Abstract
Background
Olive oil consumption has been reportedly associated with lower mortality rates, mostly from cardiovascular diseases, but its potential impact on cancer death remains controversial. Moreover, biological mechanisms possibly linking olive oil consumption to mortality outcomes remain unexplored.
Methods
We longitudinally analysed data on 22,892 men and women from the Moli-sani Study in Italy (follow-up 13.1 y), to examine the association of olive oil consumption with mortality. Dietary data were collected at baseline (2005–2010) through a 188-item FFQ, and olive oil consumption was standardised to a 10 g tablespoon (tbsp) size. Diet quality was assessed through a Mediterranean diet score. Multivariable-adjusted Cox proportional hazard models, also including diet quality, were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The potential mediating role of inflammatory, metabolic, cardiovascular and renal biomarkers on the association between olive oil intake and mortality was evaluated on the basis of change-in-estimate and associated p values.
Results
Multivariable HRs for all-cause, cancer, cardiovascular and other cause mortality associated with high (>3 tbsp/d) versus low (≤1.5 tbsp/d) olive oil consumption were 0.80 (0.69–0.94), 0.77 (0.59–0.99), 0.75 (0.58–0.97) and 0.97 (0.73–1.29), respectively. Taken together, the investigated biomarkers attenuated the association of olive oil consumption with all-cause and cancer mortality by 21.2% and 13.7%, respectively.
Conclusions
Higher olive oil consumption was associated with lower cancer, cardiovascular and all-cause mortality rates, independent of overall diet quality. Known risk factors for chronic diseases only in part mediated such associations suggesting that other biological pathways are potentially involved in this relationship.
Similar content being viewed by others
Introduction
Olive oil is possibly the most typical feature of a traditional Mediterranean diet (MD) and is the nearly exclusive source of added fat within this moderately high-fat dietary pattern [1, 2].
The health benefits of olive oil are due to its high levels of unsaturated fatty acids (up to 99% of the total weight in extra-virgin type), in particular of monounsaturated acids such as oleic, as well as to other minor components as phenolics, phytosterols and tocopherols [3], all contributing to its well-known anti-inflammatory and antioxidant properties [4], as well as anti-thrombotic functions that are relevant for cardiovascular health maintenance [5]. Furthermore, olive oil has been shown to improve cardiovascular health by a favourable modulation of the lipid profile and platelet function homoeostasis, lowering blood pressure and reducing the atherogenic process [6]. Large observational cohort studies worldwide showed that regular olive oil intake is related to a decreased risk of major non-communicable diseases such as cardiovascular diseases (CVD) [7, 8], neurodegenerative diseases [8] and certain cancers [9, 10] and higher survival [11, 12]. In one of its three arms, the large PREDIMED dietary intervention trial specifically tested an MD with free provision of extra-virgin olive oil and reported clinically meaningful reductions (versus a control group on a low-fat diet) in major CVD events [13], as well as in breast cancer incidence [14].
However, epidemiological evidence on the potential health advantages of olive oil consumption in relation to cancer mortality is less robust and provides inconsistent results. While a large number of studies analysed the association of olive oil with cancer risk [9], only six cohort studies [7, 8, 15,16,17,18] have specifically examined its relationship with cancer death, mostly conducted in Mediterranean countries [7, 15, 16, 18]. A meta-analysis that pooled the results of five out of these six studies [11] indicated an inverse association between increasing olive oil consumption and cancer mortality, although statistical significance was not held. Specifically, only one report from a large US population, where the average consumption of olive oil is substantially lower than that in Mediterranean countries, indicated a reduced risk of cancer death associated with greater olive oil intake [8]. Moreover, none of the above-mentioned cohorts examined the biological mechanisms potentially linking olive oil consumption to health outcomes.
To increase knowledge on the potential benefits of olive oil for human health and, in particular, in relation to cancer death, and fill the knowledge gap on the mechanistic pathways involved, we prospectively evaluated the association of olive oil consumption with all-cause and cause-specific mortality in the context of an MD by taking advantage of the large dataset of the Moli-sani Study on 24,325 adult men and women living in southern Italy. As a secondary aim, we examined the role of several biomarkers representative of different physiological processes (e.g., inflammatory, cardiovascular and metabolic) common to major chronic diseases as potential explanatory factors of the putative relationship between olive oil intake and mortality.
Methods
Study population
We analysed data from the Moli-sani Study, a population-based cohort established in 2005–2010 in the southern Italian region of Molise, with an enrolment of 24,325 men and women aged ≥35 years, having the main purpose of investigating genetic and environmental risk factors in the onset and progression of CVD, cerebrovascular disease and cancer [19]. Exclusion criteria were pregnancy at the time of recruitment, disturbances in mental or decision-making impairments, current poly-traumas or coma or refusal to sign the informed consent. Details of the study are available elsewhere [19,20,21].
For the purpose of the present analyses, we excluded subjects with missing data on diet, incomplete dietary or medical questionnaires, reporting implausible energy intakes (<800 kcal/d in men and <500 kcal/d in women or >4000 kcal/d in men and >3500 kcal/d in women) and without information on cause-specific death. We finally analysed 22,892 individuals (94.1% of the whole study sample). Supplementary Fig. 1 shows the flowchart for the selection of study participants.
Follow-up for vital status and ethics
The Moli-sani Study cohort was followed up for mortality since March 2005 through December 31st, 2020. Cause-specific mortality was assessed by the Italian mortality registry, validated by Italian death certificates (ISTAT form) and coded according to the International Classification of Diseases (ICD-9). Cancer death was considered when the underlying cause of death included ICD-9 codes 140–208. CVD mortality included deaths from diseases of the circulatory system, when the underlying cause of death included ICD-9 codes 390–459. Non-cardiovascular/non-cancer causes of death were included in ‘other cause mortality’ group. The Moli-sani Study complies with the Declaration of Helsinki and was granted the approval of the Ethics Committee of the Catholic University in Rome, Italy. Written informed consent was obtained from all participants.
Dietary assessment at baseline
Dietary intake was assessed at study entry (2005–2010) by an interviewer-administered semi-quantitative European Prospective Investigation into Cancer and Nutrition (EPIC) food frequency questionnaire (FFQ) validated and adapted to the Italian population to assess participants’ diet during the past 12 months. The FFQ contains 14 sections (pasta/rice, soup, meat (excluding salami and other cured meats), fish, raw vegetables, cooked vegetables, eggs, sandwiches, salami and other cured meats, cheese, fruit, bread/wine, milk/coffee/cakes and herbs/spices), with 248 questions concerning 188 different food items [22].
Participants were asked to indicate the number of times a given item was consumed (per day, week, month or year) from which the frequency of consumption was calculated. The quantity of food consumed was assessed by asking the participant to select one among several images of different food portions or a predefined standard portion when no image was available. Frequencies and quantities of each food were then linked to Italian Food Tables [23], using a specifically designed software [24], to obtain quantitative estimates of daily intake of macro- and micro-nutrients plus energy.
Total daily olive oil intake was calculated based on the participant’s reported olive oil used for cooking at home, including frying, baking and dressing and defined as number of tablespoon (Tbsp) per day. We used 10 g as the standard weight for one Tbsp [25], and derived the following five categories of consumption: none to 1.5 Tbsp/d; >1.5 to ≤2 Tbsp/d; >2 to ≤2.5 Tbsp/d; >2.5 to ≤3 Tbsp/d; and >3 Tbsp/day. For analyses purposes, olive oil intake was also modelled as a continuous variable as a 1 Tbsp-increment per day. Adherence to the traditional MD was appraised by the Mediterranean diet score (MDS) developed by Trichopoulou et al. [26] by assigning one point to healthy foods (such as fruits and nuts, vegetables, legumes, fish and cereals) monounsaturated (MUFA) to saturated fat (SFA) ratio, whose consumption was above the sex-specific medians of intake; foods presumed to be detrimental (meat and dairy products) were scored positively if their consumption was below the median. All other intakes received zero points. For ethanol, men who consumed 10–50 g/d and women who consumed 5–25 g/d received one point; otherwise, the score was zero. The MDS ranged from 0 to 9 (the latter reflecting maximal adherence) [26]. For analyses purposes, the fat component (MUFA to SFA ratio) was excluded from the MDS computation to avoid overadjustment bias, since it is a strong proxy of olive oil intake.
Assessment of covariates at baseline (2005–2010)
At baseline, information on socio-demographic variables, lifestyles and medical history was obtained by interviewer-administered questionnaires. Education was based on the highest qualification attained and was categorised as up to lower secondary (approximately ≤8 years of study), upper secondary school (9–13 years of study) and postsecondary education (>13 years of study). Urban or rural environments were defined on the basis of the urbanisation level as described by the European Institute of Statistics (EUROSTAT definition); urbanisation level was provided by the Italian National Institute of Statistics [27]. Marital status was grouped as married/in couple, separated/divorced, single and widowed. Housing tenure was classified as rented, 1 dwelling ownership and >1 dwelling ownership. Subjects were classified as never, current or former smokers (reported not having smoked at all over the previous 12 months or more). Leisure-time physical activity (PA) was assessed by an interviewer-administered structured questionnaire [28] and expressed as daily energy expenditure in metabolic equivalent task hours (MET-h/d) for sport, walking and gardening. Height and weight were measured and body mass index (BMI) was calculated as kg/m2 and grouped into three categories normal (<25 kg/m2), overweight (≥25 <30 kg/m2), or obese (≥30 kg/m2). Personal history of CVD (angina, myocardial infarction, revascularization procedures, peripheral artery diseases and cerebrovascular events) and/or cancer was self-reported and confirmed by medical records and therapy at study entry. Participants were considered to have diabetes, hypertension or hyperlipidaemia at baseline if they were taking disease-specific drugs. Menopausal status and use of hormonal contraception or hormone-replacement therapy were self-reported at baseline visits.
Selection and assessment of risk factors at baseline (2005–2010)
Biomarkers reflecting different underlying pathways common to chronic disease incidence and progression [29,30,31] were selected by subject area knowledge according to the following criteria: (1) previously studied for their relevance in pathways predisposed to non-communicable chronic disease onset; (2) shown in epidemiologic studies to be related to chronic disease onset or mortality; and (3) already analysed within the Moli-sani Study cohort. Blood samples were collected at baseline (2005–2010) in participants who had fasted overnight and had refrained from smoking for at least 6 h; lipids (total cholesterol, HDL cholesterol, triglycerides) and blood glucose were assayed in serum samples by enzymatic reaction methods using an automatic analyser (ILab 350, Instrumentation Laboratory, Milan, Italy); quality control for lipids and glucose was obtained by a commercial standard (SeraChem1 and SeraChem2). For SeraChem1 and SeraChem2, the coefficients of variability were 4.9% and 5.2%, respectively, for blood cholesterol; 3.2% and 3% for HDL; 5.2% and 5.3% for triglycerides; and 4.7% and 4.1% for blood glucose.
High-sensitivity C-reactive protein (CRP) was measured in fresh serum samples by a particle-enhanced immune-turbidimetric assay (ILab 350, Instrumentation Laboratory, Milan, Italy). Quality control for CRP was maintained using an in-house serum pool and a commercial laboratory standard whose inter-day coefficients of variability were 5.5% and 4.2%, respectively.
A hemocromocytometric analysis was performed by cell count (Coulter HMX, Beckman Coulter, IL, Milan, Italy) within 3 h of blood collection. Quality control was performed using three different levels of standards: Abnormal 1, a pathologically high control; Abnormal 2, a pathologically low control; and Normal (Coulter HMX, Beckman Coulter). Coefficients of variability for white blood cells were 6.2%, 3.3%, and 3.0% for Abnormal 1, Abnormal 2, and Normal, respectively. Markers of lipid metabolism (i.e., ApoA, apoB100, lipoprotein a), renal function (i.e., cystatin C, creatinine), glucose metabolism (i.e., insulin, C-peptide) and serum vitamin D were measured subsequently on thawed samples stored frozen in liquid nitrogen at the biological bank of the Moli-sani Study, in the framework of the collaborative BiomarCaRE (Biomarker for Cardiovascular Risk Assessment in Europe) research project, whose primary objective is to assess the value of established and emerging biomarkers for CVD risk prediction by using data from 23 cohorts across Europe [29].
Statistical analysis
Baseline characteristics of study participants across levels of olive oil consumption were presented as means and standard deviations (SDs) or as percentages for categorical traits. Differences in the distribution of baseline covariates were calculated by using generalised linear models adjusted for age, sex and energy intake (GENMOD procedure for categorical variables and GLM procedure for continuous variables in SAS software).
Hazard ratios (HRs) were used to quantify the association of olive oil intake with all-cause and cause-specific deaths. HRs with 95% confidence intervals (95% CIs) were calculated using Cox regression models with time-on-study on the time scale and adjusting for baseline age as covariate in the model. We visually assessed the proportional hazards assumption (log (−log) plots of survival curves) and identified no violation. Individuals contributed person time until their date of emigration, date of death, or loss to follow-up or until end of follow-up, whichever occurred first. In cause-specific analyses, we included participants who died from a cause other than the one under study and censored them at the date of the competing death event.
On the basis of previous literature and biological plausibility, three models for the association of olive oil with mortality risk were fitted: Model 1 was adjusted for age (continuous), sex and energy intake (kcal/d; continuous); Model 2 as in Model 1 and further controlled for educational level (up to lower secondary school; upper secondary school; postsecondary), housing (rented, ownership of one dwelling, and ownership of more than one dwelling), residence (urban/rural), leisure-time PA (continuous), smoking status (never, current, former), BMI (continuous), history of cancer (no/yes), history of CVD (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), menopausal status (no/yes/unascertained), use of hormonal contraception (no/yes/unascertained) and hormonal replacement therapy (no/yes/unascertained); Model 3 as in Model 2 and further adjusted for the MDS deprived of its MUFA to SFA ratio component (continuous; range 0–8).
To maximise data availability, missing data on covariates were handled using multiple imputations (SAS PROC MI, followed by PROC MIANALYZE; n = 10 imputed datasets).
In accordance with predefined mediation principles [32, 33], a biomarker was considered a potential mediator of the association of olive oil intake with mortality if (a) it was on the causal pathway of this association by subject area knowledge [31, 34, 35]; (b) it was associated with both the exposure and the outcome. These criteria were tested in distinct multivariable regression models for each potential explanatory factor individually (Supplementary Table 1) and through Cox models that included olive oil consumption as a covariate (Supplementary Table 2).
The multivariable Model 3 served as the reference for the analysis used to estimate the extent to which selected risk factors explained the association of olive oil intake with mortality. For the mediation analysis, we used the publicly available %MEDIATE macro in SAS [36] which calculates the point and interval estimates of the percent of exposure effect explained by one or more intermediate variables, with 95% CI and P values. Each marker was alternately, and at the end simultaneously, included (as continuous variables) into the multivariable-adjusted Model 3. To test the robustness of our findings, we ran pre-planned sensitivity analyses by (a) excluding participants with baseline CVD, cancer, diabetes, hypertension and hyperlipidaemia (therefore, assessing a potential bias resulting from modified habitual dietary intakes due to illness); (b) excluding BMI from the models since it could be in the causal pathway; (c) excluding baseline chronic health conditions and BMI as covariates; and (d) excluding deaths occurred during the first 2 years of follow-up. Additionally, to test for potential effect modifications, we ran subgroup analyses by various baseline risk factors: age groups (35–64 and ≥65 years), sex, educational levels, smoking status, BMI categories and levels of adherence to the MD. Appropriate multiplicative terms for testing interactions were included into the multivariable-adjusted models to test for a difference of effect. To test for a potential non-linear, continuous relationship between olive oil and mortality risk, we used multivariable Cox regression analysis with olive oil intake (1 tbsp/d increase) modelled as restricted cubic splines (three knots at 5%, 50% and 95% of the distribution) [37] and used the value of 1 tbsp of olive oil as the reference value.
Data analysis was generated using SAS/STAT software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The analysed sample consists of 11,976 women (52.3%) and 10,916 men (47.7%) with a mean age at enrolment of 55.4 y (SD ± 11.7), and an average olive oil consumption of 23.3 g/d (SD ± 8.9). Baseline characteristics of participants according to olive oil consumption are shown in Table 1. As compared to participants in the bottom olive oil consumption category (≤1.5 tbsp/d), participants reporting higher olive oil consumption (>3 tbsp/d) were younger, prevalently men, had higher socioeconomic status, were more physically active, and were prevalently living in rural areas. Also, they were more likely to be obese and reported lower prevalence of chronic health conditions (Table 1). Greater consumption of olive oil was associated with higher MDS and energy intake, and a greater consumption of fruits, vegetables, legumes, fish and prevalence of moderate alcohol intake (Supplementary Table 3). Furthermore, high olive oil consumers reported less cereals, meat and dairy products intakes. The contribution of fats to total energy intake increased across categories of olive oil consumption, whereas that of carbohydrates decreased, as well as dietary cholesterol intake (Supplementary Table 3). Differences in the intake of other vegetable oils and dietary saturated fat were also observed, while margarine and butter consumption did not vary across categories of olive oil consumption (Supplementary Table 3).
The cohort of 22,892 participants was followed up for mortality for a median of 13.1 years (interquartile range = 12.1–14.2 years; 291,810 person-years) during which 2566 deaths were ascertained (939 from cancer, 910 from CVD and 723 from other causes). Results from a multivariable-adjusted model also including the diet quality (i.e., the MDS deprived of its fat component) showed that, as compared to participants reporting lower olive oil consumption (≤1.5 tbsp/d), higher consumption (>3 tbsp/d) was associated with lower all-cause (HR = 0.80; 95% CI 0.69–0.94), cancer (HR = 0.77; 95% CI 0.59–0.99) and CVD mortality rates (HR = 0.75; 95% CI 0.58–0.97) (Table 2, Model 3); these results remained substantially unchanged when consumption of other vegetable oils was added to the model (HR = 0.80; 95% CI 0.69–0.93; HR = 0.76; 95% CI 0.59–0.98 and HR = 0.75; 95% CI 0.58–0.98 for all-cause, cancer and CVD mortality, respectively; data not shown). Consistently, multivariable-adjusted survival curves estimates for all-cause, cancer and CVD mortality across categories of olive oil consumption were well separated (Fig. 1A–C). No association with mortality from other causes was found (Table 2; Model 3 and Fig. 1D). The multivariable dose-response analysis between a 1 tbsp increase in olive oil consumption with all-cause and cancer mortality showed a direct linear dose-response relationship (p value for overall association = 0.051 and p value for non-linearity = 0.27 for all-cause mortality; p value for overall association = 0.051 and p value for non-linearity = 0.32 for cancer mortality) (Fig. 2A, B). Dose-response analyses for CVD or other cause mortality did not provide significant results (Fig. 2C, D).
Survival curves estimates for a all-cause, b cancer, c cardiovascular, and d other cause mortality across categories of olive oil consumption were obtained from a multivariable model adjusted for age, sex, educational level, housing tenure, marital status, smoking status, body mass index, leisure-time physical activity, history of cancer, history of CVD, diabetes, hypertension, hyperlipidaemia, place of residence, menopausal status, use of hormonal contraception, hormonal replacement therapy, and the Mediterranean diet score deprived of its fat component.
Risk estimates (hazard ratios with 95% confidence interval) for a all-cause, b cancer, c cardiovascular, and d other cause mortality were obtained from the multivariable model adjusted for age, sex, educational level, housing tenure, marital status, smoking status, body mass index, leisure-time physical activity, history of cancer, history of CVD, diabetes, hypertension, hyperlipidaemia, place of residence, menopausal status, use of hormonal contraception, hormonal replacement therapy, and the Mediterranean diet score deprived of its fat component. Olive oil consumption was considered as a continuous exposure and the reference value for hazard ratios was 1 tablespoon. The dashed lines indicate 95% confidence bands. Three knots were used, located at the 5th, 50th, and 95th percentiles of olive oil consumption.
The inverse association of olive oil (1 tbsp increment) with cancer mortality was confirmed in sensitivity analyses where baseline chronic conditions and BMI were alternately excluded, as well as deaths occurred during the first 2 years of follow-up (Supplementary Table 4).
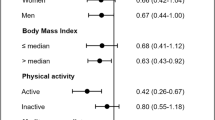
Results from pre-specified subgroup analyses for the association between olive oil intake (1 tbsp increment/day) with all-cause and cause-specific mortality are reported in Supplementary Tables 5,6. There was no evidence of effect modification for most of the variables analysed (i.e., sex, age, educational level, smoking status, PA and adherence to the MD), although the inverse associations of olive oil consumption with all-cause and cancer mortality were limited to non-obese participants (p values for interaction = 0.052 and 0.004, respectively).
Analysis of mechanistic pathways
The associations of olive oil intake with all potential explanatory factors (selected biomarkers) are shown in Supplementary Table 1. Olive oil consumption was inversely associated with biomarkers of renal function (i.e., serum levels of cystatin C and creatinine), blood glucose, total cholesterol, triglycerides, Apolipoprotein A1, CRP, the granulocyte-to-lymphocyte ratio and traditional cardiovascular risk factors, such as systolic and diastolic blood pressure and heart rate; a direct relationship with serum Vitamin D concentrations was otherwise observed (Supplementary Table 1). The HRs for all-cause, cancer and CVD mortality associated with the selected biomarkers are shown in Supplementary Table 2; all risk factors here considered, with the exception of triglycerides and Apolipoprotein A1 levels, were associated with all-cause and CVD mortality; markers associated with higher cancer mortality were blood glucose, CRP, the granulocyte-to-lymphocyte ratio, diastolic blood pressure and resting heart rate, whereas serum vitamin D concentrations were inversely related.
Of the investigated biomarkers, traditional CVD risk factors (i.e., blood pressure levels and resting heart rate) produced the highest change in the association of olive oil intake with all-cause (14% attenuation; p value = 0.0069) and cancer mortality (9.5% attenuation; p value = 0.0018) (Table 3).
Discussion
In this large cohort of middle-aged and elderly Italians, higher olive oil consumption was significantly associated with a lower rate of cancer mortality, the rate of CVD and all-cause mortality also being significantly reduced.
While previous research supported the favourable association between olive oil and cancer risk [9], only a few cohorts analysed its potential impact on cancer death. Amongst the prospective studies included in the meta-analysis by Martínez-Gonzalez et al. [11], only one [8] out of five cohorts included [7, 15,16,17] reported a significant inverse association between olive oil intake and cancer mortality. This is apparently in contrast with our findings, although a later study not included in this meta-analysis found a substantial reduction in the risk of cancer death associated with consumption of two or more tablespoons of olive oil per day in a Spanish cohort followed for nearly 20 years [18].
Our data confirm the lower rate for CVD mortality associated with higher amounts of olive oil, in line with previous cohort studies providing similar effect sizes in CVD mortality reduction in association with regular olive oil consumption [8, 38].
Our findings on survival are in agreement with prior cohort studies whose results have been recently summarised in two distinct meta-analyses; Xia et al. [12] meta-analysed findings from 11 independent cohorts for a total of 713,000 subjects and 173,817 all-cause deaths, and found that, compared with the lowest, the highest intake of olive oil was associated with 17% reduced risk of all-cause mortality. Advantages in mortality risk reduction were previously reported by a meta-analysis of Martínez-Gonzalez et al. [11] using data from 10 cohort studies and 1 RCT and showing an 11% reduced mortality risk associated with a 25 g/d increase of olive oil consumption.
There are different putative biological mechanisms through which olive oil consumption may help maintain a good health and therefore prolong survival.
The health-promoting properties of olive oil are largely attributed to its exceptional composition, which also varies according to the type of olive oil; MUFA, and, in particular, oleic acid which constitutes up to 99% of olive oil weight, as in the case of extra-virgin olive oil, were proven to have modulatory effects in a wide range of physiological functions, while some studies also suggest a beneficial effect on cancer, autoimmune and inflammatory diseases, besides its ability to ease wound healing [39]. Olive oil is also a source of polyphenols, especially hydroxytyrosol that has the ability of scavenging free radicals and reactive oxygen/nitrogen species, as well as activating endogenous antioxidant systems in the body [40] that are relevant to chronic disease initiation. Oleuropein (one of the main phenolic compounds of olive oil, which gives a bitter and pungent taste to extra-virgin olive oil) has vasodilatory and hypotensive effects [41], and is able to inhibit platelet aggregation [42] which is critical to major chronic diseases including cancer progression [43]; vitamin E, of which α-Tocopherol is its major form [44], acts as a powerful antioxidant with anti-inflammatory [45] and anti-cancer properties [46].
We found that olive oil consumption was inversely associated with several markers reflecting different biological processes linked to major chronic diseases onset, such as markers of renal function, serum lipids, blood glucose, inflammatory markers and also with established cardiovascular risk factors such as blood pressure and resting heart rate, and a novel, although still controversial cardiovascular risk factor, as serum vitamin D levels (inverse association). Of these, cardiovascular risk factors were found to act as potential explanatory factors of the inverse association between olive oil with all-cause and cancer mortality, and this supports the notion that major chronic diseases (e.g., CVD, cancer and neurodegenerative diseases) may share modifiable risk factors, and possibly molecular mechanisms of disease, as postulated by the ‘common soil hypothesis’ [31, 47, 48].
Despite the fact that the proportion explained by these factors was relatively small, our analyses offer interesting insights on the mechanisms through which olive oil can favourably impact on human health. Hypertension is a well-established risk factor for CVD, but it has also been associated with an increased risk of developing certain cancers, such as prostate cancer in men, and endometrial and breast in women and with higher cancer-related mortality [49]. Additionally, hypertension is also a known risk factor for renal cancer [50]. In line with these works, our data suggested an increased risk of cancer death in association with higher blood pressure and heart rate. The relationship between diet, heart rate and cancer is, however, less explored, with a few observational studies [51, 52] reporting an association between diet quality and this independent risk factor for CVD and all-cause mortality [53]. A large study on over 50,000 US men and women [54] found that higher resting heart rate was associated with an increased risk of overall cancer mortality, through mechanisms that include increased sympathetic nerve activity, which is reflected by higher heart rate, and that might contribute to the initiation and progression of cancer through β-adrenergic signalling on the regulation of multiple cellular processes [55]. Also, dysregulation of the sympathetic nervous system and hypothalamic–pituitary–adrenal axis may promote angiogenesis, tumour cell proliferation and survival, alteration of the immune response and exacerbate inflammatory networks in the tumour microenvironment [56]. Finally, subgroup analyses indicated that the inverse association of olive oil intake with all-cause and cancer mortality was limited to non-obese participants; this finding is relatively novel, since prior cohort studies in this field failed to observe an effect modification of BMI [7, 15, 17, 57]. Potential explanations include that obese individuals tend to underreport dietary intakes to a greater extent than non-obese individuals [58], and this might have biased the results. Moreover, BMI could be on the causal pathway between olive oil and health outcomes. However, future research is warranted to elucidate potential biological mechanisms underpinning the observed interactions between BMI and olive oil.
Strengths and limitations of the study
Strengths of the present study include a well-defined Italian Mediterranean population, where we collected information about dietary, socioeconomic and lifestyle factors using standardised and validated questionnaires, allowing to minimise sources of bias and confounding. Moreover, the robustness of our findings was reinforced by sensitivity and subgroup analyses.
The current study has, however, several limitations that need to be mentioned. First, this is an observational study and therefore causality cannot be inferred. Second, dietary data were self-reported with consequent potential measurement errors, recall and selection bias. Third, dietary information and laboratory data were obtained at baseline only, thus potential changes occurring over life course might have modified the strength of the findings; nevertheless, there is some evidence that diet in adulthood tends to remain stable over time [59] as well as most of the biomarkers here tested were not found to vary substantially over time [60]. Fourth, we were not able to distinguish different types of olive oil consumed by our participants. Although this is a common limitation of most studies in this field [57], the effect of olive oil on human health might vary according to the type, mainly because of different content in bioactive compounds [15]. Also, we acknowledge that results on cancer mortality should be interpreted carefully, considering that we incorporated different cancer types with heterogeneous prognosis.
Moreover, we acknowledge that once a condition (e.g., cancer) has been diagnosed, survival depends on various factors, including specific treatment efficacy and timing of diagnosis, which are not directly related to the health effects of olive oil consumption. However, it is unlikely that these factors could have acted as confounders of the association between olive oil and survival; for instance, it is unlikely that high olive oil consumers had earlier diagnosis than participants consuming less olive oil.
Finally, caution is warranted in generalising these findings to other populations.
Conclusions
In this large cohort of Italian Mediterranean adults, higher consumption of olive oil was associated with lower risk of cancer, cardiovascular and all-cause mortality, independent of the overall quality of the diet. The maximum benefit was observed at intakes higher than three tablespoons per day which correspond to approximately >30 g of olive oil per day.
Part of the inverse relationship between olive oil consumption and cancer mortality was explained by well-established CVD risk factors (i.e., blood pressure and resting heart rate), that might also be involved in cancer onset and progression [31, 61], therefore, reinforcing the notion of a ‘common soil’ of underlying risk factors from which these two groups of diseases originate.
However, these results do not necessarily prove causality of the observed associations since the selected mediators may merely be markers of pathogenic processes, not necessarily being on the causal pathway to mortality.
Our findings suggest to preserve or encourage the daily use of olive oil within a traditional MD, but future studies are warranted to increase knowledge on the mechanistic pathways involved.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. The data are stored in an institutional repository (https://repository.neuromed.it), and access is restricted by the ethical approvals and the legislation of the European Union.
References
Trichopoulou A, Martínez-González MA, Tong TY, Forouhi NG, Khandelwal S, Prabhakaran D, et al. Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med. 2014;12:112. https://doi.org/10.1186/1741-7015-12-112
Trichopoulou A. Olive oil, Greek Mediterranean diet heritage and honoring the past to secure our future: priorities for research and education. Front Nutr. 2022;9:1058402. https://doi.org/10.3389/fnut.2022.1058402
Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, et al. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients. 2019;11:1776. https://doi.org/10.3390/nu11081776
Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, Lopez-Miranda J. Extra virgin olive oil: more than a healthy fat. Eur J Clin Nutr. 2019;72:8–17.
Carluccio MA, Massaro M, Scoditti E, De Caterina R. Vasculoprotective potential of olive oil components. Mol Nutr Food Res. 2007;51:1225–34. https://doi.org/10.1002/mnfr.200600305
Jiménez-Sánchez A, Martínez-Ortega AJ, Remón-Ruiz PJ, Piñar-Gutiérrez A, Pereira-Cunill JL, García-Luna PP. Therapeutic properties and use of extra virgin olive oil in clinical nutrition: a narrative review and literature update. Nutrients. 2022;14:1440.
Buckland G, Mayén AL, Agudo A, Travier N, Navarro C, Huerta JM, et al. Olive oil intake and mortality within the Spanish population (EPIC–Spain). Am J Clin Nutr. 2012;96:142–9.
Guasch-Ferré M, Li Y, Willett WC, Sun Q, Sampson L, Salas-Salvadó J, et al. Consumption of olive oil and risk of total and cause-specific mortality among U.S. adults. J Am Coll Cardiol. 2022;79:101–12.
Markellos C, Ourailidou ME, Gavriatopoulou M, Halvatsiotis P, Sergentanis TN, Psaltopoulou T. Olive oil intake and cancer risk: a systematic review and meta-analysis. PLoS ONE. 2022;17:e0261649.
Psaltopoulou T, Kosti RI, Haidopoulos D, Dimopoulos M, Panagiotakos DB. Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Heal Dis. 2011;10:127.
Martínez-González MA, Sayón-Orea C, Bullón-Vela V, Bes-Rastrollo M, Rodríguez-Artalejo F, Yusta-Boyo MJ, et al. Effect of olive oil consumption on cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality: a systematic review and meta-analysis. Clin Nutr. 2022;41:2659–82. https://doi.org/10.1016/j.clnu.2022.10.001.
Xia M, Zhong Y, Peng Y, Qian C. Olive oil consumption and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Front Nutr. 2022;9:1041203. https://doi.org/10.3389/fnut.2022.1041203.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34.
Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med. 2015;175:1752–60. https://doi.org/10.1001/jamainternmed.2015.4838.
Donat-Vargas C, Lopez-Garcia E, Banegas JR, Martínez-González MÁ, Rodríguez-Artalejo F, Guallar-Castillón P. Only virgin type of olive oil consumption reduces the risk of mortality. Results from a Mediterranean population-based cohort. Eur J Clin Nutr. 2023;77:226–234. https://doi.org/10.1038/s41430-022-01221-3.
Guasch-Ferré M, Hu FB, Martínez-González MA, Fitó M, Bulló M, Estruch R, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. https://doi.org/10.1186/1741-7015-12-78.
Zhang Y, Zhuang P, Wu F, He W, Mao L, Jia W, et al. Cooking oil/fat consumption and deaths from cardiometabolic diseases and other causes: prospective analysis of 521,120 individuals. BMC Med. 2021;19:92. https://doi.org/10.1186/s12916-021-01961-2
Torres-Collado L, García-de la Hera M, Lopes C, Compañ-Gabucio LM, Oncina-Cánovas A, Notario-Barandiaran L, et al. Olive oil consumption and all-cause, cardiovascular and cancer mortality in an adult Mediterranean population in Spain. Front Nutr. 2022;9:997975. https://doi.org/10.3389/fnut.2022.997975
Iacoviello L, Bonanni A, Costanzo S, De Curtis A, Di Castelnuovo A, Olivier M, et al. The Moli-Sani Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Ital J Public Health. 2007;4:110–8.
Bonaccio M, Di Castelnuovo A, Ruggiero E, Costanzo S, Grosso G, De Curtis A, et al. Joint association of food nutritional profile by Nutri-Score front-of-pack label and ultra-processed food intake with mortality: Moli-sani prospective cohort study. BMJ. 2022;378:e070688. https://doi.org/10.1136/bmj-2022-070688
Bonaccio M, Di Castelnuovo A, Costanzo S, Ruggiero E, De Curtis A, Persichillo M, et al. Chili pepper consumption and mortality in Italian adults. J Am Coll Cardiol. 2019;74:3139–49. https://doi.org/10.1016/j.jacc.2019.09.068
Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:S152–60.
Pala V, Sieri S, Palli D, Salvini S, Berrino F, Bellegotti M, et al. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori. 2003;89:594–607.
Salvini S, Parpinel M, Gnagnarella P, Maissoneuve P, Turrini A. Banca dati composizione degli alimenti per studi epidemiologici in Italia. Milano (Italy): European Institute of Oncology; 1998.
Centro di ricerca Alimenti e Nutrizione del Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria (CREA) per l’elaborazione delle “Linee Guida per una Sana Alimentazione Italiana” – Revisione 2019, Rome, Italy. https://www.crea.gov.it/documents/59764/0/LINEE-GUIDA+DEFINITIVO.pdf/28670db4-154c-0ecc-d187-1ee9db3b1c65?t=1576850671654. Accessed April 2023.
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608.
ISTAT: Istituto Nazionale di Statistica, Atlante statistico dei comuni. Edizione 2014. https://www.istat.it/it/archivio/113712. Accessed Nov 2022.
Mannocci A, Di Thiene D, Del Cimmuto A, Masala D, Boccia A, De Vito E, et al. International Physical Activity Questionnaire: validation and assessment in an Italian sample. Ital J Public Health. 2010;7:369–76.
Zeller T, Hughes M, Tuovinen T, Schillert A, Conrads-Frank A, Ruijter HD, et al. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol. 2014;29:777–90.
Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121:2388–97.
Narayan V, Thompson EW, Demissei B, Ho JE, Januzzi JL Jr, Ky B. Mechanistic biomarkers informative of both cancer and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2726–37. https://doi.org/10.1016/j.jacc.2020.03.067
MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614.
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82.
Morvaridzadeh M, Cohen AA, Heshmati J, Alami M, Berrougui H, Zoubdane N, et al. Effect of Extra Virgin Olive Oil on Anthropometric Indices, Inflammatory and Cardiometabolic Markers: a Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Nutr. 2024;154:95–120. https://doi.org/10.1016/j.tjnut.2023.10.028.
Wang Y, Wang Y, Han X, Sun J, Li C, Adhikari BK, et al. Cardio-oncology: a myriad of relationships between cardiovascular disease and cancer. Front Cardiovasc Med. 2022;9:727487. https://doi.org/10.3389/fcvm.2022.727487
Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. Harvard T.H. Chan School of Public Health, Boston, 2012.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57.
Donat-Vargas C, Sandoval-Insausti H, Peñalvo JL, Moreno Iribas MC, Amiano P, Bes-Rastrollo M, et al. Olive oil consumption is associated with a lower risk of cardiovascular disease and stroke. Clin Nutr. 2022;41:122–30. https://doi.org/10.1016/j.clnu.2021.11.002.
Sales-Campos H, Souza PR, Peghini BC, da Silva JS, Cardoso CR. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem. 2013;13:201–10.
Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. https://doi.org/10.3390/ijms19030686
Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78:133–54. https://doi.org/10.3797/scipharm.0912-18
Benavente-Garcıa O, Castillo J, Lorente J, Ortuno A, del Rio J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68:457–62. https://doi.org/10.1016/S0308-8146(99)00221-6
Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. https://doi.org/10.1111/j.1538-7836.2010.04131
Yang CS, Luo P, Zeng Z, Wang H, Malafa M, Suh N. Vitamin E and cancer prevention: studies with different forms of tocopherols and tocotrienols. Mol Carcinog. 2020;59:365–89. https://doi.org/10.1002/mc.23160
Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. https://doi.org/10.1146/annurev.nutr.24.012003.132446
Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14:e157–65.
Donati MB. The “common soil hypothesis”: evidence from population studies? Thromb Res. 2010;125:S92–5. https://doi.org/10.1016/S0049-3848(10)70023-2
Iacoviello L, Bonaccio M, de Gaetano G, Donati MB. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin Cancer Biol. 2021;72:4–10. https://doi.org/10.1016/j.semcancer.2020.02.010.
Mohammed T, Singh M, Tiu JG, Kim AS. Etiology and management of hypertension in patients with cancer. Cardiooncology. 2021;7:14. https://doi.org/10.1186/s40959-021-00101-2
Colt JS, Schwartz K, Graubard BI, Davis F, Ruterbusch J, DiGaetano R, et al. Hypertension and risk of renal cell carcinoma among White and Black Americans. Epidemiology. 2011;22:797–804. https://doi.org/10.1097/EDE.0b013e3182300720
García-López M, Toledo E, Beunza JJ, Aros F, Estruch R, Salas-Salvadó J, et al. Mediterranean diet and heart rate: the PREDIMED randomised trial. Int J Cardiol. 2014;171:299–301.
García-López M, Martínez-González MA, Basterra-Gortari FJ, Barrio-López MT, Gea A, Beunza JJ. Adherence to the Mediterranean dietary pattern and heart rate in the SUN project. Eur J Prev Cardiol. 2014;21:521–7. https://doi.org/10.1177/2047487312467871
Jensen MT, Marott JL, Allin KH, Nordestgaard BG, Jensen GB. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: the Copenhagen City Heart Study. Eur J Prev Cardiol. 2012;19:102–8.
Gutierrez-Martinez L, Brellenthin AG, Lefferts EC, Lee DC, Sui X, Lavie CJ, et al. Resting heart rate and risk of cancer mortality. Cancer Epidemiol Biomark Prev. 2021;30:1072–8. https://doi.org/10.1158/1055-9965.EPI-20-1731
Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–6. https://doi.org/10.1158/1078-0432.CCR-11-0641
Colon-Echevarria CB, Lamboy-Caraballo R, Aquino-Acevedo AN, Armaiz-Pena GN. Neuroendocrine regulation of tumor-associated immune cells. Front Oncol. 2019;9:1077. https://doi.org/10.3389/fonc.2019.01077
Guasch-Ferré M, Liu G, Li Y, Sampson L, Manson JE, Salas-Salvadó J, et al. Olive oil consumption and cardiovascular risk in U.S. adults. J Am Coll Cardiol. 2020;75:1729–39. https://doi.org/10.1016/j.jacc.2020.02.036
Wehling H, Lusher J. People with a body mass index ⩾30 under-report their dietary intake: a systematic review. J Health Psychol. 2019;24:2042–59. https://doi.org/10.1177/1359105317714318.
Edefonti V, De Vito R, Salvatori A, Bravi F, Patel L, Dalmartello M, et al. Reproducibility of a posteriori dietary patterns across time and studies: a scoping review. Adv Nutr. 2020;11:1255–81.
Al-Delaimy WK, Jansen EH, Peeters PH, van der Laan JD, van Noord PA, Boshuizen HC, et al. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers. 2006;11:370–82.
Lau ES, Paniagua SM, Liu E, Jovani M, Li SX, Takvorian K, et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol. 2021;3:48–58. https://doi.org/10.1016/j.jaccao.2020.12.003
Acknowledgements
The present study has been performed in the context of the Fondazione Umberto Veronesi – IRCCS Neuromed framework agreement. We are grateful to the population of the Molise region who enthusiastically joined the study and wish to thank the Associazione Cuore Sano ETS (Campobasso, Italy) for its cultural support, and the Moli-sani Study Investigators (full list available in the Supplementary Information file). ER was supported by the Fondazione Umberto Veronesi, which is gratefully acknowledged. The enrolment phase of the Moli-sani Study was supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy) – Programma Triennale di Ricerca, Decreto no. 1588 and Instrumentation Laboratory, Milan, Italy.
Funding
The analyses reported here were supported by the Italian Ministry of Health (Ricerca Corrente 2022–2024). Funders had no role in the study design, collection, analysis, and interpretation of data, nor in the writing of the manuscript or in the decision to submit the article for publication. All authors were and are independent from funders.
Author information
Authors and Affiliations
Consortia
Contributions
MB and ER conceived the present study, analysed the data and drafted the manuscript; LI, MB, ER and ADiC contributed to its design and to interpretation of data; SC, SE and MP managed data collection; ADeC organised and performed laboratory tests; MBD, CC, GdG and LI originally inspired the Moli-sani Study and critically reviewed this manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruggiero, E., Di Castelnuovo, A., Costanzo, S. et al. Olive oil consumption is associated with lower cancer, cardiovascular and all-cause mortality among Italian adults: prospective results from the Moli-sani Study and analysis of potential biological mechanisms. Eur J Clin Nutr 78, 684–693 (2024). https://doi.org/10.1038/s41430-024-01442-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-024-01442-8
- Springer Nature Limited