Abstract
Background/Objectives
Posterior Capsule Opacification (PCO) is the most common long-term post-operative adverse occurrence after cataract surgery often requiring treatment with YAG laser posterior capsulotomy. This study aimed to identify potential risk factors, known at the time of cataract surgery, that influence the development of PCO.
Subject/Methods
A retrospective study of publicly funded cataract surgery from The Royal College of Ophthalmologists’ National Ophthalmology Database. Eligible for analysis were 500,872 cataract operations performed in 41 participating centres.
Results
The 500,872 operations were performed on 243,167 (48.5%) left eyes and 257,705 (51.5%) right eyes from 373,579 patients by 2196 surgeons. Post-cataract PCO was recorded for 61,778 (12.3%) eyes and the six month, one, three, five and nine year observed rates of PCO were 2.3%, 4.4%, 19.7%, 34.0% and 46.9% respectively. Different PCO profiles were observed between IOL materials and the identified risk factors that increased the risk of developing PCO included hydrophilic IOL material, axial length >26 mm, the presence of high myopia and implantation of lower IOL powers and previous vitrectomy surgery, along with younger age and female gender.
Conclusions
Many factors influence the development of PCO relating to the patient, the eye, the lens and the surgery. Some factors are modifiable such as IOL material, therefore the opportunity exists to attempt to reduce PCO rates, benefitting patients and the UK NHS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Cataract surgery is the most frequently performed surgical procedure in the UK, with around 472,000 operations funded by the NHS in England and Wales during the 2018–2019 national cataract audit year (www.nodaudit.org.uk/resources/publications-annual-report). Posterior capsule opacification (PCO) is the most frequent long-term adverse occurrence after cataract surgery, often requiring treatment with YAG laser posterior capsulotomy. As a consequence, despite YAG laser being a frequently performed and low risk procedure, posterior capsule rates are of significant public health interest due to the visual impairment they cause prior to treatment, and the resources directed towards their diagnosis and management which could be redeployed if rates were reduced.
PCO rates have been variously reported depending on definitions used and the length of follow-up in different studies. PCO has been linked to patient factors, cataract surgical techniques and implanted intra-ocular lens (IOL) materials or design, such as the established reduction in PCO rates seen with square-edged IOL designs compared to round edge IOL shown by systematic review of randomised controlled trials (RCT) [1, 2]. In addition to RCT, real-world PCO rates and risk factors have been reported [3, 4].
The Royal College of Ophthalmologists (RCOphth) National Ophthalmology Database (NOD) Cataract Audit accesses post-operative records from the majority of UK cataract service providers, and therefore provides the opportunity to explore PCO rates and risk factors at a scale not previously reported to identify potential targets for interventions to reduce PCO rates nationally.
Subjects and methods
The RCOphth NOD receives anonymised data from around 70% of centres providing publicly funded cataract surgery in England, Wales and Guernsey as described in other publications [5] and on the audit website (www.nodaudit.org.uk). Only electronic medical record (EMR) enabled centres are included in this analysis due to the in-house data collection systems submitting follow-up data at one fixed time point after surgery, instead of serial postoperative data. Only data from institutions providing a full range of ocular services were included. Centres only providing primarily cataract-related services were excluded as they have limited opportunity to see patients who are PCO-free, hence would generate artificially high PCO rates if they offer YAG laser, or artificially low rates if they do not provide this service. We excluded any centre that did not have records of at least 50 cases of PCO recorded more than one month after cataract surgery, as some institutions have pathways that systematically fail to capture certain outpatient activity on the EMR. Operations with at least one month’s follow-up are included, where the follow-up data could be for any post-surgery hospital visit for either clinical assessments or treatments. Whilst other IOL designs are used within the NHS, our analysis was restricted to cases where a monofocal single piece IOL was implanted. Other IOL designs, such as toric IOL might represent a different patient population with different visual needs or thresholds for seeking or receiving YAG laser capsulotomy so were excluded. Similarly, three-piece IOL are often used in cases where posterior capsule rupture (PCR) has occurred or capsular support for the IOL is otherwise compromised and the lens is inserted into the sulcus (with or without optic capture) rather than wholly within the capsular bag; the need for YAG capsulotomy is inherently diminished following PCR hence three-piece IOL and cases of PCR were not included. The study time period concerns operations performed between 01/04/2010 and 31/03/2018 with 31/08/2019 as the last date of any follow-up record, this enabled all operations to have the opportunity for a minimum of one year and five months follow-up. Each IOL was allocated a group based on model, material and IOL design derived from manufacturer specifications.
Excluded from the analysis were;
-
where no IOL was inserted
-
IOL was not recorded
-
operations where either a multi piece IOL or toric IOL were implanted
-
IOL’s that could not be allocated to the IOL model grouping
-
operations that experienced posterior capsular rupture
-
operations with missing patient age at surgery
-
operations with missing IOL power as this could indicate the eye was left aphakic
-
operations where the recorded IOL power is outside the range of −10 to +40 dioptres (most likely indicative of a data entry error)
-
eyes with a recorded axial length measurement <18 mm as these could be abnormal eyes or data entry errors
-
centres with fewer than 50 cases of PCO more than one month after surgery in their otherwise eligible sample.
The data was recorded on the Medisoft EMR system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK, www.medisoft.co.uk) or the Open Eyes EMR system (www.openeyes.org.uk). Anonymized database analyses of this type do not require ethical permission due to being viewed as audit or service evaluation (see http://www.hra.nhs.uk/research-community/before-you-apply/determinewhether-your-study-is-research/). This study was conducted in accordance with the declaration of Helsinki, and the UK’s Data Protection Act.
PCO definition
Post-cataract PCO was identified from recorded post-operative complications, post-cataract diagnoses or post-cataract surgical records (for YAG posterior capsulotomy) from eight days post-cataract surgery to the date of the last record of any post-cataract assessment for the patient.
The first record of PCO post-cataract surgery is used as the index “event” for PCO, and for non-PCO eyes the last assessment date for the patient is used as a surrogate for final follow-up. As PCO can occur at different points in time, the Kaplan–Meier method with the actuarial adjustment was used to graphically display the PCO (“failure event”) rates over time and create PCO rates at specific post-cataract surgery time points representing the cumulative probability of PCO occurring.
PCO risk factor modelling
To identify potential risk factors influencing the development of PCO, an accelerated failure time Loglogistic model was fitted with robust cluster adjustment of the standard error using the patients as clusters to account for patient level correlation.
The covariates considered as potential risk factors are known before cataract surgery starts. The idea behind limiting potential risk factors to those known before the start of surgery is that at the post-cataract follow-up assessment, information could be provided to patients regarding their risk of PCO occurring within specific post-cataract time periods. Attempts to account for specific diseases that could develop between cataract surgery and PCO are not feasible with data currently submitted to the RCOphth NOD.
All candidate covariates were first investigated using the Logrank test, where any covariate significant at the 10% level was considered eligible for the multivariate Loglogistic model, which was fitted using backwards selection from the ‘full’ model to the ‘best fitting’ model by removing covariates with a significance level >1%. The use of 1% significance was adopted due to the increased chance of detecting very small significant differences from the large sample size, and to try to minimise negative impacts of possible overfitting. It is feasible this approach does not produce the best model for the sample, but is practical for a very large sample where some covariates are for rare diseases, and to attempt to remove covariates with minimal clinical differences that otherwise could be found statistically significant if using a higher significance level.
Model diagnostics included comparison of the final model with other parametric modelling approaches (Weibull, Lognormal and Exponential) and plotting Cox–Snell residuals against the cumulative hazard where deviations away from the line of identity imply a poorer model fit. All analysis was conducted in STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
Patients and operation characteristics
Within the study period, 824,553 operations eligible for the national cataract audit were performed from EMR enabled centres offering a full range of ocular services with at least one case of PCO later than one-month post-cataract surgery. It was necessary to exclude 323,681 (39.3%) operations due to: 211,839 with <1 month of follow-up data recorded, 29,635 as either a multi-piece or Toric IOL was implanted, 10,443 that experienced posterior capsular rupture, 6189 from one site where there is uncertainty about the IOL information and no follow-up data since November 2016, 2565 where we were unable to match the recorded IOL to a specific IOL model, 300 were recorded as ‘no IOL inserted’, 115 with no IOL information recorded, 90 with a missing IOL power, 65 with IOL powers outside the range of −10 to +40 dioptres and 12 as the recorded axial length was <18 mm. A further 62,428 operations from 13 centres were excluded as these centres had <50 cases of PCO in their otherwise eligible sample. The 500,872 cataract operations eligible for this analysis were performed in 41 participating centres, 40 English NHS Trusts and one centre from Guernsey.
The 500,872 operations were performed on 243,167 (48.5%) left eyes and 257,705 (51.5%) right eyes from 373,579 patients by 2196 surgeons. The operations were performed by surgeons of different grades; 924 consultant surgeons performed 302,814 (60.5%) operations, 266 career grade non-consultant surgeons (associate specialists, staff grades and trust doctors) performed 57,079 (11.4%) operations, 1221 more experienced trainee surgeons (3rd year of training and beyond) performed 120,354 (24.0%) operations and 389 less experienced trainee surgeons (1st and 2nd year of training and foundation doctors) performed 20,625 (4.1%) operations.
First eye surgery was performed in 326,996 patients where 188,939 (57.8%) were female and the median age at surgery was 76.4 years (inter quartile range 68.9–82.3 years). Second eye surgery was performed in 173,366 patients where 102,287 (59.0%) were female and the median age at surgery was 77.4 years (inter quartile range 70.3–82.9 years). Immediate sequential bilateral cataract surgery (ISBCS) was performed in 255 patients. For the 127,038 (34.0%) patients who had surgery to both eyes on separate days during the study period, the median time between the two operations was 4.4 months (range two days to 7.8 years).
Intra-ocular lenses (IOL)
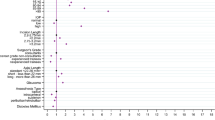
IOL were classified according to the material used (Table 1). Sixteen specific branded models were used, with two IOL models implanted in >100,000 operations each (Fig. 1). The number of different IOL models used in contributing centres varied considerably, with two (4.9%) centres using only one IOL model, four (9.8%) centres two IOL models, 11 (26.8%) centres three IOL models, two (4.9%) centres four IOL models and 22 (53.7%) centres five or more models with one centre having data for 12 IOL models.
PCO rates
Post-cataract PCO was recorded for 61,778 (12.3%) eyes, with 22,978 (37.2%) of these cases specifically documented as YAG laser being indicated or performed in the same institution that undertook the cataract surgery. The six month, one, three, five and nine year observed rates of PCO were 2.3%, 4.4%, 19.7%, 34.0% and 46.9% respectively. Different PCO profiles were observed between IOL materials, Table 2 and Fig. 2.
PCO modelling
The covariates considered for the risk factor modelling were grouped into factors related to the IOL, patient factors, cataract surgery and ocular factors. For the individual IOL models there was large variation in the hazard of developing PCO, with some models exhibiting similar patterns to overall and others a relatively flat hazard, potentially related to the tendency for certain IOL to be used predominantly in single centres whose post-operative pathways may be less or more effective at documenting PCO. To circumvent this extreme variation, the individual IOL models were not fitted as a covariate in the risk factor model, instead the IOL material was used.
At the univariate level all covariates considered for the post-cataract PCO risk factor modelling showed association at the 10% level except for the patient’s ability to cooperate (p = 0.353), the presence of corneal pathology (p = 0.947), the presence of no fundal view/vitreous opacity (p = 0.781), the presence of optic nerve/CNS disease (p = 0.888), and the presence of other retinal vascular pathology (p = 0.150). The final best fitting PCO risk factor model estimates are shown in Table 3.
Many of the differences for a covariate occur after a period of time has elapsed, for example the statistical difference in PCO rates between first or second eye surgery or the presence of glaucoma, pseudoexfoliation/phacodonesis or unspecified ‘other’ ocular co-pathology only becomes apparent after one-year post-cataract surgery. The difference between male and female patients, diabetic status, and the presence of age-related macular degeneration, diabetic retinopathy or a brunescent/white/mature cataract occur after two years post-cataract surgery. For some covariates the PCO rates diverge within the first year post-cataract surgery, for example patient age, lower power IOL’s, axial length, previous vitrectomy surgery and the presence of high myopia or other macular pathology.
The PCO risk factor model was not a perfect fit and the number of significant covariates is a concern regarding possible over-fitting. There was deviation away from the line of identity between the cumulative hazard and the Cox–Snell residuals, although the area of deviation of concern applied to ≈0.5% of the sample. As a sensitivity analysis the affected operations were removed, the PCO model re-fitted and very similar estimates found for each covariate.
Discussion
From the RCOphth NOD Cataract Audit dataset, 500,872 cataract operations performed by 2196 surgeons in 41 centres were included in this analysis with overall one-, three-, and five-year PCO rates of 4.4%, 19.7% and 34.0% respectively. These figures resonate with other large published series [3, 4, 6], and indicate the burden placed on patients and services by PCO which can be expected to grow as demand for cataract surgical provision grows over the next two decades [7]. Where opportunities exists to reduce PCO rates, there are strong economic arguments for taking these [3].
The risk factors for PCO identified in this study included an axial length >26 mm, presence of high myopia and implantation of lower IOL powers and previous vitrectomy surgery, along with younger age and female gender which could be utilised in the informed consent process.
IOL material
It has been proposed that hydrophilic IOL materials are a risk factor for PCO development. However, a Cochrane collaboration systematic review in 2010 including 66 prospective RCTs dealing with potential factors for PCO after cataract surgery failed to demonstrate statistically significant differences between different IOL optic materials, although they did conclude that silicone IOLs seem to have lower PCO rates in several studies and hydrogel (hydrophilic acrylic) IOLs tend to have higher PCO scores than other materials [2]. In the UK, the weight of evidence was insufficient for NICE, in their 2017 cataract guidelines [NG77], to promote the selection of one IOL material over another, although a meta-analysis from 2017, did report hydrophobic intraocular lenses as associated with lower Nd:YAG laser capsulotomy rates compared to hydrophilic lenses (OR = 0.38, (95% CI 0.16–0.91, P = 0.029)) [8]. Subsequent case series have also reported significantly higher rates of PCO for hydrophilic IOL [4, 9]. The findings from our study of significantly higher PCO rates with hydrophilic IOL (coefficient −0.717) compared with hydrophobic IOL is consistent with these. A report from Sweden suggests the main attraction of hydrophilic IOL may be economic, and whilst less expensive IOL save money for the surgical provider, the increased PCO rate can be shown to make the overall cost of cataract care at population level more expensive [3].
Age
The association of older age at the time of surgery with progressively lower PCO rates is consistent with other studies [9, 10]. One study estimated that each year of increased age gives an OR for PCO of 0.96 (95% CI 0.92–1.00) [10]. The observation that older patients experience less PCO does not, in itself, suggest any potential for reducing PCO rates at population level. However, should interventions that delay the onset of cataract become available, reduced PCO rates promoted by this delay in uptake of surgery may contribute to considerations of their cost-effectiveness.
Differential rates of opacification
Variation may be due to differences in the ability of clinicians to diagnose, or willingness to treat PCO. Those with small pupils had lower PCO rates, which is counterintuitive as these eyes might have been expected to have more residual soft-lens material at the end of surgery increasing their chances of PCO. A potential explanation would be that small pupils make PCO less visible to an examining clinician, hence reducing their tendency to refer for, or to offer, capsulotomy. People with diabetes and those also with diabetic retinopathy had lower PCO rates than those without diabetes, potentially explained by the increased use of anti-inflammatory medication in the immediate post-operative period, or by surgeon reluctance to perform YAG laser surgery on people with diabetes for fear of provoking macular oedema. Equally, this seemingly protective effect of diabetes could be due to retention of otherwise uncomplicated post-operative cataract patients in eye care services maintaining a larger denominator, an explanation that would serve equally for the association of lower PCO with glaucoma, age-related macular degeneration and other macular pathologies.
The higher PCO rate in eyes with an axial length >26 mm and in high myopia is potentially linked to the larger size of the capsular bag producing a less tight apposition of the square posterior edge of the IOL to the capsule reducing the effectiveness of that square edge in preventing posterior migration of lens epithelial cells. A recent meta-analysis showed clear benefit of capsular tension rings in reducing PCO rates, potentially by mitigating for looser fitting capsules [11], and it may be the use of these rings that explains the reduced PCO rates in eyes with pseudoexfoliation found in this study.
Study limitations
The use of routinely collected data, without linkage of health records, limits the inclusion of patients to those who subsequently interact with their cataract surgical provider. In some geographic areas, the assumption that patients will remain with the same provider is fairly sound, but in other settings patients move freely between providers for different treatments. Patients who have no further interaction with the cataract surgery provider leave the denominator at their last recorded visit. It is not possible to estimate the extent to which either the departure of PCO-free patients from the series creates a systematic bias towards over-estimation of PCO rates, or systematic under-estimation is produced by patients with PCO remaining undiagnosed, untreated or treated elsewhere. These biases could be reduced if the RCOphth is successful in its application for section 251 exemption which will permit repetition of this study with health data linkage between providers and other NHS databases via the NHS number.
The similarity between our estimates and those of other published series, both RCT [2] and real-world case series [3, 4, 6], suggests the extent of bias in either direction is acceptable for the purpose of risk factor evaluation, although the caveats need to be considered when citing PCO rates or absolute risks at given time points.
Conclusions
Quality in cataract service delivery is a multi-faceted concept. Reducing PCO rates feeds into most of the domains of quality suggested by the WHO (effectiveness, safety, people-centredness, timeliness, equity, integration and efficiency), as well as contributing to the proposed additional domain of planetary health [12]. Whilst the variation in observed PCO rates between centres, coupled with the tendency of many centres to adopt single IOL models for predominant use, prevented conclusions being drawn about individual IOL models, important inferences are still possible.
Centres should be strongly encouraged to undertake comparison of PCO rates for the different IOL they use, and opt for lenses that minimise the visual loss caused by PCO and the need for subsequent capsulotomy. The cost of YAG laser capsulotomy to the NHS is estimated at £132 per case on average, and it therefore represents a false economy for NHS providers to opt for less expensive IOL with higher PCO rates. Where the purchaser provider split exists, the perverse incentive to ignore PCO rates when procuring IOL needs to be resisted. It may be that commissioners could opt for lowering PCO rates as one aspect of quality on which they base remuneration, or even combine cataract and YAG laser capsulotomy services, such that no independent tariff is associated with the treatment of PCO. The incentive to lower PCO rates would then be universally felt. Future research might involve economic analyses of interventions to reduce PCO rates such as capsule tension ring usage or adoption of IOL models that perform particularly well in subsequent evaluations.
Summary
What was known before
-
Posterior Capsule Opacification (PCO) is the most common long-term post-operative adverse occurrence after cataract surgery.
-
Factors associated with increased risk of PCO can relate to the patient or their eye, the surgery, or the intra-ocular lens (IOL) material and design, such as the well-established superiority of a square-edged IOL in preventing PCO.
What this study adds
-
This is the largest published series investigating risk factors for PCO.
-
The six month, one, three, five and nine year observed rates of PCO were 2.3%, 4.4%, 19.7%, 34.0% and 46.9% respectively.
-
Risk factors that increased the risk of developing PCO included hydrophilic IOL material, an axial length >26 mm, high myopia, previous vitrectomy, younger age and female gender.
References
Maedel S, Evans JR, Harrer-Seely A, Findl O. Intraocular lens optic edge design for the prevention of posterior capsule opacification after cataract surgery. Cochrane Database Syst Rev. 2021;8:CD012516.
Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;2:CD003738.
Cullin F, Busch T, Lundstrom M. Economic considerations related to choice of intraocular lens (IOL) and posterior capsule opacification frequency - a comparison of three different IOLs. Acta Ophthalmol. 2014;92:179–83.
Ursell PG, Dhariwal M, Majirska K, Ender F, Kalson-Ray S, Venerus A, et al. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK Real World Evidence study. Eye. 2018;32:1579–89.
Buchan JC, Donachie PHJ, Cassels-Brown A, Liu C, Pyott A, Yip JLY, et al. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 7, immediate sequential bilateral cataract surgery in the UK: Current practice and patient selection. Eye. 2020;34:1866–74.
Johansson B. Clinical consequences of acrylic intraocular lens material and design: Nd:YAG-laser capsulotomy rates in 3 × 300 eyes 5 years after phacoemulsification. Br J Ophthalmol. 2010;94:450–5.
Buchan JC, Amoaku W, Barnes B, Cassels-Brown A, Chang BY, Harcourt J, et al. How to defuse a demographic time bomb: the way forward? Eye. 2017;31:1519–22.
Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis. Medicine. 2017;96:e8301.
Tokko HA, Hussain F, Al-Awadi A, Mei F, Zeiter JH, Kim C, et al. Factors associated with the development of posterior capsule opacification requiring yttrium aluminum garnet capsulotomy. Optom Vis Sci. 2019;96:492–9.
Prajna NV, Ellwein LB, Selvaraj S, Manjula K, Kupfer C. The madurai intraocular lens study IV: posterior capsule opacification. Am J Ophthalmol. 2000;130:304–9.
Zhang K, Dong Y, Zhao M, Nie L, Ding X, Zhu C. The effect of capsule tension ring on posterior capsule opacification: a meta-analysis. PLoS One. 2021;16:e0246316.
Yoshizaki M, Ramke J, Zhang JH, Aghaji A, Furtado JM, Burn H, et al. How can we improve the quality of cataract services for all? A global scoping review. Clin Exp Ophthalmol. 2021;49:672–85.
Acknowledgements
It is with gratitude that we remember our friend and colleague Robert Johnston, who sadly died in September 2016. Without his inspirational vision, determination and career long commitment to quality improvement in ophthalmology this work would not have been possible. We acknowledge the support of the hospitals that participated in this National Ophthalmology Database Audit study and thank our medical and non-medical colleagues for the considerable time and effort devoted to data collection.
The 41 centres with data in this analysis are listed in alphabetical order below separated into the regions they are located in.
English NHS trusts: Barking, Havering and Redbridge University Hospitals NHS Trust; Barts Health NHS Trust; Bradford Teaching Hospitals NHS Foundation Trust; Calderdale and Huddersfield NHS Foundation Trust; Chesterfield Royal Hospital NHS Foundation Trust; County Durham and Darlington NHS Foundation Trust; East Sussex Healthcare NHS Trust; Epsom and St Helier University Hospitals NHS Trust; Frimley Health NHS Foundation Trust; Gloucestershire Hospitals NHS Foundation Trust; Hampshire Hospitals NHS Foundation Trust; Imperial College Healthcare NHS Trust; King’s College Hospital NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Liverpool University Hospitals NHS Foundation Trust; Mid Cheshire Hospitals NHS Foundation Trust; Moorfields Eye Hospital NHS Foundation Trust*; North West Anglia NHS Foundation Trust; Nottingham University Hospitals NHS Trust; Oxford University Hospitals NHS Foundation Trust; Portsmouth Hospitals NHS Trust; Royal Berkshire NHS Foundation Trust; Royal Cornwall Hospitals NHS Trust; Royal Devon University Healthcare NHS Foundation Trust**; Royal United Hospitals Bath NHS Foundation Trust; Salisbury NHS Foundation Trust; Sandwell and West Birmingham Hospitals NHS Trust; Sheffield Teaching Hospitals NHS Foundation Trust; South Warwickshire NHS Foundation Trust; The Hillingdon Hospitals NHS Foundation Trust; The Mid Yorkshire Hospitals NHS Trust; The Newcastle upon Tyne Hospitals NHS Foundation Trust; The Shrewsbury and Telford Hospital NHS Trust; University Hospital Southampton NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; University Hospitals Bristol and Weston NHS Foundation Trust; University Hospitals Coventry and Warwickshire NHS Trust; University Hospitals Plymouth NHS Trust; Wrightington, Wigan and Leigh NHS Foundation Trust; Yeovil District Hospital NHS Foundation Trust.
Guernsey: Medical specialists group Guernsey;
*Includes data from Bedford Hospital within Bedfordshire Hospitals NHS Foundation Trust and Croydon Health Services NHS Trust as the ophthalmology services in these places are part of Moorfields Eye Hospital NHS Foundation Trust.
**This NHS Trust was formed from a merger of two former NHS Trusts since the data was collected. In this analysis only data from the former Northern Devon Healthcare NHS Trust is included.
Funding
This analysis was funded by an unconditional grant from Alcon (Geneva, Switzerland) in support of the Royal College of Ophthalmologists National Ophthalmology Database cataract audit. The funders did not have any editorial oversight, right of veto or academic input into the analysis or write up of this work. The National Cataract Audit is currently funded through participation fees from centres as well as unrestricted financial contributions from Bausch + Lomb and Alcon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Donachie, P.H.J., Barnes, B.L., Olaitan, M. et al. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: Report 9, Risk factors for posterior capsule opacification. Eye 37, 1633–1639 (2023). https://doi.org/10.1038/s41433-022-02204-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02204-1
- Springer Nature Limited
This article is cited by
-
Dislocated 4-haptic intraocular lens rescue with Gore-tex suture scleral re-fixation
International Journal of Retina and Vitreous (2024)
-
Long-term real-life outcomes of the Clareon® hydrophobic intraocular lens: the Clarte study in 191 eyes
BMC Ophthalmology (2024)
-
The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 16, influence of remuneration model on choice of intraocular lens in the UK
Eye (2023)
-
Hydrophobic versus hydrophilic acrylic intraocular lenses within public sector based on the type of funding contacts: the debate continues
Eye (2023)