Abstract
Objective An evidence-based review on the role of botulinum toxin type A (BoNTA) on diverse cosmetic applications of interest to dental practitioners and allied specialities. In this context, to identify the cosmetic treatments that have an evidence-based rationale against areas requiring further research, with a view to assess the safety and efficacy of BoNTA.
Data source and selection A comprehensive search was conducted using Cochrane Library of Systematic Reviews, Cochrane Central Register of Controlled Trials, and PubMed (Medline) electronic databases. Thirty-nine studies of variable quality were included. The Best Evidence Topics (BETs) Critical Appraisal Tool was used to facilitate the quality assessment of relevant studies.
Data extraction Based on current level II evidence, BoNTA was safe and effective to improve facial contour, reduce volume and thickness of bilateral hypertrophic masseter. Conservative doses using a combined approach of BoNTA and hyaluronic acid was recommended as a safe and effective treatment for perioral enhancement supported by level II evidence. There was limited evidence, not higher than level III, to support BoNTA effectiveness for gummy smile associated to perioral musculature hyperactivity, while jawline sculpting targeting the platysma muscle had lower level IV evidence up to this date.
Conclusion BoNTA has been widely used off-label for the investigated cosmetic orofacial conditions, with reports of 'good patient and practitioner satisfaction'. However, there is limited high-quality evidence to support the long-term safety and effectiveness of repetitive BoNTA injections. Additionally, no studies were found that provided a cost-effectiveness evaluation of BoNTA formulations against other current cosmetic interventions. Well-designed clinical trials, including long-term follow-up, would help to provide robust evidence-based recommendations for clinical practice, supporting BoNTA popularity, independently or in a combined approach.
Similar content being viewed by others
Introduction
Botulinum toxin (BoNT) is an exotoxin synthesised by gram-positive anaerobic bacteria - Clostridium botulinum, commonly found in the surrounding environment.1 At least eight antigenically different serotypes have been identified; BoNT type A and B were the most studied.2 While five distinct formulations of these two serotypes have been approved by the US Food and Drug Administration (FDA) for medical practice, botulinum toxin type A (BoNTA) formulations are used on a larger scale in comparison to BoNT type B. Type A (BoNTA) formulations include: onabotulinumtoxinA-OnaBoNTA (Botox/Vistabel), abobotulinumtoxinA-AboBoNTA (Dysport/Azzalure), incobotulinumtoxinA-IncoBoNTA (Xeomin/Bocouture), and since 2019, prabotulinumtoxinA-xvfs-PraBoNTA (Jeuveau/Nabota).3 Each of these BoNTA-containing preparations are unique and not interchangeable.4 Thus, recognition of the similarities and differences among distinct BoNTA preparations is crucial to ensure the correct application, in order to achieve optimal outcomes and safe clinical practice.1,2,3,5
Recent literature has highlighted a steady growth over the years of non-surgical and minimal invasive procedures, with BoNTA remaining the most prevalent cosmetic treatment in the US (2019).6 The range of BoNTA applications has expanded exponentially since its first introduction into the market, with considerable clinical trials in progress for registration.1,7 The toxin's widespread popularity is a reflex of the ongoing research, allowing greater knowledge of its underlying physiology, confirming that BoNTA is safe, minimally invasive, and effective for several cosmetic and therapeutic conditions.1,8 Accordingly, there is a growing number of clinical dentistry applications, as well as dentists engaging in BoNTA delivery, making up the steady growth of the BoNTA industry.8,9 In 2017, one study documented that there were approximately 2,500 dentist prescribers of BoNT in the UK.9 This figure is considerably superior when compared to any other speciality.9
Objectives
This evidence-based review aimed to identify, categorise and critically appraise current literature on the safety and efficacy of BoNTA cosmetic applications, in each of the recognised dental and orofacial conditions of interest to dentists and allied specialities. Thus, in this context, this review was performed to summarise the best available scientific evidence and potentially promote safer and effective clinical care. The Best Evidence Topic (BestBETs) methodology was used, which follows a well-structured approach, with the purpose of answering specific three-part clinical questions: 'In the treatment of cosmetic dental and orofacial conditions, is BoNTA a safe and effective intervention?'
Materials and methods
Data sources
Following the BestBETs methodology, an evidence-based review was performed using the Advance search on PubMed (Medline), Cochrane Library of Systematic reviews and Central Register of Controlled Trials databases to screen for relevant papers. Various terms combined with key text terms, which originated different constructions of search terms, have been used through the elective search engines (see online Supplementary Appendix A for keywords and Booleans).
An initial scan of the literature on this topic anticipated limited relevant evidence in which the selected studies would be highly heterogeneous, and the outcomes were likely not suitable for a meta-analysis. Therefore, the BestBETs method has been selected, as it is a suitable format to synthesise and appraise evidence when there is limited high-quality data available. First pioneered in the emergency department, the BestBETs method has disseminated to other medical specialities and has been regularly published in peer-reviewed journals. This methodology has been previously described in the literature.10
Data selection
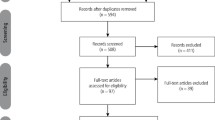
Two independent reviewers (INP and HH) have systematically searched the databases and any disagreements on inclusion and exclusion criteria were resolved through a discussion. See Figure 1 for explicit search results, with the full text evaluation narrowed down to 97 potentially relevant papers. Reference lists of all included papers were manually searched and followed the search parameters (shortlisted in Table 1). The analysis yielded 39 relevant studies on cosmetic applications. These were collected into basic BETs evidence tables, while classified and appraised for their quality for the purpose of the three-part research question, using the adapted BETs critical appraisal tool.11 Although there are other validated critical appraisal tools, the BETs checklist was selected because it is an easy and useful tool to evaluate different study types' methodological soundness to assess any confounding factors, potential bias and study limitations, having been suggested as a complement to the methodology of BestBETS.11 The data extracted and appraised for each study was confirmed by reviewer consensus.
Regarding the exclusion criteria, it is worth noting that the evolving emphasis on facial aesthetics advocates a patient-tailored panfacial approach, recognising the impact that treating one area could have on other areas. Accordingly, the facial expression muscles have compound physiologic and anatomic interactions instead of acting in isolation.12,13 Nevertheless, the study was streamlined to concentrate on the mid- and lower face, where the mouth plays a central role, therefore excluding BoNTA treatments of glabellar and forehead lines, or 'crow's feet', fundamental aspects of the upper face appearance.12,13
Assessment of study quality and risk of bias
The relevant studies found by the search strategy were firstly categorised in terms of type of study and level of evidence (LOE), following the study-quality hierarchy criteria outlined by the latest Oxford Centre for Evidence-Based Medicine ratings.16 The LOE of the studies included were classified as: level I - systematic review (SR) of randomised trials (RCTs) or n-of-1 trials; level II - individual RCTs or observational study with dramatic effect; level III - SR of non-randomised controlled cohort/follow-up study (or individual studies); level IV - SR of case series, case-control studies, or historically controlled studies (or individual studies); and level V - mechanism-based reasoning or expert opinion. Level was graded up or down based on study quality, imprecision, indirectness, inconsistencies, or effect size. We accepted the authors' quality classification of their own study or included papers and did not attempt to re-classify them.
The next step was to evaluate the detailed methodology using the adapted BETs critical appraisal checklist.11 Finally, the risk of bias assessment for included RCTs was performed according to the Cochrane Methodology for Systematic Reviews of Interventions,17 in respect of randomisation sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Each item was scored as low, unclear, or high risk of bias and expressed in Table 2. The scoring was performed independently by the reviewers (INP, HH), and any disagreement was resolved by consensus. The overall risk of bias regarding each RCT was judged based on the following: 1) low risk, if all domains were scored as 'low' or only one as 'unclear'; 2) unclear, if two or more domains were estimated 'unclear'; and 3) high, if at least one domain was scored as 'high'. Of the included SRs, we accepted the authors' own risk of bias assessment for included RCTs and did not attempt to re-classify them.
Data extraction and outcomes
Two reviewers (INP, HH) independently extracted data on study design, outcomes and quality. Clinical and patient-related outcomes and adverse events were reviewed in detail. Due to the large number of BoNTA applications being evaluated, each with several available assessment tools, when reporting, we extracted data on improvements in each individual condition-related cosmetics and concentrated on the outcomes most reported in the different studies, with either objective or subjective assessment tools. Refer to Table 3 for a brief description of the outcomes that were collected and LOE associated, where findings were simplified with symbols to facilitate comparisons.
Data analysis and reporting
The included studies were summarised in BestBET tables, using the standardised form to provide the relevant data of each study. Due to the heterogeneous nature of the outcomes in the included studies for each condition, a meta-analysis was not possible to perform. For each recognised BoNTA application, when there were sufficient studies of level I/II evidence, the studies presenting lower LOE were not considered for discussion, unless these were recent studies relevant to the three-part question. Whereas when the best evidence relied on level III/IV/V studies, these were all considered. In addition, evidence was judged 'high', 'moderate' or 'low' based on the conclusions of included studies and overall quality and risk of bias assessed.
Results
Data extraction
See Figure 1 for the PRISMA flowchart for data abstracted.18 Online Supplementary Appendix B shows the summary of papers from the initial search utilised to identify BoNTA applications concerning dental practitioners. Online Supplementary Appendix C illustrates the 39 relevant studies identified according to each aesthetic orofacial condition considering its relevance, reliability and credibility, based on those suggested by the latest Oxford Centre for Evidence-Based Medicine LOE, which acknowledges the importance of SRs of randomised trials as the strongest LOE, while giving space to the expert opinions and mechanism-based reasoning lowest level V.16 Online Supplementary Appendix D presents the domains of adapted BETs critical appraisal worksheets used with an assessment for each study.11
Summary of the findings
This evidence-based review (part two) focused on the BoNTA cosmetic applications. The graphic illustrated in Figure 2 provides an overview of relevant studies with corresponding LOE. The results are presented in a descending order of evidence.
Search and study selection - study characteristics and quality
Masseteric hypertrophy
The search regarding the safety and efficacy of BoNTA for masseteric hypertrophy produced 11 articles of sufficient quality.19,20,21,22,23,24,25,26,27,28,29 The highest evidence level I was one Cochrane review in 2013,22 that was unable to identify RCTs or controlled trials. Since then, only four level II RCTs were found;20,25,26,27 this involved 158 participants with sample sizes ranging from 20 to 98, follow-ups from 12 weeks to at least 15 months, and only one trial was double-blinded. In addition, two level III studies were included,21,29 of which one was a single-blinded prospective trial enrolling 50 participants and with long four-year follow-up.21 The other was a group-controlled cohort enrolling ten participants proving the final assessment at three months.29 Furthermore, three level IV studies were considered,19,24,28 including one clinical case series involving 680 participants,24 one single-blinded cohort enrolling 252 participants,28 from which we accepted the authors' own classification, and one evidence-based review,19 citing additional three level III30,31,32 and nine level IV studies.33,34,35,36,37,38,39,40,41 Finally, one level V literature review completed the search.23 Inclusion criteria varied among level II studies, ranging from measurement of changes in subcutaneous thickness or hard tissues alongside muscle or soft tissues effects, assessment on how to improve duration of masseter muscle reduction or the effect of a second injection. Level III studies included long-term management on bilateral masseter hypertrophy or unilateral cases. Treatment effectiveness in the East Asian population, aetiology and incidence rate of treatment complications, or the proposal of a tailored protocol based on muscle bulge-type classification, were involved in level IV studies. The level V study provided a comprehensive review on complications related to BoNTA for lower face contouring. Outcome assessment tools varied greatly among studies, ranging from ultrasonographic imaging, computerised tomography (CT) scan, photography, clinical assessment and patient self-assessment scales, 3D laser scanning or 3D-CBCT scan. Overall characteristics of included studies are available in the BestBETs table (see online Supplementary Appendix C).
Perioral lines
Research regarding BoNTA use for perioral lines produced 13 relevant papers. Of these, six were level II studies,14,15,42,43,44,45 which included one SR evaluating the safety of BoNTA in facial aesthetic treatments and five RCTs. The SR only included one RCT for perioral region and was downgraded to level II owing to the unclear risk of bias or study quality assessment performance. The five RCTs involved a combining total of 252 participants, which included only two male subjects, with sample sizes ranging from 12 to 90. Three studies reported the use of OnaBoNTA (Botox) and two selected AboBoNTA (Dysport). The majority performed the final efficacy and safety assessment approximately at six months; only one study had a longer follow-up of 36 months, and another study reported a five-month final appointment. All studies evaluated BoNTA safety and efficacy for perioral rhytids. However, the results presented different objectives, which included: comparisons between two or three doses targeting only the perioral area or the whole face, assessment of BoNTA treatment benefits in contrast to HA injections or both combined for lower face rejuvenation, with one study evaluating the efficacy of BoNTA pre-treatment to enhance chemabrasion. In addition, two level IV studies were found enrolling 32 and 18 participants each, with short follow-ups of three months and 2-3 weeks, respectively. The first level IV study reported an effective dual treatment with BoNTA and HA for the perioral area with objective measuring;46 the latter was the first case series reporting the use of BoNTA for perioral vertical rhytids.47 The remaining five studies were expert consensus providing clinical recommendations for BoNTA treatment alone, on a dual modality or multimodal approach.12,13,48,49,50 Overall characteristics of included studies are available in the BestBETs table (see online Supplementary Appendix C).
Drooping of mouth corners - marionette lines
The search conducted on the use of BoNTA to uplift mouth corners and enhance marionette lines had an overlap of seven included studies for perioral lines amelioration, which included three level II RCTs and four level V expert recommendations.12,14,44,45,48,49,50 Three additional case series were found targeting exclusively downturned mouth corners, with/without deep oral commissure and/or marionette lines caused by an hyperfunctional depressor anguli oris (DAO) muscle.51,52,53 Of these, one was level IV evidence and for the other two, we accepted the authors' classification of level V. Overall, this involved 159 participants with sample sizes ranging from 16 to 107, and final assessment at three months or one year. BoNTA formulations varied among the studies, including OnaBoNTA (Botox), IncoBoNTA (Xeomin), or PraBoNTA (Nabota). Overall characteristics of included studies are available in the BestBETs table (see online Supplementary Appendix C).
Excessive gingival display - 'gummy smile'
Most published studies on BoNTA safety and efficacy for excessive gingival display (EGD) were case series, with small sample sizes and short follow-ups. The two SRs conducted by Nasr et al.54 and Duruel et al.55, as well as a meta-analysis by Chagas et al.,56 have overall included six prospective studies (n = 145) available on this subject,57,58,59,60,61,62 in which OnaBoNTA (Botox) was the primary formulation used, with only one study reporting the administration of AboBoNTA (Dysport). The two SRs were downgraded to level IV. For one SR, we accepted the authors' own classification;54 the other had methodological limitations with unclear study quality and risk of bias assessment for included studies.55 The meta-analysis had level III classification, owing to the included prospective studies' moderate-to-poor quality score. Four additional studies have been included;63,64,65,66 of those, one was a level III prospective cohort,63 and three were level IV clinical studies.64,65,66 The highest quality of evidence was provided by the recent cohort conducted by Cengiz et al.63, enrolling 28 participants without significant gender or age differences, which investigating the efficacy of BoNTA administered to either levator labii superioris alaeque nasi (LLSAN) or orbicularis oris (OO) muscles, and the meta-analysis by Chagas et al.56 on the treatment effectiveness duration. The level IV clinical studies either evaluated the role of BoNTA as an adjunct to lip repositioning flat surgery, or following orthodontic treatment for the management of EGD with or without vertical maxillary excess, respectively.64,65 Other case series investigated BoNTA injection site according to the different EGD types due to hyperactivity of upper lip muscles.66 This involved a total of 20 female participants, with sample sizes ranging from 3-10, and the final assessment varying from 15 days to 24 weeks. Outcome assessment tools were mostly clinical measurements and standardised frontal photography to evaluate EGD severity, improvement, and duration of effects. Only one study evaluated the level of satisfaction with the VAS scale. Overall characteristics of included studies are available in the BestBETs table (see online Supplementary Appendix C).
Jawline sculpting - platysma
The search regarding BoNTA safety and efficacy for jawline contouring targeting the platysma muscle produced a total of six relevant articles of sufficient quality. Of these, three were retrospective studies,67,68,69 while one was a prospective trial and an extension of a primary study with follow-up at day 15.70,71 Overall, the LOE for three of these studies was level IV, and one level V, for which we accepted the authors' classification. This involved a total of 572 participants, with sample sizes ranging from 30 to 192. OnaBoNTA was the most recurring formulation, followed by AboBoNTA, and one study reported the use of IncoBoNTA. The outcomes reported varied among the studies. This included lower face contour improvement, overall lower face or full-face amelioration, facial sagging and neck rejuvenation, level of satisfaction with treatment and results. Outcome assessment tools were mostly standardised photography and patient/investigator questionnaires. Only one study reported the use of validated scales. In addition, two level V consensus recommendations for Asian and western populations were included.49,72 The latter was a repetitive study from research involving perioral lines. Overall characteristics of included studies are available in the BestBETs table (see online Supplementary Appendix C).
Study risk of bias
Of the 39 included studies, ten were level II evidence and nine were RCTs. Risk of bias was evaluated in the nine studies. Of these, four related to masseter hypertrophy, three for perioral enhancement (including perioral lines, marionette lines and drooping of mouth corners), and two exclusively for perioral lines (Table 2).
Masseteric hypertrophy
From the four RCTs included, the majority had small sample sizes (n = 20) and short follow-up periods, ranging from 3-6 months. Only one had a larger sample (n = 98) and longer follow-up (16 ± 2.3 months). Two were group-controlled and the other two compared the effects of a single injection to a second BoNTA injection four months apart. Risk of bias was evaluated in the four included studies (Table 2). Only one had a risk that was estimated low for most subdivided risks; however, for attrition bias, it was unclear if the loss to follow-up of three participants, with similar reasons and unlikely to be related with true outcome, may not have created an unbalanced number between groups with impact on outcome. All the remaining RCTs had an overall risk estimated unclear. None have reported the randomisation method or have addressed blinding of participants and personnel or outcome assessment. For reporting bias, there was insufficient information to permit judgement.
Perioral enhancement - perioral lines, drooping of mouth corners, marionette lines
From the five RCTs included, two were double-blind, two were single-blind and one was an open-label trial; three were parallel-design and multicentre studies, one was single-centre, and another was a split-face study. Risk of bias was evaluated in the five included studies (Table 2). Overall, all had a risk that was estimated low for most subdivided risks, except one study that was scored unclear risk of bias. For selection bias, only one study did not report the method or tool used for randomisation. For performance bias, two complemented trials were single-blind, although it was estimated that the outcome was unlikely to be influenced. Only one study did not address this outcome or the blinding of outcome assessment, for which detection bias was also estimated unclear. For attrition bias, most studies presented missing outcome data owning to loss follow-up. There was insufficient reporting to permit judgement if the missing data was likely to be related to true outcomes. For reporting bias, two studies were scored unclear due to insufficient information for key outcomes (for example, in one trial, the outcome assessment for the lower face after the four-week follow-up is not clear).
Efficacy and safety outcomes
A brief description of the outcomes that were collected, with the LOE for each key efficacy parameter, is shown in Table 3. BestBET tables available in online Supplementary Appendix C summarise the 39 identified studies and provides full detail of methods of evaluation, outcomes reported, study designs, LOE, interventions, as well as study limitations for each study. The key findings are summarised below.
Masseteric hypertrophy
The highest LOE for masseteric hypertrophy was the Cochrane review in 2013, that was unable to support or refute BoNTA safety and efficacy for benign over-developed masseter muscles.22 Since then, only four RCTs were found.20,25,26,27 Wei et al.27 illustrated a prolonged masseter muscle reduction when patients were instructed to strengthen their masticatory movements immediately after BoNTA injections, during the denervated atrophic stage of the masseter muscle, at the expense of a higher risk of paradoxical bulging of other masticatory muscles, particularly of temporalis muscle. This study (n = 98) also demonstrated the treatment to be effective and safe, with low incidence of mild and transient adverse effects (AE), and high percentage of patient satisfaction compared to baseline.27 Lee et al.25 RCT (n = 20) demonstrated greater muscle reduction and aesthetic results with a second BoNTA injection after four months. This timing was considered capable to maintain the maximum change seen at three months. The other RCTs provided by Park et al.20 in 2018 and Lee et al.26 had interesting findings on the effect of BoNTA injections into masseter muscle on soft and hard tissues. The former showed that this procedure only significantly affected the muscle thickness and not subcutaneous thickness, from muscle to skin. Moreover, the measurements of subcutaneous thickness provided by ultrasonography confirmed the need for an injection needle longer than 8 mm, and 29 G half-inch long needles were recommended.20 The latter raised concerns regarding the significant noted consequences on hard tissues with repetitive BoNTA injections. While a more effective improvement in masseteric hypertrophy and sustained facial contour results were demonstrated with a second BoNTA injection, changes in mandibular angle bone volume was reported, although without differences in intergonial angle width of mandibular angle area.26
Level III/IV studies have supported the safety and effectiveness of BoNTA for masseteric hypertrophy and acknowledged a low AE risk.19,21,24,28,29,30,31,32,33,34,35,36,37,38,39,40,41 In 2018, Peng et al.,23,24 with a large study population (n = 680), attempted to categorise the AE according to the aetiology (non-muscular, toxin effect or injection site-related) and evaluated the incidence rate, which has proven to be low. Most complications occurred within 2-4 weeks post-injection and resolved in 12 weeks, including the reduced ability to chew (30%) and bruising (2.5%), with other complications reporting an incidence rate less than 1%.24 The same author performed a level V literature review and revealed that non-specific AE, such as local pain and swelling, were also commonly reported. While the incidence rate for paradoxical bulging of the temporalis or upper part of the masseter muscle, sunken cheeks, sagging, asymmetric facial expressions, and headache varied greatly, the risk of xerostomia and neurapraxia was very low and controversial.23 A sound anatomy knowledge and 3-4 injections within the safety zone at 1 cm from all margins, with longer needle sizes to avoid injecting parotid gland, were deemed more essential to mitigate AE risk than BoNTA dose. Xie et al.28 proposed a protocol according to masseter muscle thickness and bulging type classification; this reported tailored approach improved safety, effectiveness in reducing masseter volume, and ameliorated lower face contour. In addition, Cheng et al.19 provided an evidence-based review and concluded that the ideal patients likely to benefit from BoNTA treatment were the younger presenting a wider mandible or square-face due to masseter hypertrophy. Older patients with sagging jowls were not ideal candidates, whereas larger cheekbones, excessive fat on upper masseter muscles, or associated bruxism scenarios would require special considerations. The author highlighted that several injection techniques have been described. However, the three-point triangular pattern has been recommended. The most commonly chosen location for masseteric intramuscular BoNTA injections is the safety zone located under an imaginary line connecting the earlobe and the angle of the mouth, and between the posterior and anterior easily palpable borders of the contracted masseter, 1 cm away. Based on LOE IV, this review reported that the optimal dose remains controversial, albeit men generally require higher doses, and 20 U (OnaBoNTA) was considered the minimal effective dosage for both genders.55 BoNTA was told to provide longer-lasting outcomes compared to type B.53 LOE III supported that multiple factors are likely to determine the optimal dosage, including: individual differences, injection technique, and type of BoNTA. Doses and muscular thickness tend to decrease with repetitive treatments.62 The ideal frequency for BoNTA injections was still uncertain; however, 2-4 yearly injections to maintain results have been recommended. 62 All BoNTA formulations (including newest ParaBoNTA) had comparable efficacy with the same number of injections, but no conversion ratio has been established, and without evidence-based efficacy advantage, OnaBoNTA was the most used. 57,59,63
Other level III studies showed that doses and muscular thickness tend to decrease with ongoing treatments.21,36 Shome et al.21 2019 prospective trial (n = 50) with a long four-year follow-up, recommended the frequency of injections to maintain satisfactory results to be before masseter re-hypertrophy at approximately three months, when the average of masseter muscle reduction was reported 12%. This study also revealed that lower dosages with a repetitive pattern can significantly increase short- and long-term treatment safety and effectiveness, with higher patient satisfaction regardless of age and gender, in comparison to a single dosage.21
Cha et al.29 cohort study (n = 10) reported that for facial asymmetry of muscular origin, unilateral injection on the affected masseter muscle was safe and significantly effective. Changes in muscle volume and thickness was also noted at the non-injected side, but these were not statistically significant. Overtime, the decrease in muscle thickness on the injected-side was significant, while the non-injected side remained constant, hence the lower face become less asymmetric. It was also reported that unilaterally injected muscles were likely to have less reduction in thickness and volume compared with bilateral injections.
Perioral lines
Cavallini et al.15 SR confirmed the safety profile of approved BoNTA formulations (except newest PraBoNTA) for short- and medium-term cosmetic treatment of all facial one-thirds. However, conclusions were based on only one RCT (n = 90) available on perioral treatment.14 Complications reported on the lower face were mild and transient. This involved mostly lip asymmetries and imbalance of perioral region, lip dryness, numbness, bruising, swelling, muscle weakness with functional disturbances, mild herpes simplex, and influenza-like symptoms. Chang et al.46 level IV prospective study (n = 32), one of the most recent on this subject, reported scarce quantitative evidence for specific treatment of perioral region, despite the increasing demand and proposed therapies for perioral enhancement. Based on validated tools (dynamic 3D digital image and FACE-Q questionnaire), this study concluded that BoNTA combined with hyaluronic acid (HA) resulted in significant reduction of perioral strain, with high patients' satisfaction, particularly older patients with considerable age-related changes.
Since the first prospective case series (n = 18) by Semchyshyn et al.47 in 2003, demonstrating positive effects with intradermal BoNTA injections in smoothing dynamic perioral wrinkles, there have been level II evidence studies supporting BoNTA safety and significant effectiveness on this facial area.14,42,43,44,45 These RCTs (n = 252) also showed the treatment to be well tolerated with high patient satisfaction. However, Cohen et al.43 and Hexsel et al.45 evaluated different BoNTA doses and confirmed that complications risk is dose-dependent for perioral region. While the different doses did not seem to influence the level of satisfaction or duration of effectiveness, both Carruthers et al.14,44 RCTs, and more recent level IV studies,46 have provided growing evidence of greatest efficacy, with significant improvement on duration of effect and patient satisfaction with a combined approach of BoNTA and HA. Carruthers et al.14,44 level II studies provided a comparative evaluation between treatments with BoNTA only or combined with HA, or HA independently, and showed the duration of effects to be approximately 58 days, 146 days, and 100 days, respectively. Kadunc et al.42 RCT demonstrated that pre-treatment with BoNTA injections optimised the maintenance of great clinical results of chemodermabrasion for perioral enhancement.42
There were several level V consensus reports providing clinical recommendations.12,13,48,49,50 It has been generally accepted that BoNTA injections in the perioral area should be very superficial/intradermal and symmetrical. Although the number of BoNTA injection-sites varied from two to five points, it has been recognised that these should be slightly distant from the vermillion border, at least 5 mm away from the Cupid's bow apex and medial oral commissure, to avoid flattening of the midline, or drooping and drooling of the lips, respectively. A two-week review to evaluate results and correction, if necessary, has been considered as appropriate, and the maximum dose per injection point ranged from 0.5-1 U. For a combination approach, either one session or sequential stages have been considered viable options.49 While some authors have recommended for BoNTA injections to precede a subsequent lowered dose of HA, on a single session, to avoid the resultant swelling from the fillers administration,13 the opposite has also been suggested based on the hypothesis that HA injection can provide structural support and reduce the BoNTA amount.48
Drooping of mouth corners - marionette lines
The search on the use of BoNTA to uplift mouth corners and enhance marionette lines included seven repetitive studies.12,14,44,45,48,49,50 None of the additional studies found, targeting exclusively the conditions caused by an hyperfunctional DAO muscle, were of higher LOE than level IV.51,52,53 Nonetheless, these corroborated the findings of the RCTs (n = 180) demonstrating BoNTA injections safety and significant effectiveness in lifting mouth corners and enhancing oral commissures and marionette lines.14,44,45 Moreover, simultaneous use of lower BoNTA and HA doses yielded cumulative improvements conferring greatest efficacy, safety, and patient satisfaction on the treatment of a saddened and aged appearance. Carruthers et al.14,44 RCTs demonstrated a duration effect of approximately 79 days on a dual modality compared to the 48 days seen with BoNTA standalone therapy. Jeong et al.53 level V study reported that this dual approach was effective in lifting the mouth corner in young patients intolerant to surgical intervention. Qian et al.51 case series (n = 36) reported satisfactory results for congenital unilateral cases. Visible improvement of depressed stern and aged appearance was visible at week 2, with maximal effects at the first month, that were preserved for 6-9 months.
There are no guidelines for a multimodal approach, with some authors preferring either a single or staged intervention, with BoNTA injections first following by HA administration, or vice versa.13,49 Different BoNTA injection-sites, depths and techniques have been reported, one example is the Bae et al.52 level IV prospective study (n = 16) where an additional BoNTA intramuscular injection within the mentalis muscle to rebalance perioral musculature has been recommended. However, there is consensus regarding the importance of conservative doses in the perioral area to minimise the risk of lip asymmetries, lengthening of upper lip, proprioceptive and functional disorders including nutrient intake, speech and vocalisation, when targeting the OO muscle. Whereas treating the DAO can result in the involvement of other overlapped muscles and promote drooling, oral incompetence, and smile asymmetries, thus injections into this muscle should not exceed 4-8 U.13,50
EGD - 'gummy smile'
The highest quality of evidence for BoNTA safety and efficacy on EGD involved two level III studies. One was a recent prospective cohort (n = 28) conducted by Cengiz et al.63 demonstrating that OnaBoNTA injections of 5 U into LLSAN or 2.5 U into OO can be effective to correct EGD with gingival exposure between 2-8 mm. The significant improvement in the amount of visible gingiva, increase in smile index, and level of satisfaction translated on the VAS scores were comparable for both techniques, and without differences between genders. Regardless of the technique used, the return to baseline values was estimated to start at four months; however, at six months, the visible gingiva measurements were still not at the initial values. No AEs were reported, thus the author concluded that quicker effects when targeting the OO muscle may not compensate the risk of more severe AE.
The level III meta-analysis by Chagas et al.56 also reported that BoNTA therapy had a significant impact on improving EGD caused by upper lip muscle hyperactivity, with high level of satisfaction. The maximal were obtained at two weeks, with gradual decrease up to 12 weeks. However, some of the included studies reported that the beneficial effects of BoNTA did not return to baseline values up to 24 weeks, and it has been speculated that these effects may last up to 30 or 32 weeks with repetitive injections. For this reason, the author concluded that there was limited evidence to determine an ideal BoNTA dose and effectiveness longevity.56
The two level IV SRs conducted by Nasr et al.54 and Duruel et al.55 reported that BoNTA was safe and an effective option for EGD (≥ 2 mm) associated to muscle hyperactivity, when a minimally invasive approach was preferred, or as an adjuvant to surgical interventions. Based on post-treatment surveys, patient satisfaction was high. However, the percentage of EGD improvement greatly varied among included studies, with potential correlation between the type of EGD or injection-site. Significant improvement was associated with anterior or mixed EGD type, while less improvement was linked to posterior or asymmetric EGD types, when the LLSAN was not the targeted muscle. No serious long-term AEs reported. However, short-term AEs with incidence within the first week's post-treatment occurred. This included: pain and/or twitching at the injection-site, headache, dizziness, facial or smile asymmetries, 'joker smile', ptosis of upper lip or oral commissure, lengthening of upper lip, inferior lip protrusion, drooling or difficulties with eating, speaking, or smiling. No conclusions could be drawn regarding the relation between BoNTA dose and duration of effects. However, starting with low dose and touch-up later if necessary has been recommended to minimise the risk of AEs. Duruel et al.55 SR was accompanied by a case report where 5 U BoNTA (Botox) was injected in each 'Yonsei point' located 1 cm lateral to the ala and 3 cm above lip line, to correct mixed EGD type. Great improvement lasting 20 weeks and without AEs was reported. The same author presented a level IV clinical study (n = 3) using the same injection point for several EGD types (anterior, mixed, or asymmetric) and reported 100% improvement in all cases with great level of satisfaction. The time to repeat treatment was estimated to be 24 weeks.66 The same technique with a lower dose of 3 U (Botox) was used in another level IV clinical study (n = 10) for persistent EGD (6-8 mm during smiling) due to upper lip muscle hyperfunction following orthodontic treatment.64 This study reported significant EGD improvement with BoNTA adjunctive treatment compared to baseline at a 15-day follow-up. Aly et al.65 level IV clinical trial presented seven cases of skeleton type II and prognathic maxilla with persistent EGD (5 mm when smiling) due to upper lip hypermobility and moderate vertical maxillary excess following lip repositioning flat surgery. This study evaluated EGD improvement with 2.5 U BoNTA (Botox) injections into two sites, the 'Yonsei point' and an additional point targeting the levator labii superioris muscles (LLS), two weeks post-surgery. BoNTA effects onset reported at day 15, maximum effect seen at week two post-injection, with 2 mm gingival display stable. The results were still satisfactory after one year, with gingival exposure of 3 mm reported.
Platysma - jawline sculpting
Awaida et al.70,71 (2018) crossover clinical trials (n = 30) demonstrated that microbotox dermal injection into anterior fibres of upper platysma to redefine mandibular border and the Nefertiti technique targeting upper platysma posterior fibres to address platysma bands are both safe and effective. The authors reported significant improvement of neck volume, jowls, and platysma bands at rest with microbotox technique, with approximately 150 injections, and one or two syringes used with 70 U of AboBoNTA (Dysport). A complementary approach with the Nefertiti lift performed after two weeks, if residual platysma bands present, was recommended for full lower face and neck rejuvenation. Participants and investigators demonstrated high satisfaction with both techniques. However, most participants preferred the microbotox technique for skin tightening effect and improvement in lower face contour, despite this technique being perceived as more painful. Improvement in skin texture was also noted with microbotox, but this trial was not investigating this outcome.70,71 Zhou et al.69 2019 retrospective study (n = 192) corroborated the safety and efficacy of the microbotox technique with 20 U to 30 U for mid/lower facial sagging, particularly in younger patients. A significant improvement was reported in overall mid/lower face aesthetics from both investigators and participants. Younger participants reported satisfaction, perception of younger and tightened face was significantly higher compared to investigators or older participants scores. For excessive facial fat descent seen in older patients, a combined approach, especially with surgical interventions, was suggested to improve treatment efficacy.69
Almeida et al.68 retrospective open label study (n = 161) described a novel technique for superficial BoNTA injections into upper platysma anterior portion and reported high satisfaction rate, as well as minimal AE risk for toxin spread. Not only an anterior neck lifting effect with enhancement and slimming contour was achieved, but also an improvement in horizontal rhytids at and below the chin and mandibular line, as well as in deep vertical lines lateral to oral commissures was noted. The mean dose injections per side was 16 U-20 U and seven injections distributed in two horizontal rows 2 cm apart, and one deep injection at the chin.68 The unexpected finding of lower face dynamic rhytids amelioration was also reported by Awayda et al.70,71 with both Nefertiti-lift and microbotox techniques. However, in those trials this was not statistically significant. In 2020, a retrospective cohort (n = 189) collected information regarding BoNTA units used per facial area, and related AEs for full face and neck rejuvenation treatments.67 This study observed that BoNTA mean doses were slightly higher in male participants, and injections into the platysma to improve mandibular contour was one of the most injected facial areas. The author reported that full face and neck BoNTA injections was safe, improved jawline contour and definition with facial slimming effect, marionette lines and labial commissures. While in this study the microbotox technique was only used to target the platysma muscle, the author suggested that full-face BoNTA injections with this technique was safe and with promising excellent results; however, the efficacy was not objectively assessed.67
The dosage and concentration of BoNTA injections into platysma for jawline contouring varied according to ethnic population, muscle strength, injection site, technique, or BoNTA formulation. Wu et al.73 recommended OnaBoNTA microdroplets total dose of 56 U for Asian population and suggested diluting the solution with LA to decrease the discomfort. Sundaram et al.49 recommended a maximum dose of 60 U with BoNTA and HA combined approach for western population. Most studies agreed that conservative initial doses and a two-week follow-up for touch-up if necessary is the safest option. Only one case of dysphasia and neck weakness resolved in two weeks was reported with the Nefertiti-lift.70,71 Zhou et al.69 also referred a transient 'fat-apple phenomenon' when malar fat pad is elevated to a higher position, common when dealing with excessive facial soft tissue, or disconnected jaw due to insufficient BoNTA. In the Almeida et al.68 study, there was one participant that reported a heaviness sensation and two cases of mild unilateral reduction in lower lip retraction, which was not noticeable by the affected participants. Self-limited acne and bruising at the injection site were the most common and expectable AEs.69
Discussion
This evidence-based review was performed to provide a summary of the available evidence regarding the safety and effectiveness BoNTA formulations for cosmetic interventions of interest to dental professionals and allied specialities. It was reassuring that all studies confirmed BoNTA safety profile and only a few AEs were reported for each indication, and these were mostly mild and transient. BoNTA administration into the masseter muscles reportedly encounters the risk of irreversible VII nerve injury,74 but this has not been reported. From the studies produced by the search regarding BoNTA effectiveness, only one level I study could be found for masseter hypertrophy. However, this Cochrane systematic review did not find any high-quality studies eligible in 2013. Therefore, the highest LOE was of level II.
Level II
Overall, ten level II studies could be found: four RCTs for masseter hypertrophy,20,25,26,27 one SR and five RCTs for perioral lines.14,15,42,43,44,45 Of these, one RCT was extracted from the included SR,14,15 and three RCTs also evaluated BoNTA safety and efficacy for drooping of mouth corners with/without marionette lines and/or oral commissures folds.14,44,45 For masseter hypertrophy, from the four RCTs included, only one had a larger sample size with longer follow-up. The risk of bias was estimated low only for one study, the remaining three had an unclear risk. In contrast, for perioral enhancement, the majority had an overall risk estimated low.
Masseter hypertrophy
An oval facial shape, with a soft narrowing lower-third contour and wider appearance in the mid-third of the face, is becoming increasingly desirable worldwide.19 Facial appearance is influenced by the size and thickness of the jawbone, subcutaneous fat tissue, and the large masseter muscle.21 First described by Legg in 1880, benign masseteric hypertrophy is an unusual condition characterised by a unilateral or bilateral abnormal enlargement approximately at the angle of the mandible.22 The aetiology is uncertain, and while it is more prevalent in some ethnic groups (such as Asian), and between teens and 40 years old, it is not gender-specific.21,22 It is noteworthy that most level II studies pertaining to BoNTA safety and efficacy for masseter hypertrophy are from investigations on Asian women, which is compatible with gender and population in more demand of a slimmer facial profile, albeit several consensus recommendations exist for the western population.12,48,49,50
The masseteric hypertrophy soft prominence is commonly related to negative perception of facial attractiveness particularly to women. It may be the cause of a square jaw and facial asymmetry in the unilateral cases. Occasionally, it may be associated with facial pain and trismus, intermittent or not.21 Although there is controversy, several diagnostic methods have been recommended to assist clinical findings. In conjunction, computer tomographic (CT) scan and magnetic resonance imaging (MRI) are regarded as the gold standard diagnostic tool.22 However, only two level II studies have reported the use of CT scans to assist with diagnose and outcome assessments. Ultrasonographic imaging to evaluate masseter muscle volume and thickness was the most common assessment tool used.
With varying level of success, simple pharmacotherapy with muscle relaxants, anxiolytics, and antidepressants, or the provision of occlusion adjustments, with/without the use of splints, have been reported for the treatment of symptomatic cases. More invasive surgical procedures include muscle reduction, removal of the jaw angle, buccal fat pad resection, and masseteric nerve neurectomy. Radiofrequency volumetric reduction and BoNTA injection into the masseter muscle are also considered as treatment possibilities.22
The key parameters assessed for effectiveness of BoNTA were: masseter muscle thickness and volume, measurement of change in facial contour and patient satisfaction with the degree of change. These were the outcomes most referred in level II or lower LOE studies. Level II studies consistently reported that BoNTA injections into hypertrophic masseter muscles provided significant reduction in muscle thickness and volume, with significant improvement in facial contour with high patient satisfaction compared to baseline.
Nevertheless, the inclusion criteria varied among level II studies. Alongside the evaluation of muscle or soft tissues effects some RCTs measured the changes in subcutaneous thickness or hard tissues, while other RCTs either assessed if the adjustment of masticatory movements following BoNTA injections would prolong duration of masseter muscle reduction or the effects of a second injection. The key results supported by level II studies were: prolonged aesthetic facial contour demonstrated when participants were instructed to strengthen their masticatory movements during the denervated atrophic stage of the masseter muscle, at the expense of a higher risk of paradoxical bulging of other masticatory muscles, particularly of temporalis muscle; greater muscle reduction and sustained aesthetic facial contour results reported with a second BoNTA injection. Four months was considered as the best time for the second injection, in order to maintain the maximum effects achieved at three months. This procedure did not affect subcutaneous thickness and required an injection needle longer than 8 mm and 29 G half-inch long needles; changes in the mandibular angle bone volume were demonstrated, although without differences in intergonial angle width of the mandibular angle area.
Recently, animal studies showed severe consequences in the masticatory muscle fibre constitution and proportion following BoNTA injections into the masseter muscle, such as: loss of skeletal volume, density, and fat in replacement of contractile tissue.19,75,76,77 Moreover, it has been observed in humans that repeated high doses of BoNTA injections resulted in the 'osteoporotic' effect at the condyle and mandibular ramus.26,78,79 Therefore, the findings of one level II study regarding the effects on bone tissues following BoNTA treatment for bilateral masseter hypertrophy, although it has reported fewer changes on hard tissues in comparison to animal studies, may raise some concerns. It is noteworthy that this study presented some limitations, with an unclear risk of bias score. The lack of placebo control and small sample size may have influenced the outcomes. Furthermore, the short six-month follow-up prevented being able to evaluate if a sustained reduction of bone volume or gradually recovery would be the most likely long-term effect. Thus, more well-designed RCTs are required for further clarification.
Regarding BoNTA safety, none of the level II studies reported systemic complications or serious AEs. Only one RCT observed mild and temporary AEs which involved bruising, swelling, headache, pain at injection site, muscle fatigue, bite weakness, and hollow cheek. This was in line with level IV and V studies that attempted to categorise the AEsaccording to the aetiology and evaluated the AE prevalence on BoNTA injections for masseter hypertrophy, which proven to be low. Most complications occurred within 2-4 weeks post-injection and resolved in 12 weeks. A sound anatomy knowledge and 3-4 injections within the safety zone, at 1 cm from all margins, were deemed more essential to mitigate the risk of AE than BoNTA dose.
Another important finding was that the evidence behind the use of BoNTA for unilateral masseteric hypertrophy is very limited, and primarily based on single case-reports, mostly without accurate assessment tools. Only one small cohort (n = 10) could be found showing that unilateral injection on the affected masseter muscle was safe and significantly effective. This level III study refuted previous concerns of a potential compensatory hypertrophy on the non-injected side, demonstrated only a minimal decrease on muscle thickness and volume on the untreated area. However, this is still to be validated with level II studies.
It is noteworthy that level III/IV studies also reported that lower dosages with a repetitive pattern can increase short- and long-term treatment safety and effectiveness, with higher patient satisfaction, regardless of age and gender, in comparison to a single dosage. Although the optimal dose remains controversial, and multiple factors are likely to determine the ideal dosage (individual differences, injection-technique, type of BoNTA), 20 U (OnaBoNTA) was considered the minimal effective dosage for both genders.
Perioral lines
The perioral region is characterised by great dynamism and complex interplay of several muscles. The perioral rhytids, also known as 'smoker lines', are the vertical lines radiating from the lip border to the surrounding area. These are mostly associated to the age-related multilevel changes, such as lip atrophy, perioral soft tissues volume loss with underlying bone resorption, and the OO muscle repetitive activity.13,14 The dynamic perioral wrinkles are more prevalent in women that, comparatively to men, have less blood vessels, sweat and sebaceous glands in the labial dermis, and subsequently more vertical lip lines, which in the absence of a beard also becomes noticeable.46 Thus, in general, the male facial lower third presents fewer signs of ageing, despite showing significant thicker and larger facial muscles with greater functional activity that encourages deeper rhytids.80 It is noteworthy that only one level II RCT evaluating BoNTA safety and efficacy for perioral rhytids investigated eight male participants, from a sample enrolling 90 participants, which is compatible with the gender in more demand of perioral enhancement.
The most successful treatment for this facial region subject to significant alterations during ageing is yet to be determined.46 Traditionally, surgical, ablative, or resurfacing interventions have been performed to restore the balance and harmony of the mid and lower face.42 Nowadays, dermal fillers have been widely used to restore volume and static lines around the mouth, including nasolabial folds, marionette lines and lip lines.48 BoNTA has also been introduced as a safe, well-tolerable and effective treatment option, or adjuvant to other therapies to synergistically improve the appearance in this area, tackling a broader spectrum of concerns, with longer-lasting results.13 Moreover, BoNTA ability to modulate mimic muscles activity has been linked to a preventative effect of wrinkles formation and even enhancement of skin quality with cumulative treatments.67
The key parameters assessed for effectiveness of BoNTA were: perioral strain and wrinkles severity, lip augmentation, patient reported results and satisfaction. These were the outcomes most referred in level II or lower LOE studies. Level II studies reported that BoNTA injections into OO muscle provided significant reduction in perioral strain, with significant improvement in perioral rhytids severity, and high patient satisfaction compared to baseline.
All level II and lower LOE studies reported BoNTA injections as a safe treatment for perioral lines. However, the effectiveness of perioral lines also relies on mouth functionality preservation.42,47 The recognition of lower toxin dosages importance to successfully treat perioral lines has been well stablished in all studies with high LOE. Cohen et al.43 and Hexsel et al.45 RCTs evaluated different BoNTA doses and confirmed that higher doses did not influence the level of satisfaction or duration of effectiveness, while the risk of complications was dose-dependent for perioral region.
The standard regimen in modern practice has been the combination of hyaluronic acid dermal fillers (HA) to BoNTA, which level II RCTs have proven to be safe, while significantly improving the duration of effects, the need for additional injections or higher doses, with greater patient satisfaction.42,44 For outcome assessment, all level II studies used subjective measurement tools based on facial photography, vertical lip length values, multiple investigator or participant scales and questionnaires. Chang et al.46 recent level IV study reported that the dual modality demonstrated to be highly effective. The perioral muscular strain and patient satisfaction were objectively measured with validated 3D digital imaging and FACE-Q questionnaire.
Other treatment modalities have been used in conjunction with BoNTA for perioral enhancement, including chemoabrasion, cosmeceuticals, broadband light, and fractioned lasers.13,42 The use of conservative BoNTA doses, in conjunction with other therapies, is thought to provide long-lasting and greater effects while reducing AE risks.13 However, the present evidence-based review found a highest of level II evidence to support this synergistic effect with concomitant use of BoNTA mostly with hyaluronic acid(HA),14,44 and one RCT with chemodermabrasion for perioral enhancement.42
The presence of individual asymmetries, anatomic variations, patients' needs and expectations, reflects the different choices of BoNTA dosages, injection-sites, or procedures sequence on a combined approach. Several consensus recommendations in different countries have been the only source for specific guidance on dual or multimodal approaches to enhance the lower face.12,13,48,49,50
Drooping of mouth corners - marionette lines
The presence of perioral rhytids, drooping of the lip corners with/without deep oral commissures folds and/or marionette lines, giving the impression of an old and sad appearance, are a common aesthetic concern.14 These are consequence of facial expression, genetics, ageing, photoageing, and smoking - being accentuated by the normal repetitive contraction or hypertrophic perioral musculature.81 Usually, BoNTA injection techniques target the DAO to address melomental folds or uplift mouth corners, the OO muscle to enhance perioral rhytids, and the mentalis muscle to correct chin projection and soft tissues appearance. Recently, some authors have also proposed treatment of the platysma to reduce lower face dynamic rhytids on selected patients.68
The bilateral downturned mouth corners, or reverse smile line, conveys negative feelings, while the unilateral cases can produce asymmetries, which compromises the ideal of a youth and attractive appearance.81 Several congenital and acquired factors have been involved in its aetiology, with the repetitive movements of the facial muscles associated, or not, to malocclusions or micrognathia, the soft tissues and bone changes overtime thought to exacerbate this condition.52 In the young, drooping of oral commissures is primary caused by the DAO muscle hyperactivity.53 In older individuals, the acquired downturned mouth corners are usually accompanied by deep oral commissures folds and/or marionette lines (melomental folds); a consequence of facial ageing involving reduced skin elasticity, loss of subcutaneous fat, soft tissues ptosis with bone resorption and remodelling, and to some extent also due to the overaction of the DAO, platysma, and mentalis muscles.52 The challenge of treating complex musculature and age-related changes of the highly dynamic perioral area, which often involves concomitant improvement of perioral lines, drooping of mouth corners, deep oral commissures, and marionette lines, may explain why the search for BoNTA use on these two cosmetic indications had an overlap of seven studies as well as the findings.12,14,44,45,48,49,50
The marionette lines are the vertical lines seen at the oral commissures extending downward towards the chin. The position of these folds relates to the middle of the DAO muscle upper part, which has a triangular shape with the base on the mandibular origin.52 In one study conducted by Choi et al.82 the cheilion and pupil were appointed as reliable facial landmarks of the DAO and depressor labii inferioris(DLI) based on a 3D scanning system with dissections observations; a novel approach opposing to the common practice of identifying the modiolus, upon the patient frowning, to target these muscles during BoNTA injection.
For an uplift of the mouth corners, level II RCTs reported that BoNTA injections have successfully downregulated the activity of the muscles responsible for pulling down the mouth corners, particularly the DAO.14,44,45 The key parameters assessed for effectiveness of BoNTA were: degree of drooping of the mouth, oral commissures and marionette lines severity, overall clinical improvement of sad appearance, patient tolerability or satisfaction, and preservation times. These were the outcomes most referred in level II or lower LOE studies.
All level II and lower LOE studies reported that BoNTA injections was a safe treatment for downturned mouth corners, oral commissure folds and marionette lines. Similar to what was reported for perioral rhytids enhancement, all studies with high LOE recognised the importance of BoNTA conservative doses in the perioral area to minimise the AE risk.14,44,45 Thus, there is a consensus that injections into the DAO should not exceed 4-8 U.12,48,49,50 BoNTA and HA administered in conjunction again take the central role in the literature. This dual approach has demonstrated to be well-tolerable, safest, more reliable, and effective to correct a sadness appearance caused by the drooping of mouth corners, deep oral commissures, and marionette lines.14,44 Moreover, level II studies revealed higher patient satisfaction and supported those repetitive combined treatments yielded cumulative improvements.14,44
The level IV/V studies found targeting exclusively the conditions caused by an hyperfunctional DAO muscle corroborated the findings of level II RCTs.51,52,53 There was only one level V case-series evaluating the safety and clinical efficacy of BoNTA treatment for congenital unilateral cases. Qian et al.51 reported satisfactory results that were preserved for 6-9 months. However, to ameliorate deep oral commissures and/or melomental folds associated to perioral ageing, when a nonsurgical approach is preferred, the author confirmed that a multimodal therapy was more effective.
Level III
Excessive gingival display
The diagnosis of EGD, also termed 'gummy smile', occurs when there is more than the ideal (1-2 mm) gingival exposure between the smile line and the gingival margin of the maxillary incisors, while spontaneously smiling, considering the age, gender, and periodontal health variables54. The EGD exceeding 3 mm is often considered unattractive and more preponderant in women, who tend to have a higher smile line comparatively to men.83 The smile line, usually with a convex appearance and represented by the lower margin of upper lip, significantly changes in older individuals, in whom a lower lip line and more mandible teeth display prevails.56 Published data described differing aetiologies behind the excessive gingival exposure (skeletal/dentoalveolar/soft tissue) and has highlighted the importance of stablishing the causative factor/multifactor, to determine which treatment plan is best indicated.54
Several therapeutic modalities have been informed to the correction of EGD. These are frequently multidisciplinary with either orthodontic temporary appliance, orthognathic surgery, crown lengthening techniques, lip repositioning, and muscle resection.54 When the cause is attributed to the perioral musculature hyperactivity, the use of BoNTA has been recommended, either as monotherapy or pre-/post-surgical adjunctive procedure.64 Available studies suggested that many individuals reject surgical treatment plans for the correction of lip musculature. These treatment options can be costly, complex, cause great discomfort and a risk of morbidity.64 In addition, orthodontic treatment primarily targets the hard tissue, thus when used independently is not amenable to address the problem completely.64 First documented by Polo in 2005, BoNTA use for EGD has revealed promising results, by partially denervating upper lip musculature and overcoming these obstacles, with high patient satisfaction level.54,84
To identify the muscle responsible for gummy smile, a case-series by Mazzuco and Hexsel classified EGD into four types (anterior, posterior, mixed and asymmetric), based on the area of excessive gingival display.57 Although other BoNTA injection-sites have been described, the OO site is thought to have some advantages due to its rapid effect with a lower effective dose.63 However, being this muscle involved in several basic functions (eating, drinking, speaking), it was associated with higher discomfort in the advent of injection complications.63,84 Therefore, the LLSAN has been considered as the critical muscle for BoNTA treatment success,54,84 while the 'Yonsei point', which lies at the centre of LLS, LLSAN, and zygomaticus minor muscle vectors, was the preferable site for BoNTA intramuscular injection among the available studies.55,64,66,81 Common practice in the management of EGD has been a single injection into this point, identified by Hwang et al.64,85 However, the number of injections on each side ranged from 1-3 points.54 Generally, a conversion rate of 2.5:1 between AboBoNTA and OnaBoNTA with comparable results has been accepted, with the maximum dose of 2-5 U each side being sufficient.55,66 However, the dosage, the number or site of BoNTA injection may be influenced by gender, severity of EGD, type of smile, and designated target muscle volume and size.54,84
Available level III/IV studies showed that BoNTA can be effective on EGD management, yet not without some inconsistencies.54 The key parameters assessed for effectiveness of BoNTA were: EGD severity or percentage of improvement, level of satisfaction, smile index improvement and duration of effects. Level III/IV studies reported high patient satisfaction and significant increase in smile index. However, for effectiveness longevity and EGD percentage of improvement the results greatly varied, without an obvious correlation to the number of injections or BoNTA formulations.55 Although Nasr et al.54 observed a potential correlation with the type of EGD and injection site.
While the safety of BoNTA therapy in the management of EGD was confirmed in all available studies, with no long-term or severe complications associated, short-term AEs with incidence within the first week's post-treatment were reported. Most could be corrected with additional BoNTA, others caused dysfunction that lasted several months.54 Higher doses and poor injection techniques was associated to increased AEs.54,56,84 Therefore, several authors have recommended an initial low dose of BoNTA and, if necessary, a top-up performed by experienced clinicians at a later stage.55,66,81
BoNTA for EGD has been used worldwide, with numerous variations in injection sites and doses used to target individualised characteristics contributing to the controversies surrounding this approach.84 In the absence of standardised protocols, the procedural differences and the clinician's own experience may reflect the heterogeneity among clinical results, particularly regarding the reported percentage of EGD improvement and treatment longevity values. To what extent one technique may favour more positive or negative outcomes, it is still unknown. Nevertheless, from the available level III/IV studies, BoNTA is safe and may significantly improve EGD during smiling caused by perioral muscle hyperactivity, with high patient satisfaction.
Level IV
Platysma - jawline sculpting
Initial reports of BoNTA use in the neck targeted the undesirable platysma bands. Since then, the understanding of BoNTA administration into the platysma muscle benefits has evolved to include the jawline contour improvement and, more recently, the lower facial wrinkles amelioration.68,86,87 Other procedures to reshape the lower face have been published, including dermal fillers, energy-based devices, lipofilling and/or a surgical approach.68 Currently, there is no consensus regarding the optimal management.68 It has been postulated that the ideal candidates for BoNTA injections into the platysma for lower face contour are those presenting sufficient skin elasticity, lower face hyperdynamic wrinkles or jawline distortion while exposing the lower teeth during smiling or upon platysma contraction.87
The key parameters assessed for effectiveness of BoNTA were: lower face contour improvement, overall facial sagging and neck appearance amelioration, level of satisfaction of both participants and investigators, and other facial aesthetic improvements (for example, platysma bands or wrinkles improvement).
Awaida et al.70,71 compared both Nefertiti lift and microbotox techniques and concluded that both techniques were safe and with high patient acceptance. The former was more effective to enhance platysma bands; the latter revealed superior improving the jowls, neck soft tissues ptosis, and skin quality. Without an objective evaluation tool and limited to the Asian population, Zhou et al.69 corroborated the safety and efficacy of microbotox technique for mid/lower facial sagging, particularly in younger patients. However, for excessive facial fat descent, a surgical approach was suggested to improve treatment efficacy.69 Novel injection techniques are continuously evolving with reportedly low incidence of AE and high satisfaction rate.69,70,71 One example is the new approach introduced by Almeida et al.,68 for superficial BoNTA injections into upper platysma anterior portion. The author reported clinical improvement for all key efficacy outcomes, as well as minimal AE risk for toxin spread. While D´Emilio et al.67 suggested that full-face BoNTA dermal injections were safe and with promising excellent results; however, the efficacy was not objectively assessed.67 These dermal injections of BoNTA can be painful and Wu et al.73 recommended diluting the solution with LA to decrease the discomfort.
All available studies highlighted the severe AEs that can occur when treating the thin platysma muscle that lies above the anterior muscle of the neck, including voice alterations, dysphagia, dry mouth, neck and lower face muscles weakness with subsequent lip asymmetries, altered smile or other expressions, when injecting into the upper neck. Therefore, high doses and deep BoNTA injections into the upper platysma region are not recommended. Nevertheless, all studies on jawline contouring targeting the platysma muscle, having LOE not higher than level IV, did demonstrate positive outcomes, with longevity varying from 3-6 months, with no serious AE and high satisfaction rates.49,67,68,69,70,71,72 BoNTA injections addressing the platysma muscle for enhancement of lower facial contour and particularly hyperdynamic lines is a relatively new concept, this may explain the limited evidence available. Furthermore, the jawline sculpting effect was a secondary finding while addressing platysma bands to rejuvenate the neck or sweat and sebaceous glands in the skin, with the Nefertiti-lift and microbotox technique, respectively.41,88 This discovery was the tipping point for the upsurge of many procedural variations, which have been attempting to improve safety and efficacy.89,90
Limitations
The present evidence-based review does have its weaknesses. Firstly, it did not consider unpublished or grey literature in the way that systematic reviews do, and the search was conducted using limited databases. Hence, it is possible that some evidence on the topic may have been missed. Secondly, the evolving emphasis on facial aesthetics advocates a patient-tailored panfacial approach, recognising the impact that treating one area could have on other areas. Accordingly, the facial expression muscles have compound physiologic and anatomic interactions instead of acting in isolation.12 Nevertheless, the study was streamlined to concentrate on the mid- and lower face, where the mouth plays a central role, therefore excluding BoNTA treatments of glabellar and forehead lines, or 'crow's feet', fundamental aspects of the upper face and overall facial appearance. Finally, by excluding the studies already evaluated in the included SRs or by accepting the results of these higher LOE reviews regarding the own judgement of quality and risk of bias assessment for the included primary studies, there is the risk of over-generalisation of results.
Future directions
From the encouraging results of the present evidence-based review, the authors concluded that BoNTA is safe and may be a valuable addition to the armamentarium of dentists and allied specialities. Future studies may help to provide robust evidence-based clinical recommendations for cosmetic BoNTA use, independently or on a combined approach. This would potentially contribute to reduce the risk of AEs that seems to be strongly dependent of injection techniques. High-quality studies to investigate BoNTA benefits for unilateral masseter hypertrophy or congenital unilateral drooping of mouth corners are needed. The changes on bone tissues following BoNTA injections into masseter muscles and the long-term implications remain to be elucidated. Overall, well-designed randomised controlled clinical trials with longer follow-ups enriched with a qualitative analysis are necessary, particularly to fully understand the long-term safety and cost-effectiveness associated with repeated injections of BoNTA formulations against other treatment modalities currently being used. Standardised and validated tools for patient-reported outcomes (for example, FACE-Q questionnaires) and objective outcome measures (for example, 3D digital imaging) should be incorporated to evaluate the results.
Clinical bottom line
Based on current level II studies, BoNTA was safe and effective to improve facial contour, and reduce volume and thickness of bilateral hypertrophic masseter. Conservative doses on a combined approach of BoNTA and hyaluronic acid were recommended as a safe and effective treatment for perioral enhancement supported by high evidence. There was limited evidence, not higher than level III, to support BoNTA effectiveness for gummy smile associated with perioral musculature hyperactivity, while jawline sculpting targeting the platysma muscle had lower level IV evidence up to date.
References
Carruthers A, Kane M A C, Flynn T C et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine-a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg 2013; 39: 493-509.
Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol 2018; 11: 273-287.
Lee K C, Pascal A B, Halepas S, Koch A. What Are the Most Commonly Reported Complications With Cosmetic Botulinum Toxin Type A Treatments? J Oral Maxillofac Surg 2020; DOI: 10.1016/j.joms.2020.02.016.
U.S. Food & Drug Administration. FDA Gives Update on Botulinum Toxin Safety Warnings; Established Names of Drugs Changed. 2009. Available at https://wayback.archive-it.org/7993/20170112032330/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm175013.htm (accessed February 2020).
Walker T J, Dayan S H. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol 2014; 7: 31-39.
The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics for 2019. 2019. Available at https://www.surgery.org/media/statistics (accessed May 2020).
EU Clinical Trials Register. Clinical trials for Botulinum Toxin Type A. Available at https://www.clinicaltrialsregister.eu/ctr-search/search?query=Botulinum+Toxin+Type+A (accessed February 2020).
Miller J, Clarkson E. Botulinum Toxin Type A: Review and Its Role in the Dental Office. Dent Clin North Am 2016; 60: 509-521.
Walker T W M, Gately F, Stagnell S, Kerai A, Mills C, Thomas S. Can UK undergraduate dental programmes provide training in non-surgical facial aesthetics? Br Dent J 2017; 222: 949-953.
Khan O A, Dunning J, Parvaiz A C, Agha R, Rosin D, Mackway-Jones K. Towards evidence-based medicine in surgical practice: best BETs. Int J Surg 2011; 9: 585-588.
Best BETs. Best Evidence Topics and Critical Appraisal. Available at https://bestbets.org/background/bets-and-cats.php (accessed April 2020).
Sundaram H, Signorini M, Liew S et al. Global Aesthetics Consensus: Botulinum Toxin Type A-Evidence-Based Review, Emerging Concepts, and Consensus Recommendations for Aesthetic Use, Including Updates on Complications. Plast Reconstr Surg 2016; DOI: 10.1097/01.prs.0000475758.63709.23.
Carruthers J, Carruthers A. A Multimodal Approach to Rejuvenation of the Lower Face. Dermatol Surg 2016; 42 Suppl 2: S89-S93.
Carruthers A, Carruthers J, Monheit G D, Davis P G, Tardie G. Multicentre, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg 2010; 36 Suppl 4: 2121-2134.
Cavallini M, Cirillo P, Fundarò S P et al. Safety of botulinum toxin A in aesthetic treatments: a systematic review of clinical studies. Dermatol Surg 2014; 40: 525-536.
OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Available at https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf (accessed April 2020).
Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions. Available at https://training.cochrane.org/handbook/current (accessed August 2021).
Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1.
Cheng J, Hsu S H, McGee J S. Botulinum Toxin Injections for Masseter Reduction in East Asians. Dermatol Surg 2019; 45: 566-572.
Park G, Choi Y-C, Bae J-H, Kim S-T. Does Botulinum Toxin Injection into Masseter Muscles Affect Subcutaneous Thickness? Aesthet Surg J 2018; 38: 192-198.
Shome D, Khare S, Kapoor R. Efficacy of Botulinum Toxin in Treating Asian Indian Patients with Masseter Hypertrophy: A 4-Year Follow-Up Study. Plast Reconstr Surg 2019; DOI: 10.1097/PRS.0000000000005944.
Fedorowicz Z, van Zuuren E J, Schoones J. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst Rev 2013; DOI: 10.1002/14651858.CD007510.pub3.
Yeh Y-T, Peng J-H, Peng H-L P. Literature review of the adverse events associated with botulinum toxin injection for the masseter muscle hypertrophy. J Cosmet Dermatol 2018; 17: 675-687.
Peng H-L P, Peng J-H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J Cosmet Dermatol 2018; 17: 33-38.
Lee H-H, Kim S T, Lee K-J, Baik H-S. Effect of a second injection of botulinum toxin on lower facial contouring, as evaluated using 3-dimensional laser scanning. Dermatol Surg 2015; 41: 439-444.
Lee H-J, Kim S-J, Lee K-J, Yu H-S, Baik H-S. Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: A longitudinal study. Korean J Orthod 2017; 47: 222-228.
Wei J, Xu H, Dong J, Li Q, Dai C. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type A injection. Dermatol Surg 2015; 41 Suppl 1: S101-S109.
Xie Y, Zhou J, Li H, Cheng C, Herrler T, Li Q. Classification of masseter hypertrophy for tailored botulinum toxin type A treatment. Plast Reconstr Surg 2014; DOI: 10.1097/PRS.0000000000000371.
Cha Y R, Kim Y G, Kim J H, Kim S T. Effect of unilateral injection of botulinum toxin on lower facial asymmetry as evaluated using three-dimensional laser scanning. Dermatol Surg 2013; 39: 900-906.
Kim N-H, Park R-H, Park J-B. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast Reconstr Surg 2010; 125: 1693-1705.
Kim N-H, Chung J-H, Park R-H, Park J-B. The use of botulinum toxin type A in aesthetic mandibular contouring. Plast Reconstr Surg 2005; 115: 919-930.
Lee J H, Park J H, Lee S K et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseteric hypertrophy: side-by-side comparison with onabotulinum toxin A. J Dermatologue Treat 2014; 25: 326-330.
Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, Sathaworawong A, Lektrakul N, Manuskiatti W. The efficacy of two formulations of botulinum toxin type A for masseter reduction: a split-face comparison study. J Dermatologue Treat 2017; 28: 443-446.
Lee D H, Jin S-P, Cho S et al. RimabotulinumtoxinB versus OnabotulinumtoxinA in the treatment of masseter hypertrophy: a 24-week double-blind randomized split-face study. Dermatology (Basel) 2013; 226: 227-232.
Park M Y, Ahn K Y, Jung D S. Botulinum toxin type A treatment for contouring of the lower face. Dermatol Surg 2003; 29: 477-483; discussion 483.
Choe S W, Cho W I, Lee C K, Seo S J. Effects of botulinum toxin type A on contouring of the lower face. Dermatol Surg 2005; 31: 502-508.
Kim H J, Yum K-W, Lee S-S, Heo M-S, Seo K. Effects of botulinum toxin type A on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatol Surg 2003; 29: 484-489.
Lee S H, Wee S H, Kim H-J et al. Abobotulinum toxin A and onabotulinum toxin A for masseteric hypertrophy: a split-face study in 25 Korean patients. J Dermatologue Treat 2013; 24: 133-136.
Lee S J, Kang J M, Kim Y K, Park J, Kim D Y. Paradoxical bulging of muscle after injection of botulinum neurotoxin type A into hypertrophied masseter muscle. J Dermatol 2012; 39: 804-805.
To E W, Ahuja A T, Ho W S et al. A prospective study of the effect of botulinum toxin A on masseteric muscle hypertrophy with ultrasonographic and electromyographic measurement. Br J Plast Surg 2001; 54: 197-200.
Yu C-C, Chen P K-T, Chen Y-R. Botulinum toxin a for lower facial contouring: a prospective study. Aesthetic Plast Surg 2007; 31: 445-453.
Kadunc B V, Trindade D E, Almeida A R, Vanti A A, DI Chiacchio N. Botulinum toxin A adjunctive use in manual chemabrasion: controlled long-term study for treatment of upper perioral vertical wrinkles. Dermatol Surg 2007; 33: 1066-1072.
Cohen J L, Dayan S H, Cox S E, Yalamanchili R, Tardie G. OnabotulinumtoxinA dose-ranging study for hyperdynamic perioral lines. Dermatol Surg 2012; 38: 1497-1505.
Carruthers J, Carruthers A, Monheit G D, Davis P G. Multicentre, randomized, parallel-group study of onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation: satisfaction and patient-reported outcomes. Dermatol Surg 2010; 36 Suppl 4: 2135-2145.
Hexsel D, Brum C, Porto M D et al. Full-face injections of variable total doses of abobotulinum toxin type A: A randomized, phase IV clinical trial of safety and efficacy. J Drugs Dermatol 2013; 12: 1356-1362.
Chang C S, Chang B L, Lanni M, Wilson A J, Beer J, Percec I. Perioral Rejuvenation: A Prospective, Quantitative Dynamic Three-Dimensional Analysis of a Dual Modality Treatment. Aesthet Surg J 2018; 38: 1225-1236.
Semchyshyn N, Sengelmann R D. Botulinum toxin A treatment of perioral rhytides. Dermatol Surg 2003; 29: 490-495.
de Maio M, Wu W T L, Goodman G J, Monheit G, Alliance for the Future of Aesthetics Consensus Committee. Facial Assessment and Injection Guide for Botulinum Toxin and Injectable Hyaluronic Acid Fillers: Focus on the Lower Face. Plast Reconstr Surg 2017; DOI: 10.1097/PRS.0000000000003646.
Sundaram H, Liew S, Signorini M et al. Global Aesthetics Consensus: Hyaluronic Acid Fillers and Botulinum Toxin Type A-Recommendations for Combined Treatment and Optimizing Outcomes in Diverse Patient Populations. Plast Reconstr Surg 2016; 137: 1410-1423.
Bertossi D, Cavallini M, Cirillo P et al. Italian consensus report on the aesthetic use of onabotulinum toxin A. J Cosmet Dermatol 2018; 17: 719-730.
Qian W, Zhang Y-K, Lv W, Hou Y, Cao Q, Fan J-F. Application of Local Injection of Botulinum Toxin A in Cosmetic Patients with Congenital Drooping Mouth Corner. Aesthetic Plast Surg 2016; 40: 926-930.
Bae G Y, Na J-I, Park K-C, Cho S B. Nonsurgical correction of drooping mouth corners using monophasic hyaluronic acid and incobotulinumtoxin A. J Cosmet Dermatol 2020; 19: 338-345.
Jeong T-K. Mouth Corner Lift with Botulinum Toxin Type A, Hyaluronic Acid Filler. Plast Reconstr Surg 2020; DOI: 10.1097/PRS.0000000000006605.
Nasr M W, Jabbour S F, Sidaoui J A, Haber R N, Kechichian E G. Botulinum Toxin for the Treatment of Excessive Gingival Display: A Systematic Review. Aesthet Surg J 2016; 36: 82-88.
Duruel O, Ataman-Duruel E T, Tözüm T F, Berker E. Ideal Dose and Injection Site for Gummy Smile Treatment with Botulinum Toxin-A: A Systematic Review and Introduction of a Case Study. Int J Periodontics Restorative Dent 2019; DOI: 10.11607/prd.3580.
Chagas T F, de Almeida N V, Lisboa C O et al. Duration of effectiveness of Botulinum toxin type A in excessive gingival display: a systematic review and meta-analysis. Braz Oral Res 2018; DOI: 10.1590/1807-3107bor-2018.vol32.0030.
Mazzuco R, Hexsel D. Gummy smile and botulinum toxin: a new approach based on the gingival exposure area. J Am Acad Dermatol 2010; 63: 1042-1051.
Polo M. Botulinum toxin type A (Botox) for the neuromuscular correction of excessive gingival display on smiling (gummy smile). Am J Orthod Dentofacial Orthop 2008; 133: 195-203.
Suber J S, Dinh T P, Prince M D, Smith P D. OnabotulinumtoxinA for the treatment of a 'gummy smile'. Aesthet Surg J 2014; 34: 432-437.
Sucupira E, Abramovitz A. A simplified method for smile enhancement: botulinum toxin injection for gummy smile. Plast Reconstr Surg 2012; 130: 726-728.
Somaiah M S, Muddaiah S, Shetty B P, Vijayananda K, Bhat M, Shetty P S. Effectiveness of botulinum toxin A, in unraveling gummy smile: A prospective clinical study. APOS Trends Orthod 2013; 3: 54-58.
Al-Fouzan A F, Mokeem L S, Al-Saqat R T, Alfalah M A, Alharbi M A, Al-Samary A E. Botulinum Toxin for the Treatment of Gummv Smile. J Contemp Dent Pract 2017; 18: 474-478.
Cengiz A F, Goymen M, Akcali C. Efficacy of botulinum toxin for treating a gummy smile. Am J Orthod Dentofacial Orthop 2020; 158: 50-58.
Gupta N, Kohli S. Evaluation of a Neurotoxin as an Adjunctive Treatment Modality for the Management of Gummy Smile. Indian Dermatol Online J 2019; 10: 560-563.
Aly L A, Hammouda N I. Botox as an adjunct to lip repositioning for the management of excessive gingival display in the presence of hypermobility of upper lip and vertical maxillary excess. Dent Res J (Isfahan) 2016; 13: 478-483.
Duruel O, Ataman-Duruel E T, Berker E, Tözüm T F. Treatment of Various Types of Gummy Smile With Botulinum Toxin-A. J Craniofac Surg 2019; 30: 876-878.
D'Emilio R, Rosati G. Full-face treatment with onabotulinumtoxinA: Results from a single-centre study. J Cosmet Dermatol 2020; 19: 809-816.
de Almeida A R T, Romiti A, Carruthers J D A. The Facial Platysma and Its Underappreciated Role in Lower Face Dynamics and Contour. Dermatol Surg 2017; 43: 1042-1049.
Zhou R, Fei Y, Sun L, Guo J, Zhou X, Zhang X. BTX-A Rejuvenation: Regional Botulinum Toxin-A Injection of the Platysma in Patients with Facial Sagging. Aesthetic Plast Surg 2019; 43: 1044-1053.
Awaida C J, Jabbour S F, Rayess Y A, El Khoury J S, Kechichian E G, Nasr M W. Evaluation of the Microbotox Technique: An Algorithmic Approach for Lower Face and Neck Rejuvenation and a Crossover Clinical Trial. Plast Reconstr Surg 2018; 142: 640-649.
Jabbour S F, Kechichian E G, Awaida C J, Tomb R R, Nasr M W. Botulinum Toxin for Neck Rejuvenation: Assessing Efficacy and Redefining Patient Selection. Plast Reconstr Surg 2017; DOI: 10.1097/PRS.0000000000003429.
Wu W T L, Liew S, Chan H H et al. Consensus on Current Injectable Treatment Strategies in the Asian Face. Aesthetic Plast Surg 2016; 40: 202-214.
Wu W T L. Microbotox of the Lower Face and Neck: Evolution of a Personal Technique and Its Clinical Effects. Plast Reconstr Surg 2015; 136: 92S-100S.
White S, Ahmed B, Ondhia A. The effectiveness of Clostridium botulinum toxin A (Dysport, AbobotulinumtoxinA) in the management of temporomandibular dysfunction (TMD) and a small number of other maxillofacial conditions; an open cohort study. Oral Surg 2018; 11: 175-182.
Tsai C-Y, Shyr Y-M, Chiu W-C, Lee C-M. Bone changes in the mandible following botulinum neurotoxin injections. Eur J Orthod 2011; 33: 132-138.
Rafferty K L, Liu Z J, Ye W et al. Botulinum toxin in masticatory muscles: short-and long-term effects on muscle, bone, and craniofacial function in adult rabbits. Bone 2012; 50: 651-662.
Kün-Darbois J-D, Libouban H, Chappard D. Botulinum toxin in masticatory muscles of the adult rat induces bone loss at the condyle and alveolar regions of the mandible associated with a bone proliferation at a muscle enthesis. Bone 2015; 77: 75-82.
Raphael K G, Tadinada A, Bradshaw J M et al. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: a pilot study. J Oral Rehabil 2014; 41: 555-563.
Balanta-Melo J, Toro-Ibacache V, Kupczik K, Buvinic S. Mandibular Bone Loss after Masticatory Muscles Intervention with Botulinum Toxin: An Approach from Basic Research to Clinical Findings. Toxins (Basel) 2019; 11: 84.
Trévidic P, Sykes J, Criollo-Lamilla G. Anatomy of the Lower Face and Botulinum Toxin Injections. Plast Reconstr Surg 2015; 136: 84S-91S.
Delpachitra S N, Sklavos A W, Dastaran M. Clinical uses of botulinum toxin A in smile aesthetic modification. Br Dent J 2018; 225: 502-506.
Choi Y-J, We Y-J, Lee H-J et al. Three-Dimensional Evaluation of the Depressor Anguli Oris and Depressor Labii Inferioris for Botulinum Toxin Injections [published online ahead of print]. Aesthet Surg J 2020; DOI: 10.1093/asj/sjaa083.
Tjan A H, Miller G D, The J G. Some esthetic factors in a smile. J Prosthet Dent 1984; 51: 24-28.
Polo M. Commentary on: Botulinum Toxin for the Treatment of Excessive Gingival Display: A Systematic Review. Aesthet Surg J 2016; 36: 89-92.
Hwang W-S, Hur M-S, Hu K-S et al. Surface anatomy of the lip elevator muscles for the treatment of gummy smile using botulinum toxin. Angle Orthod 2009; 79: 70-77.
Heitmiller K, Ring C, Saedi N. Facial Contouring with Neuromodulators. Adv Cosmet Surg 2020; 3: 99-107.
Bertucci V. Commentary on The Facial Platysma and Its Underappreciated Role in Lower Face Dynamics and Contour. Dermatol Surg 2017; 43: 1050-1052.
Liew S, Dart A. Nonsurgical reshaping of the lower face. Aesthet Surg J 2008; 28: 251-257.
Wu W T L. Botox facial slimming/facial sculpting: the role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am 2010; 18: 133-140.
Levy P M. Neurotoxins: Current Concepts in Cosmetic Use on the Face and Neck-Jawline Contouring/Platysma Bands/Necklace Lines. Plast Reconstr Surg 2015; 136: 80S-83S.
Acknowledgements
The authors wish to acknowledge the help and assistance of the editors and reviewers of the Evidence-Based Dentistry journal for their support and review of the research that was undertaken. Thanks to Miss R. Al-Barazanchi for proofreading.
Author information
Authors and Affiliations
Contributions
INP is a dental practitioner working for the National Health Service primary care and currently a PhD resident at the University of Porto, Faculty of Dental Medicine. This study was based on INP master's degree dissertation in Aesthetic Medicine at the Queen Mary University of London. Both authors had full access to all the data in the work and the decision to submit for publication rested with the correspondent author. HH is a clinical senior lecturer at the Academic Plastic Surgery, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London.
Corresponding author
Ethics declarations
Neither author has any conflict of interests, nor financial or non-financial competing interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Pereira, I., Hassan, H. Botulinum toxin A in dentistry and orofacial surgery: an evidence-based review - part 2: cosmetic applications. Evid Based Dent (2022). https://doi.org/10.1038/s41432-022-0277-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41432-022-0277-4
- Springer Nature Limited