH, and consequently for the well-known sectoral nature of ischemic lesions seen in AION and other ischemic disorders of the ONH. This is another example of the discrepancy between the postmortem anatomical cast findings and the in vivo circulation.
From a hemodynamic point of view, the peripapillary choroid is a low-pressure system compared to the rest of the choroid, and that has important implications in development of ischemic disorders of the ONH because peripapillary choroid supplies the ONH. Peripapillary choroid lies between the margin of the optic disc and the site of entry of temporal distal SPCAs (Fig. 3). These arteries enter the eyeball some distance away from the optic disc (Fig. 2); the lateral distal SPCAs enter the eyeball some distance away from the centre of the macular region (Fig. 3), and the medial distal SPCAs enter some distance away from the optic disc. Main branches of the distal SPCAs run away towards the equator; on the other hand, the peripapillary choroid is supplied by small branches from the distal SPCAs running towards the optic disc. Since the main direction of blood flow in the distal SPCAs is primarily towards the equator, that makes the blood pressure in their small branches to the peripapillary choroid, running in the opposite direction (Fig. 3), low – a normal phenomenon. That makes the peripapillary choroid a low pressure system compared to the rest of main choroid [26]. This tendency would be further increased by the presence of recurrent pial branches from the peripapillary choroid to the retrolaminar part of the optic nerve (Fig. 14), because that allow the blood to escape from the peripapillary choroid into the pial vessels which, being outside the eyeball, are not subjected to any pressure on them, unlike the peripapillary choroid which is subjected to the intraocular pressure (IOP)—a “functional shunt”. Therefore, when the perfusion pressure in the choroidal vascular bed falls (either due to fall of blood pressure or rise of IOP), the peripapillary choroid becomes most vulnerable to hypoperfusion or non-perfusion, as shown in Fig. 16. This phenomenon plays an important role in development of AION, glaucomatous optic neuropathy, and other ischemic disorders of the ONH. Also, presence of peripapillary degeneration in glaucoma is a well-known clinical finding.
Circle of haller and zinn (CHZ)
The arterial circle lying within the peripapillary sclera, although described first by Haller in 1754 [38] and Zinn in 1755, was in fact first fully described by Tiedemann [39] in 1824 and Huschke [40] in 1844. It is formed by anastomoses between the PCAs (Figs. 10, 14). Its prevalence, what it supplies, and how often it is a complete or incomplete circle have been controversial. More recently, scanning electron microscopic examination of plastic microvascular corrosion casts of the human ONH vasculature has provided detailed information on the circle of CHZ. Reports of its prevalence in human eyes have varied, e.g., Olver et al. [41] found it in more than 75% of 18 casts, Onda et al. [42] in 61% of 18 eyes, and Gauntt et al. [43] in 83% of 29 eyes. It is generally agreed that the CHZ gives 3 sets of branches: (i) to the lamina cribrosa, (ii) to the peripapillary choroid and (iii) recurrent pial branches to the retrolaminar region (Fig. 14) [41, 44]. Olver et al. [41, 44] described the CHZ as a microscopic, intrascleral, elliptical, microvascular anastomosis formed by branches of the medial and lateral paraoptic SPCAs; they preferred to call it “perioptic nerve arteriolar anastomoses” [41]. According to them, the complete or incomplete ellipse is divided into superior and inferior parts by the entry points of these branches into the eye and shows morphological variations (interindividual as well as interocular in the same person) in terms of its form, position and branches [22]. Of the 18 casts of the CHZ examined by them, they found complete anastomoses in 44%, complete with narrowed sections in 33% and incomplete anastomoses in 23%. The CHZ was supplied by branches of medial and lateral paraoptic SPCAs in 15 (83%), lateral paraoptic SPCAs in 2 (11%), and medial, lateral, and superior SPCAs in 1 (6%) [41]. They concluded that it lies in different planes antero-posteriorly (like a hammock) and has both extrascleral and intrascleral portions. They found that branches from the CHZ also formed arteriolar-arteriolar anastomoses. Onda et al. [42]. on examination of 18 casts found that the CHZ is formed by branches of the SPCAs; it was a complete (in 2 of 11 eyes) or a well-developed (9 of 11 eyes) anastomotic arterial circle surrounding the ONH, located approximately 200–300 µm posterior to the suprachoroidal space within the perineural sclera. Gauntt et al. [43]. on serial sectioning of 29 eyes found the CHZ incomplete in 3 and narrow in 8. They described 2 types of position of the CHZ in the sclera around the ONH—in Type 1 it was located lateral to the site of attachment of the dural sheath to the sclera (in 69.0%), and in Type 2 medial to the sheath (in 13.8%); it was more medially located in small optic discs. They postulated that a combination of small disc, medial displacement of the circle, and anatomical variation in the vascular pattern may predispose an ONH to ischemic events. Strek et al. [45]. found considerable variations in the position of the circle in 15 human foetuses aged 16–20 weeks. Heimann [46] also described the beginning of the CHZ formation in foetal studies.
Some authors claimed to have outlined the CHZ on fluorescein angiography. In my FFA studies, I found that various peripapillary choroidal arteries (Fig. 13) usually join to form a “peripapillary choroidal arterial arcade” around the optic disc, situated in front of the sclera, in the choroid and NOT in the sclera. Shimizu and Ujiie [16] in their casts of the peripapillary choroid have very nicely demonstrated the existence of the peripapillary choroidal arterial arcade. It can be easily seen when there is atrophy of the peripapillary RPE. To visualize blood vessels lying deep in the opaque scleral tissue by FFA does not seem feasible [45]. The same objection applies to claims of seeing the CHZ on Indocyanine green angiography in high myopia with peripapillary degeneration. It seems claims of seeing the CHZ on fluorescein or indocyanine angiography arise from the authors’ confusing the peripapillary choroidal arcade with the CHZ.
Submacular choroid
Because of the well-known localized involvement of the macular region in many conditions, a good deal of interest has centred on the submacular choroid. I have discussed the subject at length elsewhere [19, 47, 48]. Despite some claims about the discovery of a special macular artery, all the available evidence is against the existence of such an artery [15, 20]. All the temporal SPCAs enter the eyeball in the macular region (Fig. 3) and each artery then radiates away towards the periphery, like the spokes of a wheel; each one of the SPCAs in the macular region gives branches to the submacular choriocapillaris, as was also seen in the casts by Shimizu and Ujiie [16]. In the human eye, the sites of entry of the various temporal SPCAs are usually situated some distance away from the centre of the macular region, and each artery, near its site of entry, gives recurrent centripetal macular branches, which together supply the macular region (Fig. 3). Each SPCA supplies a segment of the choroid, with no anastomoses between adjacent segments (Fig. 8). Most of the segments of the choroid supplied by the temporal SPCAs and their watershed zones meet in the macular region (Fig. 11A). Similarly, the four quadrants of the uveal tract drained by the four vortex veins and their watershed zones meet in the macular region (Fig. 17). It has been consistently stated that the submacular choroid has a more abundant arterial supply than other parts of the choroid. This impression is based essentially on the fact that the submacular choroid is much thicker than elsewhere. This is because all the temporal SPCAs pierce the sclera in the macular region to join the choroid in the submacular choroidal region and are thus aggregated together in this region. A mere increase in the number of arteries in the submacular choroid does not increase the blood supply and nutrition to that area [15, 19]. Wybar [15], and Ring & Fujino [20] found no difference in the structure and density of the choriocapillaris in the macular region and other areas equidistant from the optic disc. Increased blood flow in the submacular choroid [49, 50] simply seems to represent increased blood flow in the large number of arteries aggregated in the submacular choroid, and not necessarily through the choriocapillaris. Terminal arterioles supplying the macular choriocapillaris arise directly from all the temporal SPCAs and other large choroidal arteries lying in the submacular choroid, and are usually short, vertical and enter the choriocapillaris perpendicularly and abruptly, as compared to those going to the choriocapillaris in the peripheral [16, 22]. This anatomical peculiarity of the submacular terminal choroidal arterioles would make the macular choriocapillaris much more vulnerable to embolism than the peripheral choriocapillaris.
Choriocapillaris
Capillaries of the choroid were first described by Hovius [51] in 1702 and called “choriocapillaris” by Eschricht [52]. According to Passera [53], the smallest choroidal arteries descend perpendicularly and break up at once into a star-shaped formation of capillaries radiating out in all directions. Winslow [54], as far back as 1733, described this layer composed of “vascular stars”.
The classical textbook description of the choriocapillaris
The choriocapillaris is arranged in one plane as a single continuous layer of capillaries forming a network on the external aspect of the Bruch’s membrane; the capillaries have a very wide lumen (10–36 µL; [9] 18–50 µL [17], 18–38 µL; [53]) so that several red blood corpuscles can pass through them side by side (ordinary capillaries have room for hardly one red blood corpuscle) and form a continuous anastomotic network over the entire choroid (Fig. 18), with no segmental distribution. Although Rohen [55] stated that “the entire choriocapillaris network undoubtedly has a continuity by capillary anastomoses” he did find that “the finer branches of the choroidal arteries run in relatively delimited sectors of the choriocapillaris”.
Cast studies
Almost all have agreed on the main features of the choriocapillaris. It has been mentioned that the characteristic feature of the choriocapillaris is a sudden transition from large choroidal vessels to the choriocapillaris, without the usual gradual change through arterioles and venules.
According to Ruskell [56] the efferent venules of the choriocapillaris travel a short distance, draining several capillaries, before turning away obliquely and quickly joining neighbouring venules, and the resulting stem shortly joins the nearest large vein.
In vivo studies
A. Embolization of choroidal arteries:
These in dogs and in cats [57] have also suggested segmental distribution of the choroidal arteries and choriocapillaris. Henkind [57] in his studies stated that “blocking a choroidal artery leads to diminished or absent flow in a large segment of the choriocapillaris; this is in spite of the fact that the choriocapillaris seems to be a continuous anastomotic network over the entire choroid”. Dollery et al. [58], on FFA of pig’s eyes with experimentally raised IOP, recorded the filling of the choriocapillaris in the form of dots which later enlarged and coalesced. Based on these studies, they stated, that “this would indicate that the choriocapillaris fills as small independent segments rather than as a continuum over the entire surface” and that “the segmental filling of the choriocapillaris resembles a pattern of patchy choroiditis or multiple drusen”.
B. Fluorescein fundus angiographic studies
My study [28] showed the following pattern of filling of the choriocapillaris:
1. Early Filling or Arterial Phase. The earliest fillings of the choriocapillaris constituted a filling of the terminal surrounded by discrete, fully filled units (Fig. 19). This suggests that there is no free communication between the adjacent units of the choriocapillaris mosaic.
2. Complete Filling Phase. The entire choriocapillaris bed is uniformly fluorescent and no mosaic pattern is now visible (Fig. 19). It is not uncommon to find well-defined geographical isolated filling defects of variable size and shape in the otherwise diffusely filled choriocapillaris bed, due to the normal spatial variation in their filling. These areas fill late and remain well-defined. Their filling sequence seems slightly delayed as compared to that of the main bed.
3. Early Emptying or Venous Phase. The fluorescent pattern at this stage (Fig. 19) is the reverse of the arterial phase (Fig. 19), i.e., a central non-fluorescent zone (corresponding to the fluorescent zone in the arterial phase—surrounded by a polygonal girdle composed of very tiny fluorescent spots about the size of microaneurysms. This produces a well-defined honeycomb pattern (Fig. 19) which is reproducible on repeated fluorescein fundus angiography, indicating that these are not artifacts. The honeycomb pattern may not always be polygonal and may show variations in size, shape, and pattern.
Thus, these FFA studies revealed that the entire choriocapillaris bed is composed of independent small lobules [28, 48]. Each lobule is supplied by a terminal choroidal arteriole situated in the centre, and its venous drainage is by venous channels situated in the periphery of the lobule. These studies further revealed that each choriocapillaris lobule is an independent unit, with no anastomoses normally with the adjoining lobules in the living eye (Fig. 19). The various lobules are arranged like a mosaic, with the margins of the mosaic formed by the venous channels (Fig. 19). The shape and size of the various choriocapillaris lobules vary in different regions of the choroid, e.g., polygonal shaped in the posterior part and elongated in the peripheral part. The choriocapillaris are arranged more compactly at the posterior pole than in the periphery, so that they gradually become less dense towards the periphery. A more detailed account of the choriocapillaris is available elsewhere [28].
Pattern of the choriocapillaris
Based on these observations, Fig. 9 has been constructed to represent schematically the choriocapillaris pattern. This shows that each terminal choroidal arteriole joins a small segment of choriocapillaris near the middle; this is strongly suggested by fluorescein angiograms of the arterial phase (Fig. 19). The radiating arrangement of the choriocapillaris from the terminal arteriole resembles somewhat the description by Winslow [54] and Passera [53] of a star-shaped pattern. The venous draining channels surround each of these segments of the choriocapillaris and thus usually help to drain adjacent segments, as suggested by fluorescein angiograms of the venous phase (Fig. 19). My further studies convinced me that the neoprene latex concept that the entire choriocapillaris network has a continuous capillary anastomosis was wrong [59]. The present concept, shown in Fig. 19, correlates fully the angiographic patterns (Fig. 19). My present concept on the choriocapillaris, as shown in Fig. 9, has been further confirmed by examination of flat preparations of the human choriocapillaris by Torczynski and Tso [60]. In their excellent study, they found a distinct lobular arrangement in the choriocapillaris, with the feeding choroidal arteriole in the centre of the lobule and the draining venule at the periphery of the lobule (Fig. 20)—a pattern having some resemblance to the pattern of a liver lobule.
Thus, Fig. 9 represents a pattern of the choriocapillaris which agrees not only with FFA findings (Fig. 19) but also with histological (Fig. 20) and neoprene latex and other injection studies.
It shows that each segment of the choriocapillaris is supplied by a terminal choroidal arteriole and is independent. The various segments communicate only via the venous channels. In a living eye, each segment acts as an independent functional unit. Because of hemodynamic factors in each segment, the blood does not flow from one segment to the other as shown very well by angiograms in Fig. 19. The question could be posed: when the blood supply to a segment is cut off, why does it not fill from the adjacent segment via the common venous draining channel? My occlusive studies in a large series have shown that there is no such direct extension of filling between the various segments. I have no definite explanation for this well-established fact. I feel it is possible that the normal IOP obliterates the area of choriocapillaris supplied by an occluded arteriole because of its intraluminal pressure having fallen to nil, and the perfusion pressure in the adjacent venule is not high enough to force blood into the collapsed choriocapillaris.
Conclusion
These studies have suggested that there are no inter-arterial anastomoses in the choroid, so that it is an end-arterial system. The only way the choroid in the occluded artery can fill is by a retrograde filling via the big choroidal veins [18], presumably due to the pumping action by the intraocular pulsation.
Thus, my studies established for the first time that the in vivo vascular pattern of the PCAs and their branches in the choroid and ONH vascular bed is strictly segmental, with no anastomoses between the adjacent segments. This indicates that the PCAs and its branches in the choroid and ONH behave as end-arteries in vivo. This helps to explain the localized nature of choroidal and ONH lesions.
In conclusion, these findings of in vivo studies showed that postmortem cast studies had misled us for almost three centuries into rejecting the fact that PCAs have segmental distribution [19]. The impressive and fascinating scanning microscopic pictures of the postmortem casts published are unfortunately, equally misleading despite their spectacular appearance. The explanation for this disparity may lie in the normally rich autonomic nerve supply (both sympathetic and parasympathetic) of the choroidal vascular bed, which influences the in vivo pattern of blood flow and circulation but is totally absent in postmortem injection studies. For example, glomus cells, controlled by nerve fibres, at arteriovenous anastomoses in the choroid form a mechanism to direct the bloodstream in vivo [61]. Thus, in vivo studies with FFA reveal the actual physiologic circulatory pattern. In the postmortem studies, by contrast, when the cast material is injected under pressure, the vessels without any neural control fill from all sources irrespective of the normal blood flow pattern; so, they give information about the morphological conduits only and not physiologic function. What matters clinically, in explaining different vascular disorders, is the in vivo circulatory pattern. The in vivo studies explain why inflammatory, ischemic, metastatic, and degenerative choroidal lesions are usually localized.
Lütjen-Drecoll [62] reported that arteries and arterioles of the choroid are surrounded by numerous nerve fibres derive from the pterygopalatine ganglion via the facial nerve. Stimulation of the facial nerve causes vasodilation of the choroidal vasculature. In the foveal region there are ganglion cells in the choroidal stroma. Choroidal ganglion cells may have mechanosensory properties and volume regulation. In glaucoma disease the number of choroidal ganglion cells is significantly reduced.
Occlusion of the various main PCAs [18] and SPCAs [19] in rhesus monkeys, caused no direct extension of filling from the normally filling sector of the choroid to the adjacent empty choroid. Similarly, watershed zones and spatial geographical filling defects were seen in normal human choroid and monkey choroid on angiography. These phenomena were difficult to reconcile with the prevalent concept of the time that the choriocapillaris forms a freely communicating vascular network.
Arterial blood supply of the anterior segment of the eye
Leber [9] in 1903 first wrote the classical description of the vascular system of the eye. Subsequent studies have mostly either confirmed or supplemented his findings. Leber [9] reported that there are usually two ACAs associated with each rectus, except the lateral rectus which has only one.
It is well-established that the anterior segment of eye is supplied by the ACAs and the LPCAs.
Based on cast studies in monkeys and humans, the arterial supply of the anterior segment of the eye has been reported by several studies. Figures 21–23 are diagrammatic representation of its various aspects.
(Modified from Van Buskirk [67]).
Anatomy of the anterior ciliary arteries
ACAs are branches of the muscular arteries in the four recti. A study [6] investigated the arteries to the four recti by intra-arterial injection of liquid latex in 58 human cadaver orbits. This study showed that the major source of muscular arteries to the various recti are branches of the medial and lateral rectus muscular arteries, which in turn are branches of the ophthalmic artery; less common are those that arise either directly from the ophthalmic artery or from its other branches. Their site of origin from the ophthalmic artery, mode of origin and the muscle(s) supplied by them are discussed at length elsewhere [6]. Medial muscular artery, a branch of the ophthalmic artery, was present in all specimens, but the lateral muscular artery was seen in only 17%. The medial muscular artery is a big artery—it arises directly from the ophthalmic artery as an independent branch in 74%, and in the rest mostly in common with a PCA. In some specimens, the muscular artery to one or more of the recti arises from the ophthalmic artery by a common trunk with one of the PCAs. The medial muscular artery usually supplies the medial, lateral, and inferior recti; and the lateral muscular artery usually supplies the lateral and superior recti [6]. Figure 1 shows the number of arterial branches to the various recti seen in the study [6].
When a muscular artery arises in common with a PCA, this has clinical importance, because GCA almost invariably involves the PCAs [23]. In such a situation, if the common trunk of the muscular artery and PCA is involved by the GCA and occluded, it can result in ischaemia of the rectus/recti supplied by the occluded artery; that manifests as diplopia—a well-known symptom of GCA. Since ACAs are branches of the muscular arteries, their occlusion can result in anterior segment ischaemia, which has been reported in GCA by many studies.
A study in 1964 [63] investigated the muscular arteries to the recti in rhesus monkeys by intra-arterial liquid neoprene latex injection. As in the human specimens (see above), there were three modes of origin of the muscular arteries to the recti from the ophthalmic arteries:
(1) The Medial Muscular Artery: This is a big artery, and it usually supplies the medial, inferior, and lateral recti.
(2) Independent Muscular Arteries Arise Directly from the Ophthalmic Artery: They always supply the superior and medial recti and rarely the lateral rectus.
(3) Muscular Branches Arising from Other Orbital Branches of the Ophthalmic Artery: They may supply one or more recti.
Ashton and Smith [64] in their human cast studies described the ACAs, divided into the following branches:
(1) Small Episcleral Twigs: These form the episcleral limbal plexus and send branches to the conjunctiva.
(2) Small Intrascleral Branches: They contribute to form an incomplete arterial circle, situated near the canal of Schlemm. There was no afferent connection between the canal and the arterioles, and they do not deal with the aqueous flow.
(3) Large Terminal Perforating Branches: These give branches to the vascular plexus in the ciliary muscle, and within the ciliary body they give branches anteriorly to the major arterial circle of the iris and posteriorly to the anterior choroid. These recurrent choroidal branches were first described by Leber [9] in 1903, and subsequently by many other studies. They also reported that the ACAs and their branches typically run in company with the veins. This arrangement is seen all over the sclera, and the small arterial branches running towards the canal of Schlemm not infrequently use the same scleral tunnel as the anastomosing outlets of the canal.
There are several reports based on studies of casts. In rhesus monkeys, Shimizu and Ujiie [16] reported that the ACAs are in the episcleral tissue and perforate the sclera to enter the ciliary body a few millimetres behind the corneal limbus. According to them, the ACAs did not communicate with the major arterial circle of the iris but divided into numerous branches to supply the ciliary muscle in the vicinity of the site of perforation. However, others [65] have stated that perforating branches of the ACAs terminate in the anterior part of the ciliary body and anastomose with LPCAs to form the “major arterial circle of the iris”. Van Buskirk [65], like Leber [9], reported that in rhesus monkeys there are 7 ACAs which arise from the recti (2 from each rectus except one from the lateral rectus). After leaving the recti, the ACAs first supply the episcleral tissues, and then perforate the sclera and anastomose with the LPCAs. ACAs arising from the lateral rectus often have no perforating branches [65, 66]. After leaving the recti muscles the ACAs interconnect via their lateral-most branches in the episcleral at the limbus to form the episcleral circle [66]. Branches of the ACAs perforate the limbal sclera and enter the ciliary muscle. Heymann et al. [67] studied ACAs emerging from the 4 recti in a radioanatomical study of 25 human eyes. They found that there were numerous individual variations. Following were their predominant findings: the ACA from the lateral rectus is usually thin and located in the superior or inferior third of the muscle. In other recti, the ACAs were rather big and frequently observed in pairs. They tend to be found on each lateral side of the muscle. By and large, vertical ACAs look bigger and more numerous than the horizontal ACAs. Johnson et al. [68]., on a photographic analysis of ACA distribution in the distal parts of 20 lateral and 22 medial rectus muscles of patients, found one major artery in the lateral rectus in 15% and in the medial rectus18%, two major arteries in the lateral rectus in 50% and in the medial rectus in 59%, and more than 2 major arteries in the lateral rectus in 35% and in the medial rectus in 23%. This showed that there was no significant difference in the mean number of major ACAS between the lateral and medial recti. Funk and Rohen [69] in human eyes reported that the perforating branches of the ACA form an intramuscular arterial circle in the posterior region of the ciliary muscle and supply the outer and posterior parts of the ciliary muscle, partly the iris, and the peripheral choroid by recurrent ACA branches.
There are several fluorescein angiographic studies of the ACAs. Meyer [70] on angiography of 13 subjects found the incidence of arteries lowest over the lateral rectus muscle. Ormerod et al. [71]. found marked individual variability and much larger vertical ACAs. Laatikainen [72], in a study of the temporal perilimbal area of 156 eyes, concluded that the first vessels to show fluorescein filling are the branches of the ACAs that are coming from the lateral, inferior, or superior rectus muscles. Meyer and Watson [73] reported that the positions of the ACAs are inconstant as they run radially towards the limbus within Tenon’s capsule. During their course over the anterior part of the globe they usually branch little, if at all. Close to the limbus they give rise to superficial (anterior episcleral) and deep (scleral) divisions. The former continues in the superficial or deeper episcleral. The latter penetrates the sclera and disappear. They found that the scleral contributions were more prominent nasally and the episcleral divisions temporally.
These anatomical findings indicate marked inter-individual variation in the number and distribution of ACAs, particularly the ACA from the lateral rectus. It has been claimed that the contribution from the lateral rectus muscle to the anterior segment circulation may be more robust than is commonly taught [68].
Direction of blood flow in the ACAS
The direction of blood flow in ACAs in normal eyes on fluorescein angiography is controversial. Some have reported that the blood flow in the ACAs is from inside the globe towards the site of attachment of the recti, while others have reported that it is centripetal in direction from the recti throughout their course till their perforating branches pass through the sclera. Still others have found the flow to be centripetal in some eyes and from inside the eye out in other; for example, Meyer [70] found that in 25 of 40 arteries the flow was away from scleral perforations. Because of the blood flow direction from inside the globe outward, some regarded the filled vessels from inside the eye as veins. Shimizu and Ojiie [16], based on their cast studies, reported them as definitely arteries and not veins. Talusan and Schwartz [74] on fluorescein angiography of 12 eyes found no difference in the filling of the ACAs by fluorescein by quadrant; and approximately 4 s after the initial appearance of the dye in the ACA, the anterior ciliary vein showed laminar filling. Meyer [70] on angiography of 13 subjects found veins were concentrated in the vertical meridian and were absent over the lateral rectus in 8 subjects, and all 8 veins drained away from the limbus. Ormerod et al. [75], based on scanning angiographic microscope study of 37 scleral perforating arteries, stated that reports of retrograde blood flow in the ACAs in most fluorescein angiographic studies are probably incorrect, being the result of unappreciated methodological problems.
Since the ACAs arise from the recti, one would normally expect that their blood flow should be from the muscles towards the limbus, but, as discussed above, some fluorescein angiographic studies have shown that it is the reverse. This paradoxical phenomenon is puzzling. Van Buskirk [65], based on his cast studies, postulated the following explanation: Flow direction in the cerebral circle of Willis can reverse when intraluminal occlusions alter relative pressure relationships in the cerebral vascular system. Moreover, regional shifts in cerebral blood flow have been demonstrated with localized cerebral activity. According to him, “By the same token, it could be postulated that since at least two complete anterior anastomotic circles exist in the anterior segment, each fed by diverse arterial trunks, spontaneous changes in flow direction may occur in response to tissue demands of the extraocular muscles, the ciliary muscle, the anterior uvea, IOP, and arterial blood pressure within the individual arterial trunks”. Direct connections between the perforating branches of ACAs and the major circle of the iris “may facilitate development of “reverse” flow in the anterior ciliary arteries with increased pressure within the eye or in the LPCA”. Nanba and Schwartz [76] found a significant positive correlation between the diameter of the ACA and IOP. They postulated that the increase in IOP may influence the circulation of the anterior uvea, resulting in decreased pressure in the ACA and an increase in diameter of the ACA. They put forward this postulate to explain the fluorescein angiographic finding of blood flow in the ACA from the inside of the eye to the outside.
However, none of these postulates fully explain the reverse blood flow documented on fluorescein angiography in ACAs in perfectly normal eyes.
Some of the ACAs, before penetrating the sclera, give branches to the intrascleral and episcleral vascular system. Some of these vessels join the so-called aqueous veins. The relationship between aqueous outflow and blood flow in the limbal vessels also plays an important role in IOP regulation. Thus, there is a close correlation between episcleral circulation and IOP regulation.
Arterial circles in the anterior segment
The following three arterial circles in the anterior segment of the eye have been described. However, there is some controversy about different aspects of the three circles.
1. Episcleral Arterial Circle: This is formed by anastomoses between the adjacent ACAs. Meyer and Watson [73], based on fluorescein angiographic studies in eight normal subjects, reported that this circle is formed by anastomoses between the adjacent ACAs, and is a fragmentary circle. According to them, the anastomotic vessels may be superficial or deep; and the anterior episcleral arterial circle supplies the episclera, anterior conjunctiva, limbus, and iris. An incomplete arterial circle close to the canal of Schlemm is a part of this group (Fig. 21) [64].
2. Intramuscular Arterial Circle: It is formed by branches from the ACAs and LPCAs and is situated in the anterior part of the ciliary muscle toward the root of the iris (Fig. 22). It supplies the ciliary muscle and also sends recurrent branches posteriorly to the choroid. According to Funk and Rohen [69], in human eyes, this circle is formed by the perforating branches of the ACAs, and it lies in the posterior portion of the ciliary muscle.
3. Major Arterial Circle of the Iris: This lies at the root of the iris and in the vicinity of the ciliary processes (Fig. 22). It supplies the iris, ciliary muscle, and ciliary processes, and also sends a few recurrent choroidal branches to the anterior part of the choroid. Leber [9] wrote that the ACAs and LPCAs join at the root of the iris to form the major arterial circle. Shimizu and Ujiie [16], in their cast studies in rhesus monkeys, could not find any communication from the ACAs to the major arterial circle of the iris. According to Funk and Rohen [69], in human eyes, this circle is formed mainly by the LPCAs and supplies the inner and anterior portion of the ciliary muscle, the iris and the ciliary processes. However, others [64, 65] have stated that perforating branches of the ACAs terminate in the anterior part of the ciliary body and anastomose with LPCAs to form the major arterial circle of the iris. According to Van Buskirk [65], this is the least continuous of the three arterial circles and consists of segments.
Blood supply of the sclera, episclera and conjunctiva
These are supplied by the ACAs. Anastomoses between the adjacent ACAs form the episcleral vascular circle. Shimizu and Ujiie [16] studied the blood supply to this region in rhesus monkeys. They showed that the episcleral vascular plexus consists of at least 3 layers, each with different characteristics. (i) The deepest episcleral layer consists of a coarse, widely variable meshwork of small-sized venules and arterioles. (ii) The middle episcleral layer comprises ACAs and their very few branches; anterior to the ACAs lie two to four tortuous vessels which frequently form vascular anastomoses. (iii) The most superficial episcleral layer consists of a vascular network which occasionally communicates with the above vascular anastomoses. They felt that all these layers possibly represent the vascular system of Tenon’s capsule. The inner vascular layer of the palpebral conjunctiva is made up of a network of venules and capillaries. The arterioles are located in an outer layer. The venules run vertically toward the lid margin. The intermediate zone, along the lid margin between the palpebral conjunctiva and the skin, has venules converging towards the site of the external outlet of the Meibomian glands. Funk and Rohen [69], in a cast study of primates, found a capillary network in the equatorial region in the episclera, conjunctiva and subconjunctival layer. In a zone 1–3 mm posterior to the limbus, they found many short typical arteriovenous anastomoses fed by arterioles from the ACAs, but such arteriovenous anastomoses were rare in a zone posterior to the limbal region. The presence of arteriovenous anastomoses in primate casts was also reported by Selbach et al. [34]. who also found capillary loops in the limbal arcade but not in the episclera itself.
Angiographic studies also have investigated the episcleral and conjunctival vasculature. Meyer and Watson [73], on fluorescein angiography of the conjunctiva and episclera in 8 normal subjects, found that the episcleral arterial circle supplies the anterior conjunctival and episcleral circulations and the limbal arcades. According to them, the episcleral circulation is supplied by the episcleral arterial circle. Arteries of the episcleral circle give rise to fine loops that run forwards into the limbal reflection of the conjunctiva before curving back radially to feed the lacework of the anterior conjunctival capillary plexus. Ormerod et al. [75], on 360° angiographic study of the episcleral and conjunctival vasculature in 11 normal human subjects, found an extensive episcleral venous plexus draining into a venous plexus around the rectus muscles, and small branches of the episcleral circle passing forward to the limbus where they looped backward in a radial direction to form the anterior conjunctival veins.
Blood supply of the limbus
The limbus is the region between the transparent cornea and opaque sclera. The blood supply to this part is entirely by the ACAs [64, 66]. Perforating branches of the ACAs, before penetrating the sclera, give branches to supply the limbus. Many arterioles from the limbal region supply the peripheral cornea. The peripheral conjunctiva is supplied by recurrent arterioles which loop posteriorly from the corneal arcade. Venous drainage from the peripheral cornea and conjunctiva, along with those from the episclera, drains posteriorly into the orbital venous system.
This region also contains a variable number of efferent vessels from the outer aspect of the canal of Schlemm, and they vary in size from capillary size to large trunks. Most of them, after leaving the canal, anastomose with one another to form the deep scleral plexus. The deep part of the intrascleral venous plexus of the anterior ciliary veins communicates with Schlemm’s canal through its efferent collector channels. Finally, they empty into the episcleral venous plexus.
Blood supply of the iris
It is well established that the blood supply of the iris comes from the major arterial circle of the iris. There are several cast studies dealing with the blood supply to the iris. Shimizu and Ujiie [16] in rhesus monkeys found the iris vessels arise from the greater arterial circle. The vessels in the iris have a radial course towards the pupil. Vessels along the collarette and the innermost pupillary border have a circular course and are few. The minor circle of the iris is not a complete circle. There are frequent interarterial communications in the iris. The venous blood from the iris flows through the ciliary process and finally into the vortex vein. Van Buskirk [65] in his cast studies in primates reported that iris arterioles usually arise from the major iris circle and occasionally from the intramuscular circle. The arterioles run centripetally toward the border of the pupil. The capillary network of the iris drains into iris venules, which join the veins that run towards the root of the iris, the bases of the ciliary processes and join the choroidal veins. Zhang and Gao [35] in casts of human subjects, found that the iris has about 180–200 radial vessels. The calibres of the iris arteries are homogeneous and measure about 50–120 microns. Song et al. [34]. in flat preparation of the human iris, found that in the pupillary margin the iris vasculature network formed the minor arterial circle of the iris - an arcade, by connecting to adjacent vessels. In the pupillary margin, the capillaries were somewhat thick and connected to the irregular traveling iris vein. Ojima and Matsuo [77] and Funk and Rohen [69], in their cast studies, reported that the iris has corkscrew-like arteries to allow iris movement. According to them, the iris vessels are arranged in the following three layers:
(i) Anterior capillary layer: This consists of many very dense, small, tortuous capillaries.
(ii) Arterio-venular layer: This lies within the iris stroma. It contains arteries and veins. The arteries have a bent spiral course, and the veins are straighter. Near the pupillary margin there is an incomplete minor arterial circle of the iris. The veins run towards the root of the iris and drain into the tributaries of the vortex veins.
(iii) Posterior capillary layer: This lies near the dilator muscle.
The iris capillaries are lined by an endothelial cell layer which has tight cell junctions, which results in the iris capillaries having a blood - aqueous barrier [78]. The iris capillaries also have pericytes.
The iris is a highly vascular structure. Rohen and Funk [79] postulated that that is meant to supply the surrounding avascular tissues of the posterior lamellae of the cornea and the trabecular meshwork with oxygen. Parodi et al. [80]. reported a rare congenital anomaly of iris arteriovenous communication on iris fluorescein angiography and indocyanine green videoangiography in eight patients. These iris arteriovenous communications consist of abnormal vascular connection bypassing the iris capillary bed. Raviola and Butler [81] in rhesus monkeys found unidirectional movement of horseradish peroxidase in the iris blood vessels. That is, when it is injected into the blood stream, horseradish peroxidase remains in the lumen of the iris vessels. However, when it is injected into the anterior chamber it permeates the iris tissues and penetrates the lumen of vessels of the iris by transcellular vesicular transport. Bill [82] reviewed the permeability of the blood-aqueous barrier to substances that are not transported by cellular mechanisms. The iris vessels seem to constitute a more efficient barrier in primates than in rabbits and cats, most probably due to the presence of tighter junctions between the endothelial cells in primates.
Fluorescein angiographic pattern of normal iris vasculature
While cast studies give useful information about the iris vessels, they do not provide any information about their in vivo circulatory pattern and properties. Fluorescein angiography of the iris vessels, however, does provide that essential information. I investigated the normal fluorescein iris angiographic vascular pattern in normal human eyes [83] as well as in the normal eyes of cynomolgus and rhesus monkeys [84].
Normal human iris vascular pattern on fluorescein angiography
In a human study [83], fluorescein angiography in patients with dark to light brown eyes the iris vessels cannot be outlined on angiography because of the brown pigment in the stroma masks the iris vessels. However, it has been shown that iris angiography using indocyanine green dye can show vessels in normal pigmented iris. Recently [85] optical coherence tomography angiography has been advocated for angiography but there are several artifacts in it. On fluorescein iris angiography, the iris vessels are outlined only in blue or green eyes. In my study [83], fluorescein iris angiography was done in 42 blue or green eyes of subjects between 10 and 64 years. Following is a very summary of the findings:
The arteries in the iris started to fill radially from the root of the iris. The sequence of filling in the various segments of the iris was highly variable. Frequently, the entire iris started to fill simultaneously, but more often the various segments filled in different sequences; often the nasal part tended to fill first and the temporal part last. Delayed filling might involve the superior temporal, inferior temporal, or the temporal sectors only. In that case the upper and lower parts of the iris filled in the intermediate phase between the nasal and temporal filling, sometimes simultaneously and other times not. It was notable that, in a few eyes, the vessels in one small segment of the pupillary region (usually the temporal part) showed a substantial delay in filling, so that it was the last part of the iris to fill. The filling of the entire iris usually took five to ten seconds or even longer. In view of this marked diversity, no one filling pattern of the iris can be described as typical.
In iris angiograms, the arterial channels could easily be recognized because they filled first of all from the root of the iris, and from their mostly radial orientation Table 1. The progress of arterial filling in the iris was fairly slow; it usually took a few seconds for the arteries to fill from the root of the iris up to the pupillary region. Although most arteries reached the pupillary region, some small arteries of variable number supplied only the peripheral part of the iris near the root.
In fluorescein fundus angiography the retinal circulation can be divided into distinct arterial, arteriovenous, and venous phases. However, in fluorescein iris angiography, after the arterial phase, all the iris vessels gradually filled with no distinct venous phase, so that at no stage in the transit of the dye were the veins the only vessels outlined in the iris. The only sure way to differentiate the arterial channels from the venous was by reference to the early arterial phase. (Also, the veins in the iris were usually more numerous and thinner than the arteries). The filling pattern of the iris vessels showed that the circulation in the iris was sluggish compared with the retinal or choroidal circulation, so that all the vessels of the iris took a much longer time to fill than in the retina, and a high concentration of the dye persisted in the iris vessels for many minutes. In view of this, a second angiogram after the second injection of fluorescein did not give significant information about the iris vasculature. The area between the collarette and the pupillary margin, which is highly variable in size, had the greatest density of fine vessels, most of them being of capillary size. At the pupillary margin the capillaries formed hairpin loops. The circulation in the pupillary and peripupillary region was often much slower than in the peripheral part of the iris. In the area between the pupillary margin and the collarette, the iris vessels were often much more distinctly outlined than elsewhere, as if the overlying iris tissue was thinnest in this area, being thickest in the region of the collarette, and of intermediate thickness between the collarette and the root of the iris; however, in other eyes, the vessels were well outlined uniformly.
Near the root of the iris, prominent arterial channels were sometimes seen, running close to the circumference, and from them branches arose that ran radially towards the pupil. It seemed as if these arterial channels were parts of the greater arterial circle of the iris and lay in the iris near its root, instead of in the ciliary body.
The collarette contained the minor arterial circle of the iris, which was seen in one form or another in most of the eyes. The circle was mostly incomplete, sometimes with big segments missing, and often fragmented. The various segments of the circle usually filled at irregular intervals and often slowly so that these did not fill completely till the veins of the iris filled, and yet they were connected to the arterial channels. The distance of the circle from the pupillary margin varied in different eyes.
Unlike the retinal vessels, the iris showed no definite arrangement of the arteries and veins.
The iris capillaries showed no fluorescein leakage during the transit of the dye and up to about two minutes thereafter except in ten eyes of this study. In these ten eyes a definite or questionable leak was seen only in the pupillary region, which has the highest concentration of capillaries. It was of a moderate degree in one eye of a girl aged 12 who had had pars planitis and secondary cataract since early childhood. The remaining eyes had only a mild or questionable leak and belonged to individuals 12 to 63 years old, with no evident ocular abnormality.
Circulation time in the iris vessels
My study [83] supports the observations of Chignell and Easty [86] that the iris circulation is much slower than the retinal circulation. Normally, the choroidal circulation is much faster than the retinal because of the much larger size of the lumen of the choriocapillaris (up to 50 µ) than of the retinal capillaries (3.5 to 6 µ [85, 87]). Thus, of the three visible ocular circulatory systems - choroidal, retinal, and iris—the iris is the slowest. It could be argued that pupil size may affect the circulation time in the iris, since the iris vessels will change their course and perhaps their resistance to flow, depending upon the area of the iris served. But the pupils were normal in every respect in all these patients, which contradicts such an argument. Since the intensity of illumination of the flash used in the camera was constant in all angiographies, any pupillary contraction produced by the bright flash was identical as well. The cause and importance of the slow circulation in the iris remain obscure at this stage; it could make the iris more vulnerable to circulatory disorders.
Regional variation in the density of iris vessels
Amalric [88] described the vascularization of the iris as maximal in the superior nasal sector, but in my study [83] there was usually no regional variation in the density of the vessels, though in some eyes it was much higher in the nasal segment than elsewhere; other minor variations were also observed. A high capillary density in the area between the pupil and collarette was seen in my study and reported by others [88, 89]. This could be a factor in the start of iris neovascularization in the pupillary region.
Sequence of filling of the vessels in different parts of the iris
Chignell and Easty [86] stated that the normal iris starts to fill 10 to 15 s after the intravenous injection of fluorescein, usually commencing above and below, with the arteries in medial and lateral quadrants filling 1 to 2 s later. My study [83], however, showed that the sequence of filling is highly variable - often the nasal part fills first and the temporal last, although frequently the entire iris filled simultaneously.
The physiological delay in the filling of various segments of the iris, pupillary region, and the minor circle of the iris could easily be mistaken for a pathological filling defect if the observer is not familiar with this phenomenon. I believe that many of the sectoral filling defects or delays in iris angiography reported by Kottow et al. [90]. in retinal vein occlusion and retinal artery occlusion [91] and by other investigators in some other disorders, may in fact be examples of just such a physiological delay. It is essential to have preoperative or pre-occlusion angiograms in order to identify abnormalities. The variability in the filling of the various segments of the iris on angiography is most probably due to the fact that the various arteries of supply to the iris vary in size and origin from the ophthalmic artery at different points and show a large amount of variation from eye to eye [6]. Sometimes a “filling defect” in the iris and/or limbal region may simply be a photographic artifact, if the area is out of focus or inadequately illuminated.
The end-on appearance of coiled capillary loops at the free margin of the iris
These resemble microaneurysms on angiograms. “Fluorescent spots” at the pupillary margin of the iris in some eyes were the end-on appearance of the coiled capillary loops.
Fluorescein leakage from the iris vessels
The existence of blood-brain, blood-retinal, and blood-optic nerve barriers is well-established. In the iris studies, using tracer material and/or electron microscopy, many investigators have demonstrated presence of a similar blood-iris barrier in the mouse, monkey, and man [79, 92]. Other investigators, however, have reported gaps in the junctions between the endothelial cells of the iris in the rat, cat, and pig [93]. Szalay et al. [94] on fluorescein iris angiography in rats, reported that they found fluorescein leak from the iris at the pupillary margin and in the radially arranged vessels of the iris - most marked in older rats. Vannas [89] reported fluorescein leakage from the iris vessels in 7% of eyes of persons under 50 and in 31% of eyes in subjects aged from 50 to 80 years. Chignell and Easty [86] found no fluorescein leakage in persons under 40 but stated that there may be leakage at the pupil margin in those over 40.
In my study, I looked for fluorescein leakage from the iris vessels during the transit of the dye and for two minutes thereafter. I did not see any fluorescein leakage from the iris vessels in nine of the 11 eyes of eight persons aged 40 to 64 years; among the eyes with fluorescein leakage in this age group, the leak was at the pupillary margin only and was seen in one eye of a 63-year-old person with thyroid myopathy and one eye of a 50-year-old patient who had had recession and resection of the horizontal recti 36 years previously. Among the 23 persons under 40, minimal to mild fluorescein leakage in the pupillary region (usually at the pupillary margin) was seen in eight eyes of seven persons (aged 12, 16, 16, 21, 22, 26, and 31 years): in one of these eyes there was a prolonged history of pars planitis with secondary cataract formation, while in the others, a history of anterior segment disease could not be definitely excluded. The previous authors reporting fluorescein leakage from the iris vessels made no attempts to ascertain whether patients had suffered any past ocular disease. However, fluorescein iris angiography findings by Satoh et al. [95]. suggested that the incidence of leakage of fluorescein from the pupillary margin and anterior chamber angle tends to increase with age and that it is important to consider the physiological changes resulting from aging.
Before any further consideration of the leak in normal eyes, I must stress the following factors: When iris angiograms are even slightly out-of-focus, the iris vessels are blurred and give an erroneous impression of fluorescein leakage. From my personal experience of taking fluorescein angiograms, it is virtually impossible to get uniform sharp focusing of the entire iris in 100% of the angiograms in every patient. It seems probable that some of the so-called “leakage” reported in the literature may well be artifacts of this kind.
Iris pseudofluorescence
In iris angiography the most used combination of excitor and barrier filters always allows visualization of the iris tissue, and that normally helps in the orientation of the iris vessels. However, it has the serious disadvantage of producing a certain degree of pseudofluorescence of the iris stroma, and the pseudofluorescence varies with the thickness of the iris tissue - the thicker the tissue, the greater the pseudofluorescence and vice versa. The pseudofluorescence is particularly great in the region of the collarette. It may not only mislead the unwary observer but also blur the underlying iris vessels, giving an erroneous impression of fluorescein leak.
Thus, all these possible artifacts need to be excluded meticulously before diagnosing iris fluorescein leakage. Having done so, I did find in a few eyes a very localized and mostly minimal fluorescein leakage from some of the capillary loops at the pupillary margin and none from the other vessels in the rest of the iris. As stressed earlier, a history of ocular disease could not be definitely excluded in these eyes.
In the brain, retina, and optic nerve the blood barrier property does not change with age. In my study, there was no evident difference in the blood-iris barrier between young and old, and it is hard to imagine any different behaviour by the iris vessels. This conflicts with some previous claims [89, 94].
From the available evidence, it can be concluded that normal iris vessels, irrespective of age, usually do not leak fluorescein during the transit of the dye or soon thereafter, and that these vessels possess a blood-iris barrier. Whether the iris vessels of the rat, cat, and pig behave differently from those of the human cannot be stated definitely.
Lens autofluorescence
The normal lens produces autofluorescence during fluorescein iris angiography. In my study, this was seen in all the eyes except those of two persons, aged 10 and 17.
Fluorescein stained aqueous
Since the ciliary body possesses no blood-ciliary body barrier, fluorescein rapidly leaks out into the posterior chamber. The fluorescent aqueous of the posterior chamber enters the anterior chamber via the pupil. Its accumulation near the pupillary margin, along with the autofluorescence of the lens, may give an erroneous impression of fluorescein leakage from the iris vessels at the pupillary margin in a slightly out-of-focus picture.
Conclusion
From all these findings of human iris fluorescein angiography, one can conclude the following:
-
1.
The blood supply of the iris is segmental, and the various segments may fill irregularly in a normal eye. This physiological delay in the filling of one or more segments may mistakenly be considered pathological.
-
2.
Blood flow in the iris is usually much more sluggish than in the choroid or retina.
-
3.
Normal iris blood vessels usually show a blood-iris barrier irrespective of age.
-
4.
Iris fluorescein angiography can show vascular abnormalities of the iris, e.g., iris vascular tufts, arteriovenous communication of the iris, iris racemose vascular anomalies, capillary haemangioma, filling defects in strabismus surgery and iris neovascularization.
Normal subhuman primate iris vascular pattern on fluorescein angiography
I felt this information was important when experimental studies are done in subhuman primates to determine their validity to the human. We investigated the normal fluorescein iris angiographic vascular pattern in 23 cynomolgus monkeys (46 eyes) and 12 rhesus monkeys (24 eyes) [84]. All eyes were normal.
The filling of the iris vessels started from the root of the iris and extended towards the pupillary margin. The iris-vessel filling was considered to be complete when the pupillary margin filled all around. The general pattern of iris vessels varied widely. The radial iris vessels in the fluorescein iris angiograms of the monkeys, unlike those of humans, were usually not seen clearly; in the majority, the iris vessels formed an irregular, uniformly distributed mesh of fine vessels with a few inconspicuous and incomplete radial vessels in between. No arteries or veins could be identified because of the intricate and lacy pattern. The distribution of the blood vessels was uniform all over the iris surface. On fluorescein iris angiography, unlike retinal angiography, no distinct arterial, arteriovenous, and venous phases could usually be differentiated during the transit of the dye; occasionally, however, during the very early arterial phase, a filling of only the radial arteries in the iris was seen. The majority of the irides filled diffusely, i.e., the filling started simultaneously at multiple points along the circumference and reached the pupillary border about the same time. In some eyes a sector of the iris would start to fill a few seconds earlier than the rest or vice versa, and the study revealed that, on repeated angiography in the same eye, the sectoral filling delay was not consistent. It is important to appreciate this fact so that this physiologic phenomenon is not considered pathologic. There was no apparent difference in the iris angiographic pattern of the rhesus and cynomolgus monkeys.
The time from the first appearance of the dye in the 46 cynomolgus monkey irides to the complete filling of the pupillary border varied from 1.4 to 6.2 (3.7 ± SD 2.3) seconds. Angiograms repeated two to six times on the same eye but on different days showed a variation in filling time from 0.1 to 5.7 (2.0 ± 2.0) seconds (in 73% ≤2 s). The difference in the filling time between the two eyes of the same animal varied from none to 5.3 (1.4 ± 1.4) seconds (in 80% ≤2 s). These variations in the iris filling time must be interpreted in the light of the various instrumental and technical limitations involved in iris angiography.
The iris vessels showed no fluorescein leakage during either the transit of the dye or the late phase, in any of the eyes. However, the aqueous became slightly fluorescent after several minutes, due to a slow leakage from the ciliary processes. It was easy to get a good quality iris angiogram in the fellow eye with a second injection within a few minutes after the first injection, because of the absence of any significant fluorescence in the iris after the first injection.
This study revealed that despite their brown irides satisfactory fluorescein iris angiograms can be produced in rhesus and cynomolgus monkeys. This opens a new avenue for the investigation of the circulation in vivo of the anterior segment in subhuman primates. As discussed above, in brown human irides it is not possible to outline the normal iris vessels by fluorescein angiography because the brown pigment masks the blood vessels. In contrast to that, no pigment evidently masks iris vessels in the monkeys, suggesting that either the brown colour of the monkey iris is due only to the dark posterior pigment epithelial layer or the melanocytes must lie deeper than the iris vessels; unfortunately, the few anatomical studies on the monkey iris available in the literature do not provide us with any information on the subject.
Our fluorescein iris angiographic studies showed the following:
-
(i)
The iris vascular pattern is identical in rhesus and cynomolgus monkeys.
-
(ii)
It is also quite like that seen in humans.
-
(iii)
In fluorescein iris angiograms, except during the initial phase, it is not possible to differentiate arteries and veins in any of these species.
-
(iv)
The various sectors of the iris do not fill simultaneously but show a physiologic spatial variation in the filling of the different sectors, so that some sectors take longer to fill than others [83]. This physiologic phenomenon should not be regarded as pathologic. From this study, it seems that any sectoral delay in filling of the iris of up to 6 s should be considered within normal limits in monkeys. Repeated iris angiographic examinations in these monkeys revealed no consistent pattern in the delayed filling of the various sectors.
-
(v)
The iris vessels in primates showed no fluorescein leakage. Using tracer material and/or electron microscopy, many investigators have demonstrated in monkey and man the presence of tight junctions between endothelial cells of iris blood vessels [79]. This provides a morphologic reason for the absence of fluorescein leakage from the iris vessels. Thus, fluorescein leakage from the iris vessels is not a normal finding in adult rhesus and cynomolgus monkeys. As discussed above, in the human iris, a mild fluorescein leakage has been reported in some older individuals and in a very few young persons [83, 96]. A slow diffusion of fluorescein from the ciliary processes is universal, because the capillaries in the ciliary processes are fenestrated [17] and the ciliary epithelium shows “leaky” junctions; this leads to fluorescein staining of the aqueous. For more information on blood-ocular, please see the excellent detailed discussion by Cunha-Vaz [97].
Blood supply of the ciliary body
The ciliary body consists of 3 parts: pars plana, pars plicata and ciliary muscle [65]. Leber [9] described an intramuscular arterial circle in the ciliary body, which has been confirmed by others in primates [98, 99] as well in the human [71]. This circle is formed by anastomoses between the perforating branches of the ACAs and LPCAs in the ciliary muscle. Based on their cast study in rhesus monkeys, Shimizu and Ujiie [16], reported that the ciliary body is supplied by branches from the ACAs and consists of collections of vessels of various sizes and directions. They found that the major portion of the blood in the ciliary muscles drains into collector veins in the pars plana and ultimately into the vortex veins. According to Funk and Rohen [69], in the human, the inner and anterior portion of the ciliary muscle is mainly supplied by arterioles from the major arterial circle of the iris, whereas the outer and more posteriorly located portion is supplied by branches of the intramuscular circle formed by the ACAs. In the posterior portion of the ciliary body the venules of the ciliary muscle run in the valleys between the ciliary processes or bend more posteriorly to the layer of the pars plana venules.
Blood supply of ciliary processes
This has been extensively investigated by many studies by examination of casts in primates and human. The pars plicata consists of about 70 major ciliary processes, interdigitated between an equal number of minor processes [65]. Major ciliary processes are separated from each other by deep grooves, which contain minor processes. The blood supply to the ciliary processes is from the greater arterial circle of the iris [100].
Shimizu and Ujiie [16] in rhesus monkeys reported that the vascular system of the ciliary processes is distinct from that of the ciliary muscle. In each ciliary process, branches of the greater arterial circle of the iris enter at its anterior part and run backwards. According to Morrison and Van Buskirk [98, 99], in primates, the ciliary process microvasculature is highly complex, with two types of branches arising from the greater arterial circle of the iris going to the ciliary processes – anterior and posterior. The anterior arterioles possess focal constrictions and supply the anterior and marginal aspects of the major ciliary processes as well as interprocess networks that connect contiguous processes. The posterior arterioles are less constricted and supply the minor ciliary processes and the central portions of the major processes. These findings, and the demonstration of multiple other channels, suggest several mechanisms by which blood can be shunted between and around the major and minor ciliary processes. Morrison and Van Buskirk felt that the presence of focal constrictions in the anterior arterioles suggests a site for possible autonomic or neurohumoral control of blood flow into the major ciliary processes. Capillaries of major and minor ciliary processes are irregularly dilated.
Funk and Rohen [69, 100], based on their human cast study of the ciliary processes’ vasculature, described the capillaries of the ciliary processes as fenestrated, as was also shown in primates [65]. From the functional point of view, they found that the vascular system of the ciliary processes consists of the following three different territories with discrete arterioles and venules.
First vascular territory
This is located at the anterior-most portion of the ciliary processes. In this region, the ciliary processes are connected with each other circumferentially by broad, leaf-like processes. The capillary network in this region is drained by separate venules which run posteriorly after they turn into the base of a neighbouring ciliary process. According to Funk and Rohen [100], this territory of the ciliary process vasculature may participate in volume regulatory mechanisms and fluid reabsorptive processes rather than aqueous formation.
Second vascular territory
This includes 2/3 of the major ciliary processes. The vasculature in the major ciliary processes resembles that of the renal glomeruli. In the anterior two-thirds of the arterioles in ciliary processes are arranged in tufts. The ciliary processes are leaf-like and contain a dense network of wide capillaries. These are supplied anteriorly by the “afferent arterioles” and are drained posteriorly by the “efferent venous segment” towards the pars plana region [100].
Third vascular territory
This includes the posterior third of the major ciliary processes and the minor processes. It is supplied by separate arterioles from the major arterial circle and shows few connections with the capillary network of the anterior portion of the ciliary processes.
The division of the ciliary process vasculature into three vascular territories, according to Funk and Rohen [69], may reflect a functional differentiation in the process of aqueous humour production.
There is dual arteriolar supply of the ciliary processes [100], and that suggests that there are mechanisms by which blood can be shunted within and around the major ciliary processes.
According to Funk and Rohen [69], although in humans the general arrangement of the ciliary process vessels is like that of the cynomolgus monkey, there are characteristic differences in the size of the territories and in the pattern of the capillary networks.
The ciliary processes are highly vascular and secrete aqueous humour. The blood flow in them, as in the choroid, is very fast, so that there is a relatively low arteriovenous pO2 difference between the anterior and posterior regions [100]. This increased perfusion of the vasculature of the ciliary processes is essential for production of aqueous humor [69], and their blood flow could directly affect aqueous humour formation by controlling hydrostatic pressure in the capillary and, thereby, ultrafiltration [65]. According to Kiel et al. [101], there is a dynamic relationship between ciliary blood flow and aqueous humour production, with production being blood flow independent above a critical level of perfusion, and blood flow dependent below it. They wrote that the plateau portion of the relationship shifts up or down depending on the level of secretory stimulation or inhibition, and that oxygen is one critical factor provided by ciliary blood flow. Bill [82] described how the tight junctions between the non-pigmented epithelial cells restrict intercellular movement of small molecules and ions from the ciliary processes into the posterior chamber. For plasma proteins, the endothelial cells of the capillaries constitute an additional barrier. The number of tight junctional strands and the electrical conductance of the epithelium are such that it can be classified as a border case between leaky and tight epithelia. Although there is no anatomical barrier, there is an efficient functional barrier between the stroma of the ciliary processes and the anterior chamber.
Venous drainage from the ciliary processes
Veins are situated along the perimeter or rim of each ciliary process. They are drained posteriorly by venules, and then they drain into the veins of the pars plana and finally into the vortex veins [69, 98,99,100].
Vasculature of the pars plana region
The pars plana lies between the posterior tips of the ciliary muscle bundles and the ora serrata. In this region, there are venules from the iris, ciliary muscle, and ciliary processes. There are numerous interconnections between these venules in the central portion of the pars plana in primates [100]. The venules finally drain into the vortex veins.
Anatomy of the cilioretinal arteries
The cilioretinal arteries belong to the PCA system. They usually arise from the peripapillary choroid or directly from one of the SPCAs. The cilioretinal artery is a congenital anomaly rather than one acquired due to some disease, inflammatory, or other morbid process. It was first described by Müller [102] in 1856, and first demonstrated histologically in 1876 by Nettleship [103], who stated that in sectioning of a human eye, he found “a small artery from the choroid turning round the edge of the disc, and running uninterruptedly for some distance in the retina, where it breaks up into capillaries”. Since then, there have been many reports of the presence of a cilioretinal artery in the literature. In 1879 Nettleship [104] published many important observations, still relevant, about the cilioretinal arteries. He stated the following:
-
1.
It is common to see healthy eyes in which a cilioretinal artery on reaching the disc turns sharply back and disappears under the rim of the sclera, as if running into (or emerging from) the choroid.
-
2.
The cilioretinal artery is generally very small but may be as large as one of the main divisions of the central retinal artery.
-
3.
They are always situated on the temporal side of the disc and pass to the foveal region of the retina.
-
4.
No practical significance can at present be attached to this peculiarity in the blood-supply of the fovea of the retina.
-
5.
Such a vessel is generally present in only one eye, but it is occasionally seen in both. It is usually single but may be two in the same eye.
-
6.
A cilioretinal artery can in most cases be readily distinguished from the other retinal arterial branches (generally of small size), which are often seen to enter (or emerge from) the optic nerve at various points between the temporal border and the physiological pit. In some cases when the vessel disappears very near to the border of the disc, it may be impossible to say whether it passes straight down the nerve or curves around the sclera.
-
7.
Anatomists do not agree as to whether those vessels, which disappear between the optic disc border and the physiological pit, are branches of the central retinal artery given off before reaching the eyeball, or branches of the ciliary vessels.
-
8.
The cilioretinal arteries do not appear to anastomose with the other retinal vessels either on the retina or optic disc.
-
9.
The presence and size of the cilioretinal artery does not appear to bear any relation to disease. It often seems to taper off a little towards its point of submergence in the choroid or sclera; this appearance being explained in the same manner as the similar apparent tapering of the central retinal artery and vein in the physiological pit, and not indicating any diminution in size.
Nettleship stated that this cilio-retinal vessel may be either an artery or a vein. We now know that it is almost always an artery and only rarely a vein.
Like Nettleship [104], Lang and Barrett [105] in 1888 defined a cilioretinal vessel as “one which dips into the nerve near the margin of the optic disc and which can be seen to arch outwards” and did not regard a vessel without this curve as cilioretinal. Jackson [106] stated that the artery curved as it emerged. This characteristic sharp hook-like appearance at its site of entry into the retina at the optic disc margin was described as a diagnostic feature of this artery by Salzmann [107] and Duke-Elder [108].
Salzmann [107] stated that a nasal cilioretinal artery was associated with anomalies of the optic disc. Collier [109] demonstrated an association of cilioretinal arteries with congenital anomalies of the optic disc and fundus and with refractive errors (he found ametropia in 195 eyes, of which 47% were astigmatic; 40·2% had hypermetropia, with or without astigmatism, and 32·5% had myopia). Glees [110] suggested some genetic association between cilioretinal vessels and various abnormalities of the retinal vessels, cerebral angiomata, and aneurysms of the cranial vessels, and stressed their value as diagnostic signs. Loring [111] stated that cilioretinal arteries were seen where the nutrition of the retina had suffered from morbid processes, but Nettleship [104] found no association with any disease. In my clinic, I have seen a cilioretinal artery as a common finding in normal eyes, varying widely in size (in few supplying almost half of the retina) with no evident association with any ocular disease.
Birnbacher [112] in 1887 rightly stated that every artery which appeared at or near the disc margin was not necessarily a cilioretinal artery. Duke-Elder and Wybar [4] described the division of the central retinal artery within the substance of the optic nerve into two, four, or even eight arteries, which emerged separately at the disc, no parent trunk being visible ophthalmoscopically. Blunt [113] found the central retinal artery bifurcating 0–4 mm. behind the disc in one case, and one of the branches passed to the margin of the lamina cribrosa to emerge from the disc at the periphery. The condition described resembles somewhat the left side of my reported cases in a rhesus monkey [114]. Bloch [115] and Salzmann [107] saw patients with arteries at the periphery of the disc and no central artery. According to Parsons [116], this possibility cannot always be eliminated. Thus, rarely the artery that looks like the cilioretinal artery ophthalmoscopically may in reality be an early branch of the central retinal artery, arising within the optic nerve, and in such cases, a normal central retinal artery would also be seen. In such cases, fluorescein fundus angiography is helpful, because normally the cilioretinal artery fills at the same time as the choroid and usually before the onset of filling of the central retinal artery or its branch. On the other hand, if it is an early branch of the central retinal artery, the branch and the main central retinal artery fill simultaneously.
Anatomical proof of the presence of the cilioretinal artery
This is very rare in the literature. Anatomically cilioretinal artery is usually one cilioretinal artery, but there may be up to 5 in number. Only Wybar [117] followed the whole course of one cilioretinal artery in latex casts, and conclusively proved that it arises from the PCAs, thus definitely proving it a cilioretinal artery. In my [114] study of two eyes of a rhesus monkey, in serial sections of the optic nerve, I was also able to trace the arteries to their origin from the PCAs and showing them to be true cilioretinal arteries. However, most previous authors have based their statistics and conclusions only on ophthalmoscopic appearances; but, as mentioned above, no absolute reliance can be placed on the ophthalmoscopic findings. Those who have based their observations on the ophthalmoscopic appearances have found the cilioretinal arteries to be very common, whereas a detailed anatomical examination of 72 eyes [113, 118] revealed no such artery (though such a negative observation is to be taken cautiously). Similarly, the anatomically based reports of the incidence of cilioretinal arteries in the rhesus monkey also seem to be low, because no example was found in about twenty eyes examined both ophthalmoscopically and either histologically or by latex injection [63].
Origin of cilioretinal arteries
In the literature, there is controversy about the origin of the cilioretinal arteries. According to Nettleship [104], these vessels were seen going to the choroid. Randall [119] found that most of them arose directly from the SPCA and passed through or around the edge of the choroid. Birnbacher [112] found one arising from the choroidal artery in a histological section and similar formations were seen by others [120,121,122]. Elschnig [120] further stated that they might arise from an artery communicating with the CHZ and with the choroid. Others [103, 108, 121] stated that they usually arose from the CHZ. According to Bailliart [123], they arose at times from the PCA and very rarely from the choroidal artery, but Elschnig [124] did not find one arising directly from the PCA. According to Parsons [116], they were mostly derived from the PCAs and not from the choroidal artery. In my [114] study of two eyes of a rhesus monkey, I was also able to trace the cilioretinal arteries to their origin from the PCAs in serial sections of the optic nerve.
Benson [125] described the existence of a retinociliary artery arising from the central retinal artery and dipping into the disc. This may represent a retinociliary collateral, which develops in some eyes after central retinal artery occlusion. In one specimen in my anatomical study [126] on the central retinal artery, a branch arose from the middle of the horizontal section of the intraneural part of the artery, and ran forwards in the centre of the nerve to enter the eyeball with the central retinal artery at the optic disc, to supply the superior part of the retina; this artery could mistakenly be considered as a cilioretinal artery.
Number of cilioretinal arteries in an eye
There is a wide variation, usually one and up to 3, in the reported incidence of the number of cilioretinal arteries in an eye, when present. Parsons [116] described them as usually small in size though rarely equal to one of the main divisions of the central vessels. Jackson [106] found large arteries in 22.5%, medium in 28.3%, and small in 49.2%. In my study [114] in a rhesus monkey, there were 8 cilioretinal arteries in the right eye and three in the left.
Coats [127] stated that the cilioretinal artery might be too small to be of any use in cases of central retinal artery occlusion, but Collier [109] in a study of 1000 subjects found in 93.7% of them to be functionally available. We know now that cilioretinal arteries may supply from a tiny part to a large part of the retina, and even the entire retina (see below).
Incidence of cilioretinal arteries
The incidence of the presence of a cilioretinal artery had been reported variably by different studies. Nettleship [104] stated in 1879: “this condition is much more common than has been reported, so common indeed that when the attention has once been drawn to the subject, scarcely a week passes without one or more examples coming under notice in the ordinary routine of ophthalmoscopic work”. Most studies were based on ophthalmoscopic evaluation; however, as mentioned before, that can be deceptive, because what looks like a cilioretinal artery on ophthalmoscopy may actually be an early intraneural branch of the central retinal artery, emerging at the optic disc as a separate artery. The most reliable way to ascertain the true incidence, as mentioned above, is by fluorescein fundus angiography. There are only two fluorescein fundus angiographic studies: (i) Justice and Lehmann [128] evaluated the incidence of cilioretinal arteries in 2,000 eyes of 1,000 consecutive patients by reviewing stereoscopic colour fundus photographs and fluorescein angiograms. One or more cilioretinal arteries were present in 49.5% of all patients or in 32% of the eyes. The arteries occurred bilaterally in 14.6%. Great variation in size, number, and distribution of cilioretinal vessels was observed. The incidence reported by this study is much higher than those previously reported on ophthalmoscopic evaluation, because in this study early-phase fluorescein angiography helped in the detection of these vessels that might otherwise have been missed. (ii) Wang [129] in a study of 2,050 eyes found it in 40.2%. He found 129 eyes in which a cilioretinal artery was believed to have been found on ophthalmoscopy but that was not confirmed on fluorescein angiography. This shows that cilioretinal arteries are actually more common than most ophthalmologists believe.
Clearly, ophthalmoscopic examination is not a true guide to the presence or absence of the cilioretinal artery, and no absolute reliance can be placed on the ophthalmoscopic appearance of the fundus and the location of the arteries on the disc in reaching a correct diagnosis of the cilioretinal arteries. Fluorescein fundus angiography is the only reliable guide.
Location of the cilioretinal artery
Most authors have described them as situated on the temporal side of the disc and supplying the macula, but Randall [119] stated that they might be seen elsewhere and might pass to widely separated areas of the retina. Jackson [106] found an artery like a hook in the disc in 44.5%, running from the edge of the disc in 38.7%, starting from the choroid in 16.8%, and distributed to the macula in 98.4%, an incidence which he thought too great to be accidental. Salzmann [107] and Duke-Elder [108] described them as occurring very rarely on the nasal side. Collier [109] in a series of 250 eyes with cilioretinal arteries found them in the superior temporal quadrant in 47%, inferior temporal in 25%, macular in 15%, superior nasal in 10%, inferior nasal in 3%, and all over the retina in one case. Justice and Lehmann [128] in their study of 2000 eyes of 1,000 consecutive patients found large unilateral temporal cilioretinal vessels in 164 patients and bilateral in another 23 patients, so that in 19% of the patients, they contributed some portion of the macular circulation. Forty-three eyes had the cilioretinal arteries originating nasally in 43 eyes, superiorly in 45 eyes, and in only four inferiorly. Shihab et al. [130]. described the distribution of 57 cilioretinal arteries in their study: superior temporal in 25%, temporal in 14%, inferior temporal in 48%, nasal in 5%, and superior nasal in 7%. In my studies, I have also found that, although most cilioretinal arteries are situated on the temporal side, they can also be seen on other sides (see below).
Size and area of the retina supplied by a cilioretinal artery
In my studies, I have found that the size and area of the retina supplied by a cilioretinal artery vary widely - from a minute artery supplying a tiny area of the peripapillary retina, to supplying about half of the retina. Wang [129] reported that in his study of 195 eyes with cilioretinal arteries, in 52.3% they did not supply the macula.
I have seen two patients where cilioretinal arteries supplied the entire retina [131]. Hedge et al. [132] reported a similar case. Parsa et al. [133] reported a case with cilioretinal arteries supplying the entire retina in both eyes. The phenomenon of a missing central retinal artery, where the entire retina was supplied by the cilioretinal arteries, was first reported by Bloch [115] in 1906 and Salzmann [107] in 1912—each reported one case of complete absence of the central retinal artery, substituted by 2 cilioretinal arteries. Collier [109] described complete absence of the central retinal artery in one case. These findings are in sharp contrast to the findings of Nettleship [134], who was of the opinion that, even when large numbers of cilioretinal arteries were seen and even in the lower mammals, a rudimentary central retinal artery lying in the central fibrous tract of the optic nerve near the eye was always present. This is not supported by my anatomical study of a rhesus monkey where both eyes showed multiple cilioretinal arteries (eight in the right, and three in the left) [114]. The central retinal artery was absent on the right side, and on the left it supplied only the upper part of the retina. Ophthalmoscopic examination showed nothing grossly abnormal except the peripheral location of the arteries on the disc, but a detailed anatomical examination revealed the true picture. In my anatomical study of the central retinal artery in man [126, 135], in 106 eyes, I never found an eye where the central retinal artery was absent. Similarly, in another anatomical study of 16 specimens in the rhesus monkey [63], the central retinal artery was always present. It may be worth mentioning here that the basic vascular pattern of the eye and optic nerve in the rhesus monkey closely resembles that in man [63], so that the findings in one should be applicable to the other for all practical purposes.
In addition to supplying of the retina, cilioretinal retinal arteries may supply the corresponding part of the optic disc surface; when a cilioretinal artery stops short of reaching the retina and ends in the surface layer of the optic disc, it has been called a “cilio-papillary artery”.
Most consider the cilioretinal arteries to be end-arteries. However, Collier [109] found a cilioretinal artery anastomosing on the optic disc in 4·5%, between a branch of the central retinal artery in 1·5% and between the retinal and choroidal systems in 0·6%. No other author has described any such anastomosis. This may be due to old central retinal artery occlusion where the retinociliary collateral can develop [131].
Cilioretinal arteries in lower mammals
NettIeship [134] found that, in lower mammals with a well-developed retinal blood supply, the cilioretinal vessels took a major part in the retinal circulation and, in some species, even of the whole of the retina. But even when a large number of cilioretinal arteries were seen, a rudimentary central retinal artery lying in the central fibrous tract of the optic nerve near the eye was always observed. In some species (lemur, camel, otter), the central vessels enter the nerve very close to the eye, suggesting that they are cilioretinal vessels from the sclera or choroid rather than separate twigs; according to Nettleship, the system of retinal blood vessels is the same in monkeys as in man. Mann [136] stated that cilioretinal vessels are very commonly seen in lower animals. She described the development of the cilioretinal artery by the enlargement of an anastomosis of one of the PCAs with a small branch from the hyaloid artery on the disc. This anastomosis is located in man at the edge of the optic disc where traces of the SPCAs enter the nerve near the lamina cribrosa in the 3–4-month-old human foetus, but these seldom reach the disc. She further added that the presence of the cilioretinal artery in man may have some atavistic implication. Johnson [137] thought it might be a vestigial relic in the human eye. Duke-Elder [110] stated that, in the lower mammals, the ciliary system tends to assume more and more importance, so that in some the whole of the retina is supplied by it. Franck [138] and Bach [139] described the absence of the true central retinal artery, and Langenbacher [140] found it anastomosing with the ciliary arteries. In the dog, cat, and the fox there are many ciliary vessels in addition to the retinal vessels [140]. In man the absence of the central retinal artery is rare, but many arteries may emerge from the margin of the disc, and these are probably due to a division of the central retinal artery within the nerve substance. Salzmann [141] reported the disappearance of the cilioretinal arteries in his own eyes with age and he believed the process to be like the disappearance of the hyaloid artery. My histological study [114] of two eyes of a rhesus monkey showed multiple cilioretinal arteries (8 in the right and 3 in the left); the central retinal artery was absent in the right eye and supplied only the upper part of the retina in the left.
Anatomy of Uveal venous vascular bed
Vortex veins
The various veins from the uveal vascular bed merge to form a vortex vein. There are usually 4 vortex veins. Each drains the corresponding segment of the iris, ciliary body, and choroid. They have a whorl pattern, seen in the equatorial region. Before perforating the sclera, each vortex vein has an ampulla, i.e., a dilated portion. Lim et al. [142] in 46 autopsy eyes found more than four vortex veins in 70%. The number of second or third vortex veins found in the nasal quadrants was significantly higher than the number found in the temporal quadrants (P < 0.01). Kutoglu et al. [143]., on a study of 60 cadaver orbits, found that the number of vortex veins per eye varied from four to eight. Most frequently there were four (35%) or five (30%) vortex veins. Three eyes (5%) had eight. Although the incidence of the vortex veins was variable, there was at least one vein in each quadrant of the sclera. Intervortex venous anastomosis in pachychoroid, central serous chorioretinopathy, peripapillary pachychoroid syndrome, and pachychoroid-associated neovascularization have been reported [144].
Watershed zones between the various vortex veins
There are poor communications between the adjacent vortex veins [145]. The watershed zones between the various vortex veins therefore extend:
-
(1)
Antero-posteriorly through the entire length of the uveal tract - a horizontal watershed between the upper and lower vortex veins passes through the optic disc and macular region, and a
-
(2)
Vertical watershed between the temporal and nasal vortex veins passes between the optic disc and macular region (Fig. 18).
The superior vortex vein drains into superior ophthalmic vein, to join the cavernous sinus. The inferior vortex veins drain into Inferior ophthalmic vein and join either the superior ophthalmic vein or the cavernous sinus directly.
Chorio-vaginal vein
In addition to the vortex veins, in some eyes a vein may be seen which passes from the choroid through the sclera closely adjacent to the optic nerve-head (Fig. 24) and drains into the venous plexus of the pial sheath of the optic nerve [146]. This chorio-vaginal vein occurs more frequently in highly myopic eyes than others [4].
Choroidopial veins
Ruskell [147] investigated the peripapillary venous drainage from the choroid histologically in 10 human ONHs. Peripapillary veins varying in number and size were present in seven preparations and none in the other three. All veins penetrated the sclera from the choroid close to the ONH and entered the pia mater directly, receiving small veins from the laminar and postlaminar ONH. No other locations of posterior venous penetrations of the sclera were found. He called them “choroidopial veins”. He concluded that choroidopial veins represent a minor and inconstant route for blood drainage from the choroid, with a role in ONH circulation.
Flugel-Koch et al. [148] in 21 human eyes found a network of nonvascular alpha-smooth-muscle-actin-positive cells (NVSMC) in the entire choroid, most densely arranged in the posterior part of the suprachoroidal of the submacular region. Posteriorly the NVSMC net reached the optic nerve, anteriorly it ended in the region of the vortex veins but did not continue into the ciliary muscle. The elastic net of the choroid was firmly connected with the posterior elastic tendons of the ciliary muscle. During accommodation, the ciliary muscle pulls this net forward, presumably influencing the position and diameter of the choroidal vessels. They assumed that the network of NVSMC of the choroid counteracts the ciliary body movements during accommodation, thus guaranteeing the three-dimensional architecture of the choroid and the position of the retina, particularly in the macular region.
Retinociliary Vein
I found this to be present only rarely, running along with a cilioretinal artery.
Venous drainage of the ciliary body
This comprises two systems.
-
(i)
Main Venous Drainage System: This consists of all the venous drainage from the highly vascular ciliary processes, and also from the ciliary body and the iris. The veins from this venous drainage go posteriorly and join the vortex veins behind the equator.
-
(ii)
A Subsidiary Venous Drainage System: Blood from the anterior and outer part of the ciliary muscle drains into the anterior ciliary venous system.
Anterior ciliary venous system
It lies in the outer portion of the ciliary muscle. It drains blood from the anterior and outer parts of the ciliary body. From this system, anterior ciliary emissary veins pass through the sclera a little posterior to the limbus, to the episcleral region, and some of them accompany branches of the ACAs [64]. These veins link up with the efferent vessels from Schlemm’s canal before they emerge into the episcleral venous plexus. The anterior ciliary venous system is important because it takes part in aqueous humour drainage. The anterior ciliary veins usually form a complex intrascleral venous plexus within the anterior part of the sclera. This plexus consists of superficial and deep parts. (i) The deep part forms a dense network, and it communicates with Schlemm’s canal through its efferent collector channels. Some of the veins from the deep plexus go to the episcleral plexus. (ii) The superficial part is flat and consists of smaller veins, with few communications with the deep plexus. It drains independently into the episcleral plexus.
Aqueous veins
From the outer aspect of the canal of Schlemm there are a variable number of efferent vessels. They vary in size from capillary size to large trunks. Most of them anastomose with one another after leaving the canal to form the deep scleral plexus. The deep part of the intrascleral venous plexus of the anterior ciliary veins communicates with Schlemm’s canal through its efferent collector channels. Finally, they empty into the episcleral venous plexus.
Watershed zones in uveal vascular bed
Watershed zones in PCA vascular bed
The existence of watershed zones in the uveal vascular bed was first described in 1990 [149]. When a tissue is supplied by two or more end-arteries [150], the border between the territories of distribution of any two end-arteries is called a “watershed zone” (WSZ). The occurrence of such watershed zones between the various cerebral arteries is well-known, as is also the case in some other organs having end-arterial systems. The significance of the WSZs is that in the event of a fall in the perfusion pressure in the vascular bed of one or more of the end-arteries, the WSZ, being an area of comparatively poor vascularity, is most vulnerable to ischaemia. Development of watershed infarcts in the cerebral cortex is well known [151, 152].
From the previous discussion, it is evident that PCAs and their subdivisions, right down to the choroidal arterioles, are end-arteries. Therefore, this vascular bed has WSZs (1) between the PCAs, (2) between the SPCAs, (3) between LPCA and SPCAs, and (4) between ACAs and SPCAs. These are arranged as follows:
Watershed Zones Between the Main PCAs [149]
Fluorescein fundus angiographic studies in man clearly show the presence of WSZs between the various PCAs (Fig. 25). These can be outlined only in high-quality fluorescein fundus angiography, performed at a fast speed, because normally the blood flow in the choroid is so fast that the filling pattern of the areas supplied by the various PCAs and of the WSZs between them cannot be photographed by the slow speed of angiography equipment currently available - unless, of course, the choroidal circulation is markedly slowed down, as it often is seen in conditions including AION, carotid insufficiency and high IOP [149, 153]. High-resolution videoangiography or cineangiography can provide this information more reliably.
(LPCA Lateral PCA, MPCA medial PCA). A Right eye of a 78-year-old woman with GCA and mild AION, shows non-filling of the watershed zone (indicated by WSZ). B Fluorescein fundus angiogram, of right eye of a 60-year-old man with non-arteritic AION, shows non-filling of the watershed zone (indicated by WSZ). C Fluorescein fundus angiogram, of left eye of a 74-year-old man with arteritic AION, shows non-filling of a watershed zone (indicated by WSZ). D Fluorescein fundus angiogram, of left eye of a 45-year-old woman with non-arteritic AION, shows non-filling of the watershed zone (indicated by WSZ).
1. When there are two (medial and lateral) PCAs: As discussed above, the area of the choroid supplied by the medial and lateral PCAs shows a marked inter-individual variation which must result in a wide variation in the location of the WSZ between the two. Figure 26 is a diagrammatic representation of some of the locations of the WSZ between the medial and lateral PCAs; it shows the WSZ location may vary widely, as depicted in Fig. 26A–D. It may:
(a) be situated temporal to the peripapillary choroid (Fig. 26A);
(b) pass through the temporal peripapillary choroid (Fig. 26B);
(c) pass through one or the other part of the optic disc; or the entire optic disc may lie in the WSZ (Fig. 26C);
(d) pass through the medial peripapillary choroid (Fig. 26D); or
(e) various combinations of the above.
In fluorescein fundus angiographic studies in eyes with glaucoma and low-tension-glaucoma, where the WSZ could be outlined, the commonest (60%) site being the temporal part of the optic disc and the adjacent peripapillary choroid.
ii. When there are three or more PCAs: The location of the WSZs varies according to the number of the PCAs and their locations. With three PCAs, the WSZ usually assumes the shape of the letter “Y”, passing through a part of the optic disc [153] (Fig. 7); or the entire optic disc may lie in the WSZ [153]. The various combinations of WSZs which can occur when an eye has more than two PCAs; if one or more of the PCAs in such circumstances has low perfusion pressure, the eye will show a filling defect in the corresponding WSZ, which may be vertically or obliquely oriented and involve only one half of the WSZ, in contrast to a WSZ seen in the entire vertical length when there are only two PCAs [153].
Watershed Zones Between SPCAs [19, 149]
Each SPCA supplies a well-defined sector of the choroid. Figure 11 is a diagrammatic representation of the WSZs between the various short PCAs, as revealed by fluorescein angiographic studies in experimental occlusion of the temporal SPCAs [19]. These studies revealed that the area supplied by each SPCA has a well-defined margin, with WSZs situated between adjacent SPCAs. All the temporal SPCAs enter the eyeball in the macular region and spread out to the periphery of the fundus to supply the temporal half of the choroid (Fig. 3). It is, therefore, natural that most of the segments of the choroid supplied by the temporal SPCAs and their WSZs meet in the macular region. This was a consistent pattern in my studies. It is well-established that an area where numerous WSZs meet is an area of comparatively poor vascularity and in the event of circulatory disorders, most vulnerable to ischaemia—for example, the frequent occurrence of age-related macular degeneration, and submacular choroidal neovascularization represent response to chronic ischaemia, as discussed elsewhere in detail [19, 154].
I have found that the large peripapillary choroidal filling defect in the macular region (produced by the meeting of multiple WSZs of the SPCAs) has erroneously been considered as a part of the WSZ between the main PCAs; however, the two obviously are totally different in nature, and have very different clinical implications.
Watershed zones between the short and long PCAs
Experimental studies revealed that there are no anastomoses between the LPCA and the adjacent SPCAs [11]. Thus, there is a WSZ between the LPCA and its adjacent SPCAs.
Watershed zones between the PCAS and the anterior ciliary arteries
Experimental and clinical studies of PCA occlusion [18, 155,156,157,158] or ACA occlusion [26, 159], have clearly shown that there are no anastomoses between the PCAs and ACAs, because in the event of PCA occlusion the choroid does not fill by extension from the ACAs. Similarly, clinical and experimental studies on ACA occlusion following resection of the various recti revealed that PCAs do not safeguard the anterior uvea from the development of anterior segment ischaemia in these eyes [26, 159]. Also, Takahashi et al. [37], on wide-angle indocyanine green angiography in humans, found absence of functional anastomoses between terminal branches of the PCAs and ACAs. Thus, there is a WSZ between the PCAs and ACAs, situated in the equatorial region of the choroid (Fig. 11) [149].
Watershed zones between the choriocapillaris lobules
The WSZs between the various lobules of the choriocapillaris are arranged like a honeycomb [28] (Fig. 9). That explains the presence of empty lobules with fully filled surrounding lobules of fluorescein angiography in Fig. 27b.
Fundus photograph (a) and fluorescein angiogram (during the late phase) (b) of right eye of a rhesus monkey with malignant arterial hypertension (day 78; blood pressure 190 mmHg). (a) It shows multiple acute focal retinal pigment epithelial lesions with serous retinal detachment. (b) It shows fluorescein staining of the acute focal retinal pigment epithelial lesions. (c) Fundus photograph shows the typical Elschnig’s spots.
Watershed zones between the vortex veins
Experimental studies on vortex vein occlusion in rhesus monkeys revealed that the various vortex veins show no free anastomoses and that the WSZs between the four vortex veins extend anterior-posteriorly through the entire length of the uveal tract—a horizontal WSZ between the upper and lower vortex veins passes through the optic disc and macular region, while a vertical WSZ between the temporal and nasal vortex veins passes between the optic disc and macular region [19, 145] (Fig. 18).
These findings on the in vivo end-arterial nature of the PCAs and their branches, and the presence of WSZs have since been confirmed by several subsequent studies [35, 59].
Conclusion
Although, no doubt, meticulously conducted postmortem injection cast preparations gave us useful information on the morphology of the choroidal vascular bed since 1700; [14] these studies misled us greatly for centuries about the in vivo situation. Fluorescein angiographic studies in the living eye have since clearly revealed that the postmortem studies did not reflect the physiological anatomy and the pattern of blood flow in the choroidal vascular bed. Studies have shown that the PCAs and their branches, right down to the terminal choroidal arterioles, and the choriocapillaris, have a segmental distribution in the choroid, and that PCAs and choroidal arteries function as end-arteries, which explains the basis pf occurrence of isolated vascular chorioretinal lesions. Thus, fluorescein angiography can truthfully be said to have revolutionized our concept of the choroidal circulation in health and disease. The choroidal vascular pattern described above is based mainly on the fluorescein fundus angiographic studies, and that should form the basis for a fuller understanding of the choroidal vascular bed in health and disease.
References
Ramon Y Cajal S Recollections of My Life. Santiago Ramon Y Cajal. Translated by EH Craigie from the third Spanish edition (1923) 1937; p. 564-65. Philadelphia: American Philosophical Society.
Hayreh SS. Blood supply of the optic nerve. In: Shaffer RN, Glaucoma Research Conference. Am J Ophthalmol. 1966;61:564.
Hayreh SS. Blood supply of the optic nerve head and its Competing interestsrole in optic atrophy, glaucoma and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–48.
Duke-Elder S, Wybar KC System of Ophthalmology, Vol. 2: The anatomy of the visual system. 1961; p. 345, 351, London: Kimpton 358,69.
Hayreh SS, Dass R. The ophthalmic Artery: I. Origin and intra-cranial and intra-canalicular course. Br J Ophthalmol. 1962;46:65–98.
Hayreh SS. The ophthalmic artery III. Branches. Br J Ophthalmol. 1962;46:212–47.
Meyer F. Zur Anatomie der Orbitalarterien. Morphol Jahrüch. 1887;12:414–58.
Sudakevitch T. The variations in the system of the trunks of the posterior ciliary arteries. Br J Ophthalmol. 1947;31:738–60.
Leber T Die Circulations- und Ernährungsverhältnisse des Auges. Graefe-Saemisch Handbuch der Gesamten Augenheilkunde, 2nd ed. 1903 vol 2. p. 33-35 Leipzig: Wilhelm Engelmann, 43-6.
Ducournau D. Les arteres ciliaires posterieures courtes para-optiques. Une entite anatomo-clinique. [Short para-optic posterior ciliary arteries. An anatomo-clinical entity. Bull Soc Ophtalmol Fr. 1982;82:1527.
Hayreh SS. The Long posterior ciliary arteries. Graefes Arch Clin Exp Ophthalmol. 1974;192:197–213.
Siam AL, El-Mamoun TA, Ali MH. A restudy of the surgical anatomy of the posterior aspect of the globe: an essential topography for exact macular buckling. Retina 2011;31:1405–11.
Ducournau D La systématisation vasculaire de la choroide. [Vascularization of the choroid]. Annee Ther Clin Ophtalmol. 1979:17–22.
Wedel C, Ruysch F. Epistola anatomica, problematica ad Fredericum Ruyschium. De oculorum tunicis. Amstelaedami: Janssonio-Waesbergios; 1737.
Wybar KC. Vascular anatomy of the choroid in relation to selective localization of ocular disease. Br J Ophthalmol. 1954;38:513–17.
Shimizu K, Ujiie K. Structure of ocular vessels. 54. New York: Igaku-Shoin; 1978. p. 70–9. 108-24
Hogan MJ, Alvarado JA, Weddell JE. Histology of the human eye: an atlas and textbook. 50. Philadelphia: Saunders; 1971. p. 189. 326, 372.
Hayreh SS, Baines JA. Occlusion of the posterior ciliary artery. I. Effects on choroidal circulation. Br J Ophthalmol. 1972;56:719–35.
Hayreh SS. Segmental nature of the choroidal vasculature. Br J Ophthalmol. 1975;59:631–48.
Ring HG, Fujino T. Observations on the anatomy and pathology of the choroidal vasculature. Arch Ophthalmol. 1967;78:431–44.
Uyama M, Ohkuma H, Itotagawa S, Koshibu A, Uraguchi K, Miki K. [Pathology of choroidal circulatory disturbance. Part 1. Angioarchitecture of the choroid, observation on plastic cast preparation (author’s transl)]. Nihon Ganka Gakkai Zasshi. 1980;84:1893–909.
Olver JM. Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye 1990;4:262–72.
MacFaul PA. Ciliary artery involvement in giant cell arteritis. Br J Ophthalmol. 1967;51:505–12.
Hayreh SS. Anterior ischaemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol. 1974;58:964–80.
Hayreh SS, Scott WE. Fluorescein iris angiography. II. Disturbances in iris circulation following strabismus operation on the various recti. Arch Ophthalmol. 1978;96:1390–1400.
Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–48.
Hayreh SS. The blood supply of the optic nerve head and the evaluation of it: myth and reality. Prog Retin Eye Res. 2001;20:563–93.
Hayreh SS. The choriocapillaris. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192:165–79.
Wagenmann A. Experimentelle Untersuchungen über den Einfluss der Circulation in den Netzhaut- und Aderhautgefässen auf die Ernährung des Auges, insbesondere der Retina, und über die Folgen der Sehnervendurchschneidung. Albrecht Von Graefes Arch Ophthalmol. 1890;36:1–120.
Bossalino D. Ricerche sperimentali sulle alterazioni dell’occhio consecutive al taglio dei vasi ciliari. Ann di ottalmologia. 1910;39:866–914.
Freeman HM, Hawkins WR, Schepens CL. Anterior segment necrosis. An experimental study. Arch Ophthalmol. 1966;75:644–50.
Hayreh SS. Choroidal circulation in health and in acute vascular occlusion. In: Cant JS, editor. Proceedings, 3rd William Mackenzie Memorial Symposium (1974: Glasgow). St. Louis: Mosby; 1976. p. 157–70.
Hayreh SS, Baines JA. Occlusion of the posterior ciliary artery. II. Chorio-retinal lesions. Br J Ophthalmol. Br J Ophthalmol. 1972;56:736–53.
Selbach JM, Rohen JW, Steuhl KP, Lütjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res. 2005;30:337–44.
Zhang HR, Gao J. [Electron microscopy of the angioarchitecture of the human iris and ciliary body]. [Zhonghua Yan Ke Za Zhi] Chinese. J Ophthalmol. 1989;25:148–51.
Song Y, Song YJ, Ko MK. A study of the vascular network of the iris using flat preparation. Korean J Ophthalmol. 2009;23:296–300.
Takahashi K, Muraoka K, Kishi S, Shimizu K. Watershed zone in the human peripheral choroid. Ophthalmology 1996;103:336–42.
von Haller A. Arteriarum oculi: historia et tabulae arteriarum oculi. Gottingen: Typis Abrami Vandenhoeck; 1754.
Tiedemann F Der antheil des sympathischen nervens an den verrichtungen der sinne. In: Friedrich T, Treviranus GR, editors. Zeitschrift für Physiologie. 1824. 1. p. 237-90. Heidelberg: August Osswald.
Huschke E. Eingeweide und Sinnesorgane. In: von Sömmerring ST, editor. Vom Bau des menschlichen Körpers, bd 5. Leipzig: Voss; 1844. p. 710.
Olver JM, Spalton DJ, McCartney AC. Quantitative morphology of human retrolaminar optic nerve vasculature. Invest Ophthalmol Vis Sci. 1994;35:3858–66.
Onda E, Cioffi GA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol. 1995;120:92–102.
Gauntt CD, Williamson TH, Sanders MD. Relationship of the distal optic nerve sheath to the circle of Zinn. Graefes Arch Clin Exp Ophthalmol. 1999;237:642–47.
Olver JM, Spalton DJ, McCartney AC. Microvascular study of the retrolaminar optic nerve in man: the possible significance in anterior ischaemic optic neuropathy. Eye 1990;4:7–24.
Strek P, Strek W, Nowogrodzka-Zagorska M, Pitynski K. Angiomorphology of the retrobulbar part of the optic nerve. A scanning electron microscopy of vascular casts. Folia Morphol (Warsz). 1996;55:143–50.
Heimann K. The development of the choroid in man. Ophthalmic Res. 1972;3:257–73.
Hayreh SS. Microangioarchitecture of optic papilla [comment]. Jpn J Ophthalmol. 1989;33:519–25.
Hayreh SS. Controversies on submacular choroidal circulation. Ophthalmologica 1981;183:11–9.
Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29.
Stern WH, Ernest JT. Microsphere occlusion of the choriocapillaris in rhesus monkeys. Am J Ophthalmol. 1974;78:438–48.
Hovius J. Tractatus de circulari humorum motu in oculis. 1716. Lugdunum Batavorum: Langerak.
Eschricht DF. Beobachtungen an dem Seehundsauge. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin. 1838;1716:575–99.
Passera E. La rete vascolare sanguigna della membrana coriocapillare dell’uomo. Ricerche (fatte nel laboratorio Di Anat Norm della R Univá di Roma). 1896;5-6:133–67.
Winslow J-B. An anatomical exposition of the structure of the human body. Translated from the French original, by G Douglas. 1733; p.77 London: Printed for N. Prevost.
Rohen JW. Morphology of the uveal tract. Int Ophthalmol Clin. 1965;5:581–667.
Ruskell GL. Choroidal vascularization in the rabbit. Am J Ophthalmol. 1961;52:807–15.
Goldor H, Gay AJ. Chorioretinal vascular occlusions with latex microspheres (a long-term study). II. Invest Ophthalmol. 1967;6:51–8.
Dollery CT, Henkind P, Kohner EM, Paterson JW. Effect of raised intraocular pressure on the retinal and choroidal circulation. Invest Ophthalmol. 1968;7:191–98.
Hayreh SS. Vascular pattern of the choriocapillaris. Exp Eye Res. 1974;19:101–4.
Torczynski E, Tso MO. The architecture of the choriocapillaris at the posterior pole. Am J Ophthalmol. 1976;81:428–40.
Loewenstein A. Glomus cells in the human choroid as the basis of arteriovenous anastomoses. Am J Ophthalmol. 1949;32:1651–59.
Lütjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006;82:357–61.
Hayreh SS. The Orbital Vessels of Rhesus Monkeys. Exp Eye Res. 1964;3:16–30.
Ashton N, Smith R. Anatomical study of Schlemm’s canal and aqueous veins by means of neoprene casts. III. Arterial relations of Schlemm’s canal. Br J Ophthalmol. 1953;37:577–86.
Van Buskirk EM. The ciliary vasculature and its perturbation with drugs and surgery. Trans Am Ophthalmol Soc. 1988;86:794–841.
Morrison JC, Van, Buskirk EM. Anterior collateral circulation in the primate eye. Ophthalmology 1983;90:707–15.
Heymann V, George JL, Sirbat D, Rauber G, Raspiller A. Apport artériel au segment antérieur de l’oeil. Etude radio-anatomique d’une série de vingt-cinq globes humains. [Arterial supply to the anterior segment of the eye. Radioanatomical study of a series of 25 human eyes]. J Fr Ophtalmol. 1985;8:697–703.
Johnson MS, Christiansen SP, Rath PP, Watanabe TM, Merten G, Bothun ED, et al. Anterior ciliary circulation from the horizontal rectus muscles. Strabismus 2009;17:45–8.
Funk R, Rohen JW. Scanning electron microscopic study on the vasculature of the human anterior eye segment, especially with respect to the ciliary processes. Exp Eye Res. 1990;51:651–61.
Meyer PAR. Patterns of blood flow in episcleral vessels studied by low-dose fluorescein videoangiography. Eye. 1988;2:533–46.
Ormerod LD, Fariza E, Webb RH. Dynamics of external ocular blood flow studied by scanning angiographic microscopy. Eye. 1995;9:605–14.
Laatikainen L. Perilimbal vasculature in glaucomatous eyes. Acta Ophthalmol Suppl. 1971;111:54–73.
Meyer PA, Watson PG. Low dose fluorescein angiography of the conjunctiva and episclera. Br J Ophthalmol. 1987;71:2–10.
Talusan ED, Schwartz B. Fluorescein angiography: Demonstration of flow pattern of anterior ciliary arteries. Arch Ophthalmol. 1981;99:1074–80.
Ormerod LD, Fariza E, Hughes GW, Doane MG, Webb RH. Anterior segment fluorescein videoangiography with a scanning angiographic microscope. Ophthalmology 1990;97:745–51.
Nanba K, Schwartz B. Increased diameter of the anterior ciliary artery with increased intraocular pressure. Arch Ophthalmol. 1986;104:1652–5.
Ojima M, Matsuo N. Angioarchitecture of rabbit iris. Acta Med Okayama. 1985;39:47–52.
Raviola G. Effects of paracentesis on the blood-aqueous barrier: an electron microscope study on Macaca mulatta using horseradish peroxidase as a tracer. Invest Ophthalmol. 1974;13:828–58.
Rohen JW, Funk RH. Functional morphology of the episcleral vasculature in rabbits and dogs: presence of arteriovenous anastomoses. J Glaucoma. 1994;3:51–7.
Parodi MB, Bondel E, Saviano S, Da Pozzo S, Bergamini L, Ravalico G. Iris arteriovenous communication: clinical and angiographic features. Int Ophthalmol. 1998;22:1–5.
Raviola G, Butler JM. Unidirectional transport mechanism of horseradish peroxidase in the vessels of the iris. Invest Ophthalmol Vis Sci. 1984;25:827–36.
Bill A. The blood-aqueous barrier. Trans Ophthalmol Soc U K 1986;105:149–55.
Hayreh SS, Scott WE. Fluorescein Iris Angiography. I. Normal Pattern. Arch Ophthalmol. 1978;96:1383–9.
Virdi PS, Hayreh SS. Normal fluorescein iris angiographic pattern in subhuman primates. Invest Ophthalmol Vis Sci. 1983;24:790–3.
Iovino C, Peiretti E, Braghiroli M, Tatti F, et al. Imaging of iris vasculature: current limitations and future perspective. Eye (Lond). 2022;36:930–40.
Chignell AH, Easty DL. Iris fluorescein photography following retinal detachment and in certain ocular ischaemic disorders. Trans Ophthalmol Soc U K 1971;91:243–59.
Ishikawa T. Fine structure of retinal vessels in man and the macaque monkey. Invest Ophthalmol. 1963;2:1–15.
Amalric P. The angiography of the anterior segment of the eye. In: Shimizu K, editor. Proceedings of the International Symposium of Fluorescein Angiography, Tokyo, 1972. Tokyo: Igaku Shoin Ltd; 1974. p. 281–318.
Vannas A. Fluorescein angiography of the vessels of the iris in pseudoexfoliation of the lens capsule, capsular glaucoma and some other forms of glaucoma. Acta Ophthalmol Suppl. 1969;105:1–75.
Kottow M, Metzler D, P H Iris angiographic findings in retinal vein occlusion. In: Cant JS, editor. Vision and circulation. 1976. p. 251–4 London: Kimpton.
Kottow M Iris angiography in vascular diseases of the fundus. In: DeLaey JJ, editor. Proceedings of the International Symposium on Fluorescein Angiography. 1976. Vol. 9. p. 465-71. Documenta Ophthalmologica Proceedings Servies. The Hague: W. Junk.
Shiose Y, Oguri M. Electron microscopic studies on the blood-retinal barrier and the blood-aqueous barrier. Jpn J Ophthalmol. 1970;14:73–87.
Shakib M, Cunha-Vaz JG. Studies on the permeability of the blood-retinal barrier. IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp Eye Res. 1966;5:229–34.
Szalay J, Nunziata B, Henkind P. Permeability of iridial blood vessels. Exp Eye Res. 1975;21:531–43.
Satoh K, Takaku Y, Ohtsuki K, Mizuno K. Effects of aging on fluorescein leakage in the iris and angle in normal subjects. Jpn J Ophthalmol. 1999;43:166–70.
Van Nerom PR, Rosenthal AR, Jacobson DR, Pieper I, Schwartz H, Greider BW. Iris angiography and aqueous photofluorometry in normal subjects. Arch Ophthalmol. 1981;99:489–93.
Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–96.
Morrison JC, Van, Buskirk EM. Ciliary process microvasculature of the primate eye. Am J Ophthalmol. 1984;97:372–83.
Morrison JC, Van Buskirk EM. Microanatomy and modulation of the ciliary vasculature. Trans Ophthalmol Soc U K. 1986;105:131–9.
Rohen JW, Funk RHW. Vasculature of the anterior eye segment. Prog Retin Eye Res. 1994;13:653–85.
Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. 2011;30:1–17.
Müller H. Anatomisch-physiologishe Untersuchungen über die Retina des Menschen und der Wirbelthiere. Z für wissenschaftliche Zoologie. 1856;8:1–122.
Nettleship E. Unusual distribution of retinal blood-vessels: three cases. Br Med J. 1876;1:161–2.
Nettleship E. Cilio-retinal blood vessels (Additional cases and remarks). R Lond Ophthalmic Hosp Rep. 1879;9:161–7.
Lang W, Barrett JW. On the frequency of ciliary-retinal vessels and of pulsating veins. R Lond Ophthalmic Hosp Rep. 1888;12:59–60.
Jackson E. Cilio-retinal and other anomalous retinal vessels. Ophthalmic Rev. 1911;30:264–9.
Salzmann M. The anatomy and histology of the human eyeball in the normal state; its development and senescence. 183. Chicago: University of Chicago Press; 1912. p. 184.
Duke-Elder S. Textbook of ophthalmology. 2. St. Louis: C.V. Mosby; 1932. p. 1389.
Collier M. Frequence des vaisseaux cilioretiniens, leur rapport avec les ametropies, leur association avec d’autres anomalies du fond de l’oeil. Bull Soc Ophtalmol Fr. 1957;64:598–601.
Glees M. Über zilioretinale und optikoziliare Gefässe als allegemein diagnostischer Hinweis [Cilio-retinal and optico-retinal vessels as diagnostic pointers]. Klin Monatsbl Augenheilkd. 1956;128:580–92.
Loring EG. Anomalous circulation in the eye. Arch Ophthalmol Otolaryngol. 1872;2:1–10. plate B.
Birnbacher A. The cilio-retinal blood-vessels. Arch Ophthalmol. 1887;16:32–4.
Blunt MJ. Implications of the vascular anatomy of the optic nerve and chiasma. Proc R Soc Med. 1956;49:433–9.
Hayreh SS. The Cilio-Retinal Arteries. Br J Ophthalmol. 1963;47:71–89.
Bloch H. Ueber abnormen Verlauf der Papillengefässe. Klin Monatsbl Augenheilkd. 1906;44:413–8.
Parsons JH The ocular circulation (Arris and Gale Lecture Series). 903; p. 31 London: John Bale, Sons & Danielsson, Ltd.
Wybar KC. Anastomoses between the retinal and ciliary arterial circulations. Br J Ophthalmol. 1956;40:65–81.
Steele EJ, Blunt MJ. The blood supply of the optic nerve and chiasma in man. J Anat. 1956;90:486–3.
Randall BA. Cilio-retinal or aberrant vessels. Trans Am Ophthalmol Soc. 1887;4:511–7.
Elschnig A. Cilioretinale Gefässe. Albrecht von Graefes Arch Ophthalmol. 1897;44:144–71.
Fuchs E. Ueber Anomalien der Blutfässe im Sehnerveneintritt. Klin Monbl Augenheilkd. 1923;71:583–9.
Czermak W. Aus der Augenklinik des Prof. Fuchs. Beitrag zur Kenntniss der sog. Cilioretinalen Gefässe. Wien Klin Wochenschr. 1888;1:251–4.
Bailliart P. La circulation retinienne a l'Ètat normal et pathologique. Paris: G. Doin; 1923. p. 10–12.
Elschnig A. Ueber opticociliare Gefässe. Klin Monatsbl Augenheilkd. 1898;36:93–6.
Benson AH. Drawing showing an unusual course taken by a branch of the central artery of the disc. Trans Ophthalmol Soc U K 1883;3:101–2. plate IV, fig 101.
Hayreh SS A study of the central artery of the retina in human beings [Thesis for the Master of Surgery]: Panjab University (India); 1958.
Coats G. Visible anastomoses on the papilla after obstruction of the central artery. R Lond Ophthalmic Hosp Rep. 1913;19:78–81.
Justice J Jr., Lehmann RP. Cilioretinal arteries. A study based on review of stereo fundus photographs and fluorescein angiographic findings. Arch Ophthalmol. 1976;94:1355–58.
Wang RS. [Cilioretinal arteries under fundus fluorescein angiography] (in Chinese). Zhonghua Yan Ke Za Zhi. 1991;27:219–20.
Shihab ZM, Beebe WE, Wentlandt T. Possible significance of cilioretinal arteries in open-angle glaucoma. Ophthalmology 1985;92:880–3.
Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30:359–94.
Hegde V, Deokule S, Matthews T. A case of a cilioretinal artery supplying the entire retina. Clin Anat. 2006;19:645–7.
Parsa CF, Cheeseman EW, Maumenee IH. Demonstration of exclusive cilioretinal vascular system supplying the retina in man: vacant discs. Tr Am Ophthalmol Soc 1998;96:95–106.
Nettleship E. Notes on the blood-vessels of the optic disc in some of the lower animals. Trans Ophthalmol Soc U K 1905;25:338–9.
Singh (Hayreh) S, Dass R. The central artery of the retina. I. Origin and course. Br J Ophthalmol. 1960;44:193–212.
Mann IC. Development of the Human Eye. Cambridge: Cambridge University Press, for the British Journal of Ophthalmology; 1928. p. 230–4.
Johnson GL. Contributions to the comparative anatomy of the mammalian eye, chiefly based on ophthalmoscopic examination. Philos Trans R Soc Lond B. 1901;194:1–82.
Franck JL. Handbuch der Anatomie der Hausthiere, mit besonderer Berücksichtigung der Pferdes. 2 ed. Stuttgart: Schickhardt & Ebner; 1883.
Bach L. Ueber die Gefässe des Pferdeauges mit besonderer Berücksichtigung der Gefässversorgung der Aderhaut. Arch fűr wiss und praktische Tierheilkd. 1894;20:241–56.
Langenbacher L. Vergleichend anatomische Untersuchungen über die Blutfässe in der Netzhaut des Auges. Österreichische Vierteljahresschr für wiss. 1880;53:121–50.
Salzmann M. Über Rückbildung (Schwund) von cilio-retinalen arterien [Atrophy of the cilio-retinal arteries]. Albrecht Von Graefes Arch Ophthalmol. 1953;153:451–8.
Lim MC, Bateman JB, Glasgow BJ. Vortex vein exit sites. Scleral Coord Ophthalmol. 1995;102:942–6.
Kutoglu T, Yalcin B, Kocabiyik N, Ozan H. Vortex veins: anatomic investigations on human eyes. Clin Anat. 2005;18:269–73.
Spaide RF, Ledesma-Gil G, Gemmy Cheung CM. Intervortex venous anastomosis in pachychoroid-related disorders. Retina 2021;41:997–1004.
Hayreh SS, Baines JA. Occlusion of the vortex veins. An experimental study. Br J Ophthalmol. 1973;57:217–38.
Coats G. On the pathology of chorio-vaginal (posterior vortex) veins. Ophthalmol Rev. 1906;25:99–112.
Ruskell GL. Peripapillary venous drainage from the choroid: a variable feature in human eyes. Br J Ophthalmol. 1997;81:76–9.
Flugel-Koch C, May CA, Lutjen-Drecoll E. Presence of a contractile cell network in the human choroid. Ophthalmologica 1996;210:296–302.
Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye (Lond). 1990;4:273–89.
Hayreh SS. Anterior ischemic optic neuropathy. Arch Neurol. 1981;38:675–8.
Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke 1984;15:221–3.
Bogousslavsky J, Regli F. Unilateral watershed cerebral infarcts. Neurology 1986;36:373–7.
Hayreh SS. Inter-individual variation in blood supply of the optic nerve head. Its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985;59:217–46.
Hayreh SS. Submacular choroidal vascular bed watershed zones and their clinical importance. Am J Ophthalmol. 2010;150:940–1.
Hayreh SS, Baines JA. Occlusion of the posterior ciliary artery. III. Effects on the optic nerve head. Br J Ophthalmol. 1972;56:754–64.
Hayreh SS. Anterior ischaemic optic neuropathy. I. Terminology and pathogenesis. Br J Ophthalmol. 1974;58:955–63.
Hayreh SS. Acute ischemic disorders of the optic nerve: pathogenesis, clinical manifestations, and management. Ophthalmol Clin North Am. 1996;9:407–42.
Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509–20.
Virdi PS, Hayreh SS. Anterior segment ischemia after recession of various recti. An experimental study. Ophthalmology. 1987;94:1258–71.
Funding
Supported by research grants from the British Medical Research Council, EY-1151, EY-1576, EY3330, and RR-59 from the U.S. National Institutes of Health, in part by unrestricted grants from Research to prevent Blindness, Inc., New York.
Author information
Authors and Affiliations
Contributions
SSH was responsible for the writing and editing of the final manuscript. SBH provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Due to Dr. Hayreh's death, some errors and omissions may exist in the article as published.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayreh, S.S., Hayreh, S.B. Uveal vascular bed in health and disease: uveal vascular bed anatomy. Paper 1 of 2. Eye 37, 2590–2616 (2023). https://doi.org/10.1038/s41433-023-02416-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02416-z
- Springer Nature Limited