Abstract
Objective
To investigate the relationship of serum uric acid (Uacid) and derived parameters as predictors of insulin resistance (IR) and elevated liver transaminases in children and adolescents
Methods
Data of 1648 participants aged 10–18 years was analyzed using nationwide survey. Logistic regression analysis was performed with IR and elevated liver transaminases as dependent variables, and odds ratios (ORs) and 95% confidence intervals (CIs) for tertiles 2 and 3 of each parameter in comparison to tertile 1, which served as the reference. Receiver operating characteristic (ROC) curves were generated to assess predictability of the parameters for IR and elevated liver transaminases.
Results
Hyperuricemia, IR, and elevated liver transaminases were significantly associated with each other. All Uacid and derived markers showed continuous increase in ORs and 95% CIs for IR and elevated liver transaminases across the tertiles of several biochemical and metabolic variables of interest (all p < 0.001), and were also significantly predictive in ROC curve. Overall, Uacid combined with obesity indices showed higher ORs and area under the curve (AUC) compared to Uacid alone. Uacid-body mass index (BMI) standard deviation score presented the largest AUC for IR. For elevated liver transaminases, Uacid-BMI and Uacid-waist-to-height ratio showed the largest AUC.
Conclusions
Uacid combined with obesity indices are robust markers for prediction of IR and elevated liver transaminases in children and adolescents. Uacid and derived markers have potential as simple markers which do not require fasting for screening of IR and elevated liver transaminases in children and adolescents.
Similar content being viewed by others
Introduction
Steatotic liver disease (SLD) is a chronic liver condition characterized by accumulation of excessive fat in the liver, often accompanied by elevated hepatic enzymes [1, 2]. MASLD is a SLD accompanied by metabolic dysfunction without significant alcohol drinking, replacing the classic concept of non-alcoholic fatty liver disease (NAFLD) [2]. MASLD is recognized as a significant contributor to liver fibrosis, advanced liver disease, and is closely linked to various cardio-metabolic risk factors, such as obesity, dyslipidemia, and insulin resistance (IR) [3]. In the United States, the prevalence of SLD and MASLD were high at 38.9% and 32.5%, respectively [4]. To prevent progression of MASLD and related complications, early screening is important. Although alanine aminotransferase (ALT) is recommended for a screening method for pediatric NAFLD and various biomarkers has been suggested for MASLD in adults, investigations on screening strategy including biomarkers for pediatric MASLD is limited yet [1, 5,6,7].
Meanwhile, IR plays a key role in the development of metabolic diseases, including MASLD [3, 8]. IR leads to increase in serum level of insulin, glucose, and fatty acids, which in turn promotes accumulation of fatty acids and triglycerides in the liver [1]. The glucose clamp technique, a classical method for measuring IR, is highly invasive, involving intravenous catheters and continuous monitoring and impractical for large-scale studies [9]. Therefore, an alternative approach, the homeostasis model assessment of IR (HOMA-IR) index, has been introduced as a reliable method for quantifying IR [10, 11]. However, HOMA-IR relies on fasting blood samples, which can be clinically relevant for some individuals. Moreover, there is no standardized protocol for measuring insulin levels, and such measurements are not routinely conducted in children. Therefore, investigations on development of simple markers for IR are required.
Meanwhile, Uacid has emerged as a valuable biomarker for predicting and understanding metabolic conditions including MASLD, and does not require fasting [12, 13]. This connection stems from Uacid’s role in promoting IR, a central factor in the pathogenesis of hepatic steatosis, as well as its correlation with other metabolic risk factors, such as obesity and dyslipidemia [12]. A systematic review reported that OR of NAFLD was 1.92 times higher when comparing individuals with the highest serum Uacid levels to those with the lowest levels [13]. Moreover, a population-based study reported a recent increase in the serum Uacid level among Korean children and adolescents. In addition, obesity indices, such as BMI, waist circumference (WC), and waist-to-height ratio (WHtR), are closely related with IR and MASLD [1, 9]. Thus, some studies have reported that biomarkers for the prediction of IR or fatty liver could be more powerful when they were combined with obesity indices [8, 14, 15]. In addition, Uacid combined with other biomarkers, such as creatinine (Cr) or high-density lipoprotein (HDL), have been suggested as alternative markers for hepatic steatosis in adults [7, 16]. However, research exploring the association of Uacid with IR, MASLD, and abnormal liver enzymes considering obesity indices or other biomarkers among children and adolescents is currently limited.
Therefore, we aimed to investigate the predictive value of Uacid-derived markers, which combined Uacid and Cr and HDL, as well as obesity indices, including BMI, BMI SDS, WC, and WHtR, for IR and elevated liver transaminases in the youth by analyzing data from the Korea National Health and Nutrition Examination Survey (KNHANES). Our objectives were to: 1) examine the association between hyperuricemia and IR and elevated liver transaminases, 2) compare Uacid and Uacid-derived markers for prediction of IR and elevated liver transaminases, and 3) establish the optimal cutoff value of Uacid and derived marker for prediction of IR and elevated liver transaminases.
Methods
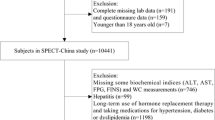
We retrospectively evaluated data from 1648 children and adolescents aged 10–18 years who participated in the KNHANES conducted between 2019 and 2021. Figure 1 illustrates the study design and workflow. KNHANES is a nationally representative survey conducted in Korea, employing a complex, stratified, multistage probability sampling method to select participants from the entire population. The survey is administered by the Korea Centers for Disease Control and Prevention and encompasses health surveys, medical examinations, and nutrition assessments. Sample weights were applied to address variations in selection probabilities and non-response rates, and these weighted data were subsequently adjusted to accurately reflect the demographics of the Korean population by sex and age groups [17].
Participants’ weights were determined with a scale (Giant 150 N, HANA, Seoul, South Korea) accurate to the nearest 0.1 kg, while their heights were measured using a stadiometer (range: 850–2060 mm; Seriter, Holtain Ltd., Crymych, UK) with precision to the nearest 0.1 cm. BMI was computed as the weight in kilograms divided by the square of the height in meters. The height, weight, and BMI were then expressed as standard deviation score (SDS) values, referencing the 2017 Korean National Growth Charts [18]. Children were categorized based on their BMI into four groups: underweight (BMI <5th percentile), normal weight (BMI 5th—85th percentile), overweight (85th—95th percentile), and obesity (BMI ≥95th percentile). The measurement of WC was taken at the midpoint between the costal margin and iliac crest during a normal exhalation using a tapeline by trained nurses. WHtR was calculated by dividing the waist circumference (cm) by the height (cm).
Blood samples were obtained from the antecubital vein after an overnight fast of 8 h. These samples were then processed and promptly stored in a refrigerator. The levels of aspartate aminotransferase and ALT in the serum were determined using commercially available kits (Pureauto S ALT, Daiichi Pure Chemicals, Tokyo, Japan) without employing the pyridoxal-5-phosphate method, relying instead on ultraviolet light measurement. Serum insulin levels were assessed using the Wizard 1470 gamma counter (Perkin-Elmer, Turku, Finland). Plasma concentrations of fasting glucose, Uacid, total cholesterol, HDL, and triglycerides were measured using the Hitachi Automatic Analyzer 7600/7600-210 (Hitachi, Tokyo, Japan). Serum Cr was measured using Cobas c702 (Roche Diagnostics, Mannheim, Germany).
Low-density lipoprotein cholesterol (LDL) concentrations were determined using the Friedewald formula, which was computed as follows: LDL = total cholesterol − [HDL + (triglycerides/5)] [19]. Non-HDL levels were calculated by subtracting HDL from the total cholesterol. The HOMA-IR was calculated as fasting insulin (mg/dL) multiplied by fasting glucose (mg/dL) and then divided by 22.5, and IR was defined as having a HOMA-IR value exceeding the 95th percentile for each gender and age group, as determined by the Korean HOMA-IR reference data [20]. ALT elevation was defined as having ALT levels higher than 26 IU/L for males and greater than 22 IU/L for females, provided there was no concurrent hepatitis B viral infection [5, 21]. Hyperuricemia was defined as higher uric acid levels based on age-specific reference value [22]. Uacid derived markers were defined and calculated as follows: Uacid divided by Cr (Uacid/Cr), Uacid divided by HDL (Uacid/HDL), Uacid × BMI (Uacid-BMI), Uacid × BMI SDS (Uacid-BMI SDS), Uacid × WC (Uacid-WC), Uacid × WHtR (Uacid-WHtR) [23,24,25].
All categorical variables are presented as numbers and weighted percentages, and continuous variables are presented as weighted means and standard errors. Student’s t-test was used to compare the mean values of continuous variables. The Rao-Scott chi-square test was used to compare categorical variables. Logistic regression analyses were performed with IR and elevated liver transaminases as dependent variables to investigate the relationship among obesity, central obesity, hyperuricemia, IR, and elevated liver transaminases. ORs and their corresponding 95% confidence intervals (CIs) were calculated for tertiles 2 and 3 of each parameter, with tertile 1 serving as the reference point for comparison. Sensitivity and specificity were determined as the optimal cutoff values for the markers, employing Youden’s index. Receiver operating characteristic (ROC) curves were generated to assess and compare the relative diagnostic effectiveness of these parameters in identifying IR and elevated liver transaminases. Pairwise comparisons of the parameters’ area under the curve (AUC) values were carried out using the bootstrap method. Statistical significance was established at a p value of less than 0.05. All statistical analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC) and R version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria), accounting for the complex survey design with clustering, stratification, and unequal weighting of the KNHANES sample.
Results
Table 1 presents a comparison of the clinical characteristics of participants with or without IR and elevated liver transaminases. Obesity-derived anthropometric indices, including BMI, BMI SDS, WC, and WHtR, and biochemical indices, including total cholesterol, LDL, non-HDL, triglycerides, glucose, insulin, HOMA-IR, AST, ALT, and hyperuricemia, were higher in the IR and ALT elevation groups compared to the non-IR and normal ALT groups, respectively (p = 0.002 for total cholesterol and p = 0.011 for glucose in comparison between ALT elevation and normal ALT, and p < 0.001 for the other comparisons). In contrast, HDL level was lower in the IR and ALT elevation groups compared to those in the non-IR and normal ALT groups (all p < 0.001). Overall, the IR and ALT elevation groups had higher values of Uacid and Uacid-derived markers than those in the non-IR and normal ALT groups (all p < 0.001).
In logistic regression analyses with IR and ALT elevation as dependent variables, ORs of general obesity for IR was 4.38 (95% CI, 2.23–8.63; p < 0.001), and the corresponding values for ALT elevation were 4.00 (95% CI, 2.01–7.94; p < 0.01), respectively (Table 2). The hyperuricemia group had OR value of 1.91 (95% CI, 1.33–2.76; p < 0.01) for IR and 1.81 (95% CI, 1.04–3.17; p 0.037) for ALT elevation. In addition, OR of IR for ALT elevation was 1.63 (95% CI, 1.13-2.34; p = 0.009)
Table 3 shows OR and 95% CI of the biochemical and metabolical variables for IR and ALT elevation among the whole population, with tertiles calculated using tertile 1 as the reference point for comparison. The ORs and 95% CIs for IR and ALT elevation progressively increased across tertiles of each variable, including Uacid, Uacid-Cr, Uacid-HDL, Uacid-BMI, Uacid-BMI SDS, Uacid-WC, Uacid-WHtR, total cholesterol, triglycerides, LDL, and non-HDL, among the total subjects (Table 3). Uacid combined with obesity indices exhibited ORs of tertile 3 ranging as 8.17–18.74 compared with those of tertile 1, while the Uacid, Uacid-Cr, and Uacid-HDL exhibited ORs of tertile 3 as 4.39. 4.94, and 5.23 compared with those of tertile 1 for IR in all individuals. For ALT elevation, Uacid combined with obesity indices exhibited ORs of tertile 3 ranging as 11.73–19.22 compared with those of tertile 1, while the Uacid, Uacid/Cr, and Uacid/HDL exhibited ORs of tertile 3 as 9.86, 3.83, and 6.95 compared with those of tertile 1 in all individuals. Among the indices, Uacid-BMI SDS presented the highest ORs and 95% CIs for IR in the total subjects (OR, 18.74), and Uacid-WHtR for ALT elevation (OR, 19.22), respectively. Overall, Uacid combined with obesity indices presented higher ORs and 95% Cis for IR compared to Uacid alone, Uacid/Cr, Uacid/HDL and lipid parameters. The same results were shown for ALT elevation, and it was also superior to insulin and HOMA IR.
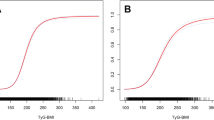
Table 4 and Fig. 2 summarize the results of ROC curve analyses and AUCs with the corresponding 95% CIs for Uacid and Uacid-derived markers to predict IR and ALT elevation. All variables predicted IR and ALT elevation significantly (all p < 0.001). The cut-off values for IR prediction were 5.95, 9.13, 0.11, 139.89, 3.54, 438.38, and 2.56 for Uacid, Uacid-Cr, Uacid-HDL, Uacid-BMI, Uacid-BMI SDS, Uacid-WC, and Uacid-WHtR, respectively. All Uacid combined with obesity indices showed higher AUC compared to Uacid, Uacid/Cr, and Uacid/HDL for IR. Uacid-BMI SDS showed the largest AUC for IR detection at 0.837. In addition, Uacid/HDL presented higher AUC compared to Uacid. The cutoff values for ALT elevation prediction were 5.55, 9.17, 0.12, 137.88, 3.76, 421.86, 2.49, 15.45, and 3.04 for Uacid, Uacid/Cr, Uacid/HDL, Uacid-BMI, Uacid-BMI SDS, Uacid-WC, Uacid-WHtR, insulin, and HOMA-IR, respectively. All Uacid combined with obesity indices showed higher AUC compared to Uacid, Uacid/Cr, Uacid/HDL, insulin, and HOMA-IR for ALT elevation. Uacid-WHtR showed the largest AUC for ALT elevation detection at 0.803. Uacid-BMI SDS presented significantly higher AUC values and 95% CIs than all other parameters for IR prediction (all p < 0.001) (Supplementary Table S1). For ALT elevation prediction, all Uacid combined with obesity indices presented higher AUC compared to Uacid, Uacid/Cr, Uacid/HDL, insulin, and HOMA-IR. In addition, Uacid-BMI and Uacid- WHtR had the highest AUC among the parameters.
ROC curves to predict IR in (A) and ALT elevation in (B). The dot on the curves represents the position of cutoff point in ROC curve. ROC receiver operating characteristic, ALT alanine transaminase, IR insulin resistance, Uacid uric acid, Cr creatinine, BMI body mass index, WC waist circumference, WHtR waist-to-height ratio, HOMA-IR homeostasis model assessment of insulin resistance.
Discussion
This study demonstrated that Uacid combined with obesity indices can be useful predictors for IR and ALT elevation in children and adolescents. These parameters were superior to Uacid, Uacid/Cr, and Uacid/HDL for prediction of IR and ALT elevation. Moreover, Uacid combined with obesity indices was superior to insulin level and HOMA-IR for ALT elevation prediction, although insulin and HOMA-IR were also useful for ALT elevation prediction. In addition, hyperuricemia, IR, and ALT elevation were closely related to each other.
In our study, Uacid was related to IR and ALT elevation, which showed a strong interconnection with each other. A retrospective study reported that HOMA-IR was positively associated with Uacid among youth with obesity [26]. Another retrospective study reported that serum Uacid level was related to non-alcoholic steatohepatitis among children with obesity [27]. Kim et al. suggested Uacid and HOMA-IR as predictors for NAFLD in children with obesity [28]. A cross-sectional study reported that high serum Uacid could be a useful marker for prediction of fatty liver disease in youth [29]. Relationship between Uacid and IR and hepatic steatosis can be explained by the following reasons: 1) Hyperuricemia decrease nitric oxide synthase activity, which induce IR; 2) Intracellular Uacid stimulates adenosine monophosphate dehydrogenase enzyme activity while inhibiting adenosine monophosphate kinase enzyme activity, and adenosine monophosphate dehydrogenase, in turn, stimulates hepatic gluconeogenesis while intracellular adenosine monophosphate kinase inhibits this process; 3) Endothelial dysfunction, oxidative stress, inflammation, and IR induced by hyperuricemia contribute to development of hepatic steatosis [13].
Uacid combined with obesity indices were more powerful than Uacid alone, Uacid/HDL, and Uacid/Cr for IR prediction in our study. This is because IR is closely associated with obesity and central obesity [9, 30]. Adipose tissue generates hormones, cytokines, and adipokines, such as leptin and adiponectin; and an imbalance in these molecules, frequently seen in obesity, can trigger inflammation and IR [31]. Moreover, in obesity, increased free fatty acids can enter the liver through portal circulation, leading to an increase in hepatic lipid synthesis, gluconeogenesis, and the development of IR within the liver [32, 33]. Among the Uacid-derived markers, Uacid-BMI SDS was the most powerful predictor for IR prediction in our study. In children, obesity is defined as having a BMI at or above the 95th percentile of sex- and age-specific reference values, making BMI SDS generally more appropriate than BMI alone for assessment [34]. In our previous study, triglyceride glucose index was more powerful for IR prediction when combined with BMI SDS, compared to BMI [9].
For ALT elevation, Uacid combined with obesity indices were more powerful than Uacid alone. Obesity induces excessive fat tissue throughout the body, which can be stored in the liver, resulting in hepatic steatosis, which is a key characteristic of hepatic steatosis [35]. Moreover, the excess fat in obesity, especially visceral fat, produces pro-inflammatory cytokines that can promote inflammation in the liver, worsening hepatic steatosis [32]. In addition, IR induced by obesity accelerates progression of hepatic steatosis [1, 8]. In a pediatric study, OR for children with BMI SDS above 3 was 2.56 compared to those with 2 ≤ BMI-SDS < 3 [36]. In a nationwide study, OR per BMI SDS unit was 4.67 for NAFLD risk among children with obesity [37]. Especially, Uacid-WHtR was a powerful predictor for ALT elevation in our study. Accumulation of visceral abdominal fat leads to an increase in circulating free fatty acids, contributing to the onset of hepatic steatosis, which is more closely associated with WC rather than BMI [8, 38]. A systematic review reported that WHtR showed a significant increase in NAFLD patients compared to the control group, with a mean difference of 0.073 [39]. A cross-sectional study reported that overweight individuals with central adiposity or IR faced a higher risk of NAFLD compared to those with milder central adiposity or IR, even if their obesity status was similar.
Uacid/HDL was significantly associated with both IR and ALT elevation. The mechanism regarding the negative relationship between HDL and IR can be explained by the following reasons: 1) Ceramide, a sphingolipid positively associated with IR, may be taken up by HDL from adipose tissue [40]. Thus, lower HDL levels could indicate higher tissue ceramide concentrations, which related to IR; 2) In individuals with IR, HDL level decreases due to increased inflammatory cytokines, which are elevated due to the visceral obesity typically seen in the metabolic syndrome [41, 42]. In addition, the association of HDL with obesity and IR might contribute to the relationship between HDL and hepatic steatosis [1, 7, 43]. A cross-sectional study reported that Uacid/HDL was related to HOMA-IR in adults with type 2 diabetes [44]. Moreover, a cohort study reported that Uacid/HDL was significantly related to NAFLD in adults with normal serum Uacid [7].
Our research had some limitations. First, it was a cross-sectional study limited to the Korean population. Second, we were unable to consider factors such as pubertal status, dietary habits, and physical activity. Third, imaging studies or liver biopsies for hepatic steatosis were not included. Thus, we investigated elevated liver transaminase instead of MASLD. Although there are many other causes of transaminase elevation and SLD, the KNHANES only provides information on hepatitis B virus for children; therefore, only this factor could be considered. Fourth, we were not able to consider measuring both lean and fat body mass in our study. However, this study assessed Uacid and derived markers as predictors of IR and ALT elevation across a large number of children and adolescents. In addition, we proposed new parameters, Uacid combined with obesity indices, as predictive markers for IR and ALT elevation in youth.
In conclusion, this study demonstrates the value of combining Uacid with obesity indices as robust predictors of IR and ALT elevation as well as intricate interplay among hyperuricemia, IR, and ALT elevation in children and adolescents. These combined parameters outperform individual Uacid alone and other derived markers in predicting both IR and ALT elevation. Notably, they also exhibit superior predictive power compared to insulin levels and HOMA-IR for ALT elevation. Combination of Uacid with obesity indices does not require fasting, making it a convenient tool for early detection and prevention strategies in the pediatric population. Therefore, this research underscores the potential of Uacid combined with obesity indices for the screening of IR and MASLD among children and adolescents.
Data availability
The data used in this study is available in the KNHANES website. https://knhanes.kdca.go.kr/knhanes/main.do.
References
Song K, Kim HS, Chae HW. Nonalcoholic fatty liver disease and insulin resistance in children. Clin Exp Pediatr. 2023;66:512–9.
Kamani L, Rahat A, Yilmaz Y. Addressing the looming epidemic of metabolic dysfunction-associated steatotic liver disease in Pakistan: a call for action. Hepatol Forum. 2024;5:1–2.
Bansal SK, Bansal MB. Review article: Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024. https://doi.org/10.1111/apt.17930.
Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M Prevalence of Steatotic Liver Disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clin Gastroenterol Hepatol. 2023. https://doi.org/10.1016/j.cgh.2023.11.003.
Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010;138:1357–64. 64 e1-2.
Kouvari M, Valenzuela-Vallejo L, Guatibonza-Garcia V, Polyzos SA, Deng Y, Kokkorakis M, et al. Liver biopsy-based validation, confirmation and comparison of the diagnostic performance of established and novel non-invasive steatotic liver disease indexes: Results from a large multi-center study. Metabolism. 2023;147:155666.
Zhang YN, Wang QQ, Chen YS, Shen C, Xu CF. Association between Serum Uric Acid to HDL-cholesterol ratio and nonalcoholic fatty liver disease in lean chinese adults. Int J Endocrinol. 2020;2020:5953461.
Song K, Lee HW, Choi HS, Park G, Lee HS, Kim SJ, et al. Comparison of the modified TyG indices and other parameters to predict non-alcoholic fatty liver disease in youth. Biol (Basel). 2022;11:685.
Song K, Park G, Lee HS, Choi Y, Oh JS, Choi HS, et al. Prediction of insulin resistance by modified triglyceride glucose indices in youth. Life (Basel). 2021;11:286.
Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005;54:206–11.
Ha J, Oh YR, Kang E, Nam HK, Rhie YJ, Lee KH. Single Point Insulin Sensitivity Estimator for predicting type 2 diabetes mellitus in obese adolescents. Ann Pediatr Endocrinol Metab. 2022;27:201–6.
Sertoglu E, Ercin CN, Celebi G, Gurel H, Kayadibi H, Genc H, et al. The relationship of serum uric acid with non-alcoholic fatty liver disease. Clin Biochem. 2014;47:383–8.
Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Biochem. 2016;49:636–42.
Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19:167–73.
Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963.
Sookoian S, Pirola CJ. The serum uric acid/creatinine ratio is associated with nonalcoholic fatty liver disease in the general population. J Physiol Biochem. 2023;79:891–9.
Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43:69–77.
Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018;61:135–49.
Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol. 1988;62:345–6.
Yi KH, Hwang JS, Kim EY, Lee SH, Kim DH, Lim JS. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pr. 2014;103:106–13.
Song K, Park G, Lee HS, Lee M, Lee HI, Ahn J, et al. Trends in prediabetes and non-alcoholic fatty liver disease associated with abdominal obesity among korean children and adolescents: based on the Korea National Health and Nutrition Examination Survey between 2009 and 2018. Biomedicines. 2022;10:584.
Uric Acid, Serum - Mayo Clinic Laboratories, Pediatric Catalog. 2021. https://pediatric.testcatalog.org/show/URIC.
Kocak MZ, Aktas G, Erkus E, Sincer I, Atak B, Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras (1992). 2019;65:9–15.
She D, Xu W, Liu J, Zhang Z, Fang P, Li R, et al. Serum uric acid to creatinine ratio and risk of metabolic syndrome in patients with overweight/obesity. Diabetes Metab Syndr Obes. 2023;16:3007–17.
Zhong D, Liu D, Guo Y, Huang H, Li L, Wu F, et al. Association of the serum uric acid to creatinine ratio with metabolic syndrome in the middle age and older population in China. Front Endocrinol (Lausanne). 2022;13:1060442.
Niu Y, Tang Q, Zhao X, Zhao X, Mao X, Sheng J, et al. Obesity-induced insulin resistance is mediated by high uric acid in obese children and adolescents. Front Endocrinol (Lausanne). 2021;12:773820.
Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031–6.
Kim A, Yang HR, Cho JM, Chang JY, Moon JS, Ko JS. A nomogram for predicting non-alcoholic fatty liver disease in obese children. Pediatr Gastroenterol Hepatol Nutr. 2020;23:276–85.
Di Bonito P, Valerio G, Licenziati MR, Di Sessa A, Miraglia Del Giudice E, Morandi A, et al. Uric acid versus metabolic syndrome as markers of fatty liver disease in young people with overweight/obesity. Diabetes Metab Res Rev. 2022;38:e3559.
Kim HY, Kim JH. Temporal trends in the prevalence of metabolically healthy overweight and obesity in Korean youth: data from the Korea National Health and Nutrition Examination Survey 2011-2019. Ann Pediatr Endocrinol Metab. 2022;27:134–41.
Kojta I, Chacinska M, Blachnio-Zabielska A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020;12:1305.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–223.
Choe J, Kim J, Moon JS. Cutoff values of body mass index for severe obesity in Korean children and adolescents: the 99th percentile versus 120% of the 95th percentile. Ann Pediatr Endocrinol Metab. 2023;28:131–7.
Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102:709–57.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89.
Peng J, Wu F, Tian H, Yang H, Zheng Y, Deng Z, et al. Clinical characteristics of and risk factors for small airway dysfunction detected by impulse oscillometry. Respir Med. 2021;190:106681.
Putri RR, Casswall T, Hagman E. Risk and protective factors of non-alcoholic fatty liver disease in paediatric obesity: A nationwide nested case-control study. Clin Obes. 2022;12:e12502.
Song K, Yang J, Lee HS, Kim SJ, Lee M, Suh J, et al. Changes in the prevalences of obesity, abdominal obesity, and non-alcoholic fatty liver disease among korean children during the COVID-19 outbreak. Yonsei Med J. 2023;64:269–77.
Ismaiel A, Hosiny BE, Ismaiel M, Leucuta DC, Popa SL, Catana CS, et al. Waist to height ratio in nonalcoholic fatty liver disease - Systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2023;47:102160.
Hoofnagle AN, Vaisar T, Mitra P, Chait A. HDL lipids and insulin resistance. Curr Diab Rep. 2010;10:78–86.
Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96.
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57.
Zhu W, Liang A, Shi P, Yuan S, Zhu Y, Fu J, et al. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterol. 2022;22:196.
Zhou X, Xu J. Association between serum uric acid-to-high-density lipoprotein cholesterol ratio and insulin resistance in patients with type 2 diabetes mellitus. J Diabetes Investig. 2024;15:113–20.
Funding
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2021-0150).
Author information
Authors and Affiliations
Contributions
Youngha Choi was responsible for conceptualization, methodology, formal analysis, investigation and writing - original draft. Hyejin Yang was responsible for resources, data curation, formal analysis and writing - original draft. Soyoung Jeon was responsible for resources, data curation, formal analysis and writing - original draft. Kyoung Won Cho, Seo Jung Kim and Sujin Kim was responsible for conceptualization, methodology and writing - review & editing. Myeongseob Lee was responsible for methodology, investigation and writing - review & editing. Junghwan Suh was responsible for conceptualization, methodology and writing - review & editing. Hyun Wook Chae was responsible for conceptualization, methodology and writing - review & editing. Ho-Seong Kim was responsible for supervision and writing - review & editing. Kyungchul song was responsible for conceptualization, methodology, formal analysis, investigation and writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (IRB, 9-2023-0215).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41430_2024_1475_MOESM1_ESM.docx
Supplementary Table S1. Comparison of areas under receiver operating curves among each parameter for predicting IR and ALT elevation
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, Y., Yang, H., Jeon, S. et al. Prediction of insulin resistance and elevated liver transaminases using serum uric acid and derived markers in children and adolescents. Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01475-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41430-024-01475-z
- Springer Nature Limited