Abstract
Objective
Attention Deficit/Hyperactivity Disorder (ADHD) is a common behavioral disorder among children. Based on literature, it has been hypothesized that the higher intake of rich sources of phytochemicals may be inversely related to the risk of ADHD. We investigated the association of dietary phytochemical index (DPI) with odds of ADHD.
Methods
This case-control study was conducted on 360 children and adolescents 7–13 years old in Yazd, Iran. Subjects were categorized into the case (n = 120) and control groups (n = 240) based on matching age and sex. To diagnose ADHD, the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSMIV-TR) was used. Food frequency questionnaire was used to measure food intake. DPI was calculated by percent of daily energy intake from phytochemical-rich foods. The association of DPI with the odds ratio of ADHD was examined by logistic regression.
Results
Subjects in the highest quartile of DPI have higher intake of macronutrient, eicosatetraenoic acid, docosahexaenoic acid, calcium, zinc, iron, vitamins B12, B6, and folic acid compared to lowest quartile. After adjusting for potential confounders, subjects in the highest quartile of DPI compared with subjects in the lowest quartile showed a lower risk of ADHD (OR: 0.44; 95% CI: 0.18–0.90). There was a significant decreasing trend in the odds of ADHD across increasing quartile of DPI (P for trend: 0.02).
Conclusion
We found that higher DPI score is associated with lower risk of ADHD in children. Cohort and clinical studies are necessary to approve our results.
Similar content being viewed by others
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common behavioral disorders in children [1]. The global prevalence of ADHD among children and adolescents is reported to be 5% [2]. Its prevalence among boys and girls of Iran has been estimated to be 5.03% and 2.79%, respectively [3]. The prevalence of ADHD is increasing and imposes high socioeconomic costs on health care as well as educational systems, and reduces children’s quality of life [4, 5]. Although the pathogenesis of ADHD is not fully clear, both genetic [6] and environmental factors such as brain damage, prenatal exposures, and unhealthy dietary patterns are the main risk factors [7]. Diet modifications plus pharmacological treatments have been proposed in the treatment of ADHD [8].
Evidence suggested that quality of diet plays an important role in the health status of hyperactive children [9]. Diet modifications including low intake of refined sugars, food additives, and allergenic foods, as well as high intake of rich sources of antioxidants such as fruits and vegetables can reduce severity of ADHD symptoms [10]. Phytochemicals are health-protective compounds with antioxidant properties and are found in high amounts in plant foods such as vegetables, fruits, whole grains, nuts and legumes [11]. Phenolic acids, carotenoids, terpenoids, allium, and phytosterols are the important phytochemicals [12]. Oxidative stress and inflammation are involved in the pathogenesis of chronic diseases, and phytochemicals by eliminating oxidant agents and scavenging free radicals as well as inhibiting inflammatory markers activity can exert their protective effects [13, 14]. Furthermore, evidence reported the neuroprotective properties of phytochemicals [15]. Previous studies indicated that consumption of phytochemical-rich foods inversely is associated with severity of ADHD [16, 17]. Considering the beneficial effects of phytochemicals on health status, the dietary phytochemical index (DPI) was presented by McCarty [18]. Previous studies have investigated the relationship between DPI and health status [19]. For example, a study found that women with higher DPI scores had a lower risk of depression, anxiety, and psychological distress [19]. Another study reported that a higher DPI score significantly decreased risk of cardio-metabolic disorders [20]. Evidence confirmed that oxidative stress and inflammation are important factors in the pathogenesis of psychiatric disorders [21].
Due to the anti-inflammatory and antioxidant properties of phytochemical-rich foods [22], it has been hypothesized that high DPI scores can play a protective role in the prevention and management of behavioral diseases. To the best of our knowledge, there is no study evaluating the relationship between DPI and ADHD. Moreover, the efficacy of phytochemicals in the treatment of ADHD is not fully clear. Accordingly, we designed the present case control study to investigate the relationship between dietary phytochemicals content using DPI and ADHD among school-age Iranian children.
Materials and methods
Study design and population
In this age and gender-matched case-control study (approved by the research council of Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences and Health Services in Yazd, Iran), 120 primary school students diagnosed with ADHD were recruited from two referral clinics of Shahid Sadoughi University of Medical Sciences. Cases were assessed and diagnosed based on Text Revision of Statistical Manual of Mental Disorders-Fourth Edition criteria (DSM-IV TR) [23]. For each case, two healthy controls were enrolled from primary schools in Yazd, from the same urban region of the cases. Exclusion criteria for controls were as follows: adherence to a specific diet in the past year, psychological diseases, premature, parental addiction at birth. A total of 360 primary school students (120 students with ADHD and 240 healthy controls) from 6 to 13 years old were selected and their parents signed a written informed consent approved by the ethical committee of Shahid Sadoughi University of Medical Sciences and Health Services in Yazd, under code: IR.SSU.SPH.REC.1395.158.
Dietary assessment
Dietary intakes were assessed using a validated semi-quantitative food-frequency questionnaire (FFQ) containing 186 food items [24]. The validity of the FFQ for nutrients was confirmed in the two cohort studies in Iran [24, 25]. Questionnaires were filled in the presence of the children and their parents by a trained nutritionist to estimate the children’s food intake inside and outside the home. To record the frequency of food items consumption during the previous year, parents were asked about their children’s intake (never, daily, weekly, monthly, and yearly). Portion sizes were converted to grams using household scales [25]. The Nutritionist IV software was used to extract the energy and nutrients intake.
Phytochemical index calculation
The DPI was computed based on the method presented by McCarty in 2004 as follows: DPI = (daily energy derived from phytochemical-rich foods (kcal)/total daily energy intake (kcal)) × 100 [18]. Fruits, vegetables, legumes, whole grains, nuts, soy products, seeds, and olive oil were considered as phytochemical-rich foods. The phytochemicals content of potatoes is low and potatoes were not included. Due to the high phytochemical content of natural fruit and vegetable juices as well as tomato sauces, these sources of phytochemicals were included in the fruit and vegetable groups [26].
Anthropometric assessment
Weight was measured using the Seca scale (made in Germany, with an accuracy of 100 g) with minimal clothing and no shoes. Height was measured by a tape (with an accuracy of 0.5 cm) in standing position without shoes. Body mass index (BMI) was calculated as weight (kg)/height squared (m2).
ADHD detection
ADHD was diagnosed using DSM-IV TR criteria by a pediatric neurologist [27]. Based on DSM-IV TR criteria, a child with ADHD must have at least six symptoms of inattention or hyperactivity–impulsivity for at least 6 months to an extent that is disruptive and inappropriate for the person’s developmental level [27].
Assessment of other variables
General characteristics including age, medical history, current use of medications, family history of ADHD, economic situation, parental educational level were obtained in the presence of the children and their parents using a demographic questionnaire. Physical activity was assessed by a validated questionnaire [28, 29]. The level of physical activity was classified to less than once per week, 2–3 times per week, 3–5 times per week, and more than 5 times per week. Family history of ADHD and taking ADHD medications were assessed by asking questions from parents.
Statistical analysis
To compare the mean differences of quantitative and qualitative variables across DPI of quartiles categories, the one-way analysis of variance and chi-square tests were performed, respectively. To determine the odds of ADHD in each quartile of DPI, the binary logistic regressions were used in crude and multivariable-adjusted models. Adjustment for confounding variables including total energy intake, family history of, and physical activity was performed in the first model. Additional adjustment for BMI was done in the second model. Statistical analysis was performed using SPSS version 23. Finally, P value < 0.05 was considered as the significant level.
Results
Characteristics of the study population
The characteristics of the study participants are represented in Table 1. The mean age of the children was 8.7 years. There was no significant difference between children with ADHD and controls in the Parents’ educational level, economic status, and quartiles of DPI. There were significant differences between case and control groups in physical activity and family history of ADHD. The family history of ADHD in children with ADHD was higher than controls.
Dietary intakes of participants
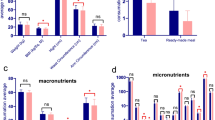
Some food groups and nutrient intakes in quartiles of DPI are shown in Table 2. Children in the highest quartile of DPI compared to lowest quartile had higher intake of macronutrient, eicosapentaenoic acid, docosahexaenoic acid, calcium, zinc, iron, vitamin B12, B6, and folic acid. Moreover, intake of fruits, vegetables, whole grain, and legumes in fourth quartile was higher than first quartile.
ADHD and DPI score
Crude and multivariate-adjusted odds ratios for ADHD across quartiles of DPI are represented in Table 3. There was a significant protective association between DPI and risk of ADHD (OR: 0.79; 95% CI: 0.64–0.97) in crude model. Moreover, a significant decreasing trend in the odds of ADHD across increasing quartiles of the DPI (P-trend = 0.02) was observed. In addition, children in the fourth quartile of DPI had 58% lower odds of ADHD than the first quartile. This association remained significant after adjusting potential confounders including energy intake, BMI, family history of ADHD, physical activity, and (OR: 0.78; 95% CI: 0.61–0.98; p for trend: 0.03).
Discussion
To the best of authors’ knowledge, the present study was the first case-control study investigating the association between DPI and the odds of ADHD. Our findings showed that after adjusting for potential confounders, the higher score of DPI can decrease the odds of ADHD. Recent studies have been examined the association between food groups and dietary patterns with ADHD [30, 31]. Healthy dietary patterns containing rich sources of phytochemicals such as food grain, wheat, beans, vegetables, and fresh fruit/vegetable juices have a protective role in ADHD [32]. Moreover, evidence suggested that higher intake of fruits and vegetables is associated with lower risk of ADHD in children [31, 33]. Contrary to our findings, some studies found no association between the risk of ADHD and “healthy” as well as “traditional” dietary patterns containing high amounts of condiments, vegetables, tofu/soymilk, mushrooms, fruits, vegetable oils, whole grains, legumes, and dairy products [34, 35]. A logical reason for the discrepancy between the results of these studies and our findings is that some food groups of these patterns are not a phytochemical source and therefore, are not used in the calculation of DPI. Moreover, differences in adjusted confounder factors between previous studies and our study, probably is another reason for this discrepancy. There are some hypotheses about the etiology of relation between DPI and ADHD. Evidence showed that oxidative stress is one of the important factors in the pathogenesis of ADHD [36]. A meta-analysis confirmed that children with ADHD have insufficient response to the oxidative stress [36, 37]. Phytochemicals are the powerful antioxidants that can improve oxidative stress by reducing lipid peroxidation and inhibiting production of free radicals [38]. In addition, vegetables and fruits are rich sources of vitamin C and folate as the essential factors in the synthesis of dopamine, norepinephrine, and serotonin. Studies demonstrated that children with ADHD have decreased levels of dopamine and norepinephrine and also disturbed catecholamine neurotransmission [39, 40]. As a result, children’s diet preferences may be changed [41]. The change in the eating habits of ADHD children is a probable reason for these results. It has been reported that phytochemicals have multiple neurotrophic and neuroprotective roles [42]. Some studies reported the association between ADHD and inflammation [43,44,45,46]. Inflammatory markers such as interleukin-1β and interleukin-6 (IL-6) can lead to neurotransmission changes in patients with ADHD [47]. Evidence showed that polyphenols of fruits and vegetables such as quercetin and resveratrol can reduce IL-6, TNF-α, hs-CRP, and IL-10 levels [48,49,50]. Nuts, which are used in the calculation of DPI, are important sources of omega-3 fatty acids [51]. Evidence reported that omega-3 fatty acids are necessary for normal function of brain, and low intake of omega-3 may contribute to the pathogenesis of ADHD [52, 53].
The present study has several strengths. The case and control groups were matched for age and sex. In addition, we controlled the important confounders. We selected the newly diagnosed children with ADHD, thereby, the children did not change their dietary patterns. The FFQ has been filled out by trained dietitian that was blind about the groups of case and control. However, our study has some limitations. First, the case-control design cannot report the causal association between the DPI and ADHD. Second, in the design of case–control, individual’s health status influence food intake.
In conclusion, there is an inverse relationship between DPI score with risk of ADHD in children. To clarify our vision, cohort and clinical studies in this field are required.
References
Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry. 1999;46:1234–42.
Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5:175–86.
Mohammadi MR, Ahmadi N, Salmanian M, Asadian-Koohestani F, Ghanizadeh A, Alavi A, et al. Psychiatric disorders in Iranian children and adolescents. Iran J Psychiatry. 2016;11:87.
Pawaskar M, Fridman M, Grebla R, Madhoo M. Comparison of quality of life, productivity, functioning and self-esteem in adults diagnosed with ADHD and with symptomatic ADHD. J Atten Disord. 2020:1087054719841129.
Gupte-Singh K, Singh RR, Lawson KA. Economic burden of attention-deficit/hyperactivity disorder among pediatric patients in the United States. Value Health. 2017;20:602–9.
Thapar A, Langley K, Asherson P, Gill M. Gene–environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. Br J Psychiatry. 2007;190:1–3.
Mash EJ, Barkley RA. Assessment of childhood disorders: Guilford Press; 2009.
Lange KW. Micronutrients and diets in the treatment of attention-deficit/hyperactivity disorder: chances and pitfalls. Front Psychiatry. 2020;11:102.
Rucklidge JJ, Taylor MR, Johnstone J. Do diet and nutrition affect ADHD? Facts and clinical considerations. Psychiatr Times. 2018;35:15–6.
Schnoll R, Burshteyn D, Cea-Aravena J. Nutrition in the treatment of attention-deficit hyperactivity disorder: a neglected but important aspect. Appl Psychophysiol Biofeedback 2003;28:63–75.
Winston C, Beck L. Phytochemicals: health protective effects. Can J Dietetic Pract Res. 1999;60:78.
Sakthinathan B, Nandhini U. Phytochemicals—a nutraceutical source of vegetables. Chem Sci Rev Lett. 2017;6:2133–7.
Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr. 2018;58:1260–70.
Zhang Y-J, Gan R-Y, Li S, Zhou Y, Li A-N, Xu D-P, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–56.
Kumar GP, Anilakumar K, Naveen S. Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn J. 2015;07:01–17.
Curtis LT, Patel K. Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): a review. J Altern Complementary Med. 2008;14:79–85.
dela Peña IC, Yoon SY, Kim Y, Park H, Kim KM, Ryu JH, et al. 5, 7-Dihydroxy-6-methoxy-4’-phenoxyflavone, a derivative of oroxylin A improves attention-deficit/hyperactivity disorder (ADHD)-like behaviors in spontaneously hypertensive rats. Eur J Pharmacol. 2013;715:337–44.
McCarty MF. Proposal for a dietary “phytochemical index”. Med Hypotheses 2004;63:813–7.
Mofrad MD, Siassi F, Guilani B, Bellissimo N, Azadbakht L. Association of dietary phytochemical index and mental health in women: a cross-sectional study. Br J Nutr. 2019;121:1049–56.
Bahadoran Z, Golzarand M, Mirmiran P, Saadati N, Azizi F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J Hum Nutr Diet. 2013;26:145–53.
Firth J, Veronese N, Cotter J, Shivappa N, Hebert J, Ee C, et al. What is the role of dietary inflammation in severe mental illness? a review of observational and experimental findings. Front Psychiatry. 2019;10:350.
Serafini M, Peluso I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr Pharm Des. 2016;22:6701–15.
Carter MJ. Diagnostic and statistical manual of mental disorders. Therapeutic Recreation Journal. 2014;48:275
Salehi-Abargouei A, Zimorovat A, Moghtaderi F, Amiri M, Raeisi-Dehkordi H, Mohyadini M, et al. Validity and reproducibility of a semi-quantitative multiple-choice food frequency questionnaire in adults living in central Iran. Res Sq. 2020; https://doi.org/10.21203/rs.3.rs-15529/v1.
Asghari G, Rezazadeh A, Hosseini-Esfahani F, Mehrabi Y, Mirmiran P, Azizi F. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br J Nutr 2012;108:1109–17.
Farhangi MA, Najafi M, Jafarabadi MA, Jahangiry L. Mediterranean dietary quality index and dietary phytochemical index among patients candidate for coronary artery bypass grafting (CABG) surgery. BMC Cardiovasc Disord. 2017;17:114.
Owens J, Hoza B. Diagnostic utility of DSM-IV-TR symptoms in the prediction of DSM-IV-TR ADHD subtypes and ODD. J Atten Disord. 2003;7:11–27.
Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35:1196–202.
Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN Study. Bull World Health Organ. 2007;85:19–26.
Ríos-Hernández A, Alda JA, Farran-Codina A, Ferreira-García E, Izquierdo-Pulido M. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. 2017;139:e20162027.
Kim KM, Lim MH, Kwon H-J, Yoo S-J, Kim E-J, Kim JW. et al. Associations between attention-deficit/hyperactivity disorder symptoms and dietary habits in elementary school children. Appetite. 2018;127:274–9.
Yan S, Cao H, Gu C, Ni L, Tao H, Shao T, et al. Dietary patterns are associated with attention-deficit/hyperactivity disorder (ADHD) symptoms among preschoolers in mainland China. Eur J Clin Nutr. 2018;72:1517–23.
Wang L-J, Yu Y-H, Fu M-L, Yeh W-T, Hsu J-L, Yang Y-H, et al. Dietary profiles, nutritional biochemistry status, and attention-deficit/hyperactivity disorder: path analysis for a case-control study. J Clin Med. 2019;8:709.
Azadbakht L, Esmaillzadeh A. Dietary patterns and attention deficit hyperactivity disorder among Iranian children. Nutrition. 2012;28:242–9.
Woo HD, Kim DW, Hong Y-S, Kim Y-M, Seo J-H, Choe BM, et al. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients. 2014;6:1539–53.
Oztop D, Altun H, Baskol G, Ozsoy S. Oxidative stress in children with attention deficit hyperactivity disorder. Clin Biochem. 2012;45:745–8.
Joseph N, Zhang-James Y, Perl A, Faraone SV. Oxidative stress and ADHD: a meta-analysis. J Atten Disord. 2015;19:915–24.
Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42.
Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–e57.
Wallace DL, Aarts E, Uquillas FdO, Dang LC, Greer SM, Jagust WJ. et al. Genotype status of the dopamine-related catechol-O-methyltransferase (COMT) gene corresponds with desirability of “unhealthy” foods. Appetite. 2015;92:74–80.
Bahramsoltani R, Farzaei MH, Farahani MS, Rahimi R. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci. 2015;26:699–719.
Oades RD, Dauvermann MR, Schimmelmann BG, Schwarz MJ, Myint A-M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism-effects of medication. Behav Brain Funct. 2010;6:29.
Misener VL, Schachar R, Ickowicz A, Malone M, Roberts W, Tannock R, et al. Replication test for association of the IL-1 receptor antagonist gene, IL1RN, with attention-deficit/hyperactivity disorder. Neuropsychobiology. 2004;50:231–4.
Lasky‐Su J, Anney RJ, Neale BM, Franke B, Zhou K, Maller JB, et al. Genome‐wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet Part B: Neuropsychiatr Genet. 2008;147:1355–8.
Lasky‐Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, et al. Genome‐wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet Part B: Neuropsychiatr Genet. 2008;147:1345–54.
Anand D, Colpo GD, Zeni G, Zeni CP, Teixeira AL. Attention-deficit/hyperactivity disorder and inflammation: what does current knowledge tell us? A systematic review. Front Psychiatry. 2017;8:228.
Lapuente M, Estruch R, Shahbaz M, Casas R. Relation of fruits and vegetables with major cardiometabolic risk factors, markers of oxidation, and inflammation. Nutrients. 2019;11:2381.
Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–12.
Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–31.
Vos E, Weir E. Nuts, omega-3s and food labels. Circulation. 1999;99:733–5.
Richardson AJ. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int Rev Psychiatry. 2006;18:155–72.
Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins, leukotrienes Essent Fat Acids. 2006;75:299–308.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darabi, Z., Sangouni, A.A., Darand, M. et al. Dietary phytochemical index and attention-deficit/hyperactivity disorder in Iranian children: a case control study. Eur J Clin Nutr 76, 456–461 (2022). https://doi.org/10.1038/s41430-021-00952-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-00952-z
- Springer Nature Limited
This article is cited by
-
Nutrition in the Management of ADHD: A Review of Recent Research
Current Nutrition Reports (2023)
-
The association between dietary phytochemical index with depression and quality of life in iranian adolescent girls
BioPsychoSocial Medicine (2022)