Abstract
Background
The analysis of computerised tomography (CT) images to provide body composition data has grown exponentially. Despite this, there remains limited published data defining the normal range of skeletal muscle area and adipose tissue area using CT. The aim of this study was to determine age- and sex-specific body composition values using CT at the level of the third lumbar vertebrae, in a Caucasian population with a healthy body mass index (BMI). In addition, we sought to develop threshold values for low skeletal muscle mass using these data.
Methods
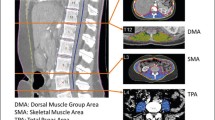
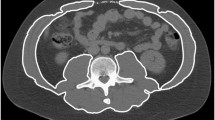
We included 107 healthy Caucasian patients (46 males; 61 females) with a healthy BMI (18–25 kg/m2) for analysis. Body composition data were obtained from a single transverse CT image at the mid-third lumbar vertebrae using ImageJ software. Tissue segmentation was performed using Hounsfield unit thresholds of −29 to +150 for muscle and −190 to −30 for adipose tissue.
Results
The mean age of the study cohort was 47.8 ± 11.0 years (range 21–73) with a median BMI of 23.7 kg/m2 (interquartile range 22.3–24.8). Patients were sub-divided into age above or below 50 years. Cut-offs for low muscle quantity, representing two standard deviations below the young healthy population mean values, were 43.5 cm2/m2 for males and 30.0 cm2/m2 for females.
Conclusions
Our data provide an insight into the distribution of skeletal muscle and adipose tissue surface area values measured on CT from a healthy Caucasian population. Our CT-derived cut-offs for low muscle quantity, based on international guidelines, are much lower than those previously suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Loss of skeletal muscle mass (SMM) and quality have a significant impact on health outcomes across a broad range of populations [1,2,3,4]. With the increasing availability of computed tomography (CT) for assessment of illness, the utilisation of this imaging modality to provide information on body composition has grown exponentially. In 2004, Shen et al. presented data from a healthy cohort that demonstrated skeletal muscle and adipose tissue surface area on a single slice of cross-sectional magnetic resonance imaging (MRI) strongly correlates with total body MRI muscle and fat stores [5].
In 2008, Prado et al. measured skeletal muscle area at the level of the third lumbar vertebrae and corrected for height (in metres) squared, to provide the skeletal muscle index (SMI). Sex-specific low SMI cut-offs associated with mortality were published in a population of obese patients with cancer [6]. Sex-specific low SMI cut-offs associated with mortality were published in a population of obese patients with cancer [6]. Subsequently, many researchers have used these cut-offs to define low muscle quantity in studies examining the relationship of SMM to morbidity and mortality in different populations [3, 7,8,9].
However, normative data using CT are scarce to confirm that these thresholds are in accordance with guidelines which define low muscle quantity as two standard deviations (SD) below the age- and sex-specific mean values [10,11,12,13]. The European Working Group on Sarcopenia in Older People (EWGSOP) recommends this definition as part of a multi-component method (muscle quantity, muscle strength and physical performance) of diagnosing ‘sarcopenia’ [10].
There is also a need to define the distribution of adipose tissue in healthy populations. The Japan Society for the Study of Obesity has suggested that a visceral adipose tissue (VAT) area >100 cm2 be used to diagnose ‘visceral obesity’ based on data from a healthy Japanese cohort [14]. However, it well recognised that there are significant ethnic variations in adipose and muscle stores.
The aim of this study was to determine age- and sex-specific body composition values using CT at the level of the third lumbar vertebrae, in a healthy Caucasian population with normal body mass index (BMI). In addition, we sought to provide threshold values representing 1, 2 and 2.5 SD below the sex-specific mean to assist diagnosis of low muscle quantity.
Patients and methods
Ethics approval was granted from the Metro South Human Research Ethics Committee (HREC/14/QPAH/23) and the study performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We included 107 healthy Caucasian patients (46 males; 61 females) with a healthy BMI (18–25 kg/m2) for analysis. Patients having a CT abdomen for trauma evaluation (n = 17), or as part of a living donor renal transplant assessment (n = 90) were included. The clinical records were evaluated and patients excluded if they had illnesses (pre-existing malignancy (n = 4); unexplained iron deficiency anaemia (n = 1)), medications (hormonal therapy (n = 6); angiotensin inhibitors (n = 4); statins (n = 3)) or a CT not suitable for analysis (n = 24) [15].
CT scans were assessed for quality as previously described [16]. Data were obtained from a transverse CT image at the mid-third lumbar vertebrae using ImageJ software (National Institute of Health, Bethesda, MD, USA). Tissue segmentation was performed using Hounsfield unit thresholds of −29 to +150 for muscle and −190 to −30 for adipose tissue [16, 17]. The cross-sectional area of subcutaneous adipose tissue (SAT), VAT and muscle were measured and normalized for stature (cm2/m2), and described as subcutaneous adipose tissue index, visceral adipose tissue index and SMI as previously reported [18]. Patients were divided into sex- and age-specific groups since these two variables are known to have significant impact on body composition variables. Twenty subjects had a CT with only partial SAT area and were classified as missing data for analysis.
All images were analysed by a single trained observer (AW). Intra-observer variability was assessed by re-analysing 10 randomly selected scans after a minimum 6-month interval. The intraclass correlation coefficient (ICC) for SMA was 1.00 (95% CI 0.99–1.00), VAT 0.99 (95% CI 0.98–1.00) and SAT was 1.00 (95% CI 1.00–1.00), while the coefficients of variation were 0.7%, 6.5% and 2.4%, respectively. Variation in software was assessed by re-analysing 10 scans with Slice-O-Matic (Tomovision, Montreal, QC, Canada). This analysis showed ICCs of 0.99 (95% CI 0.97–1.00), 0.99 (95% CI 0.97–1.00) and 1.00 (95% CI 1.00–1.00) for SMA, VAT and SAT, respectively. Using 10 randomly selected scans, inter-observer variability was assessed by a blinded second trained analyst (SK). The ICC for SMA, VAT and SAT for this comparison were 1.00 (95% CI 0.99–1.00), 1.00 (95% CI 1.00–1.00) and 1.00 (95% CI 1.00–1.00), respectively.
Statistical analysis
Statistical analyses were performed using SPSS version 24 (IBM, New York, USA). Two-tailed (p = 0.05) tests of significance were used. Continuous data were assessed for normality using a Shapiro–Wilk test and are presented as means ± SD, or medians and interquartile range (IQR), depending on distribution of the data. Non-normally distributed data were transformed where possible for statistical analysis.
Comparison of continuous variables was examined using independent t-tests (normal distribution) or Mann–Whitney U tests (non-normal distribution). Correlation was assessed using Pearson’s or Spearman’s test of correlation depending on the distribution of data. For interpretation of correlation coefficients, Cohen’s levels were used [19].
Patients were divided into sex- and age-specific groups since these two variables are known to have significant impact on body composition variables [20]. To provide a ‘young’ reference data set, an age cut-off of <50 years was used. This age cut-off has been used in other studies to define ‘young cohorts’, and provided an even distribution of data in our cohort [11, 12]. Prior population-level studies have demonstrated that ‘no noticeable loss of skeletal muscle occurs until after the fifth decade’ [21]. Regression analysis of our data did not detect a significant decline in CT SMI with age (Supplementary Fig. 1), including no noticeable decline in those over the age of 50 years.
The median age of our young healthy males and females was 39.8 years (IQR 27.4–46.0) and 45.5 years (IQR 40.5–47.9), respectively. Comparison of age- and sex-specific groups was performed using one-way ANOVA analysis and Tukey’s post hoc test (normal distribution) or Kruskal–Wallis analysis and Dunn’s post hoc test (non-normal distribution).
Results
The mean age of the study cohort was 47.8 ± 11.0 years (range 21–73) with a median BMI of 23.7 kg/m2 (IQR 22.3–24.8). Sex-specific patient characteristics are shown in Table 1. CT body composition results are shown in their sex- and age-specific groups in Tables 2 and 3.
Sex- and age-specific differences in body composition were explored. One-way ANOVA analysis showed a significant difference in mean SMI between groups (F = 31.29; p < 0.001). Tukey’s post hoc test showed that young males had a significantly higher mean SMI than both female groups (p < 0.001); however, no difference compared with older males (p = 0.798) (Fig. 1). There was also no significant difference in mean SMI between the two female groups (p = 0.980).
Between group comparison of SAT using one-way ANOVA demonstrated similar findings, with significant sex differences (higher SAT in females), but not age (Supplementary Fig. 2). Analysis of VAT also showed sex-specific differences in groups, with both male cohorts having higher VAT compared with females (Supplementary Fig. 3). However, there was also a significant difference between the two male cohorts (p = 0.025), with older males having higher VAT.
Thresholds representing 1, 2 and 2.5 SDs below the mean sex-specific values are shown in Table 4.
Discussion
The use of cross-sectional imaging for clinical care is expanding and can provide important body composition information on patients. Multiple methods have been reported to quantify skeletal muscle on CT, however the most common method is the measurement of total SMA on a single transverse image. There is a paucity of normative CT data using this technique. Our aim was to provide age- and sex-specific body composition values using CT at the level of the third lumbar vertebrae, in a healthy Caucasian population.
These data allow comparison to previously published CT SMA threshold values for low muscle quantity. To date, the most frequently used CT skeletal muscle cut-offs defining low muscle quantity have been derived using statistical methods (optimal stratification) to predict mortality in cohorts with cancer [6, 22].
The mean CT SMI in our sex-specific groups of healthy subjects approached the previously utilised cut-offs from Prado et al. (52.4 cm2/m2 for males and 38.5 cm2/m2 for females) [6]. These cut-offs were derived from a population with a BMI >30 and it is likely that a proportion of this cohort had higher SMM contributing to the elevated BMI values reported. Subsequently, Martin et al. published data from a cohort of cancer patients, which suggested lower cut-offs are associated with mortality in those patients with normal BMI (<43 cm2/m2 for males and <41 cm2/m2 for females) [22]. It should be noted that we restricted our cohort to those with a BMI 18–25, a range considered as ‘normal’ for Caucasian ethnicity according to the World Health Organisation and representing a ‘healthy young’ cohort [23]. Using this BMI category can provide healthy normative data on both SMM and adipose tissue, allowing the potential to distinguish entities such as sarcopenic obesity.
Our threshold values representing two SDs below the sex-specific reference values were similar to those published by Hamaguchi et al. from a large Japanese population [13]. CT muscle and adipose surface area values at the level of the third lumbar vertebrae were collected on 657 healthy Japanese liver transplant donors. Their thresholds for low muscle quantity were 40.31 cm2/m2 for males and 30.88 cm2/m2 for females [13].
The threshold values from our study and Hamaguchi et al. differ substantially from the widely used cut-offs from Prado et al. and are not representative of low muscle quantity according to the EWGSOP definition. This is highlighted by the fact that in our ‘healthy cohort’, according to the thresholds from Prado et al., 18 male subjects and 12 female subjects met criteria for ‘sarcopenia’. However, the Prado et al. thresholds have been linked to morbidity and mortality in many different cohorts suggesting that less marked degrees of muscle loss are likely to be clinically significant [6, 22]. We propose that stages of SMM loss should be considered in future studies using CT, similar to what was suggested by Janssen et al. for studies using Bioelectrical impedance analysis [24]. Thus, patients more than one SD below the healthy population mean would be classified as ‘Class I SMM depletion’, while those more than two SDs would have ‘Class II SMM depletion’.
We have not provided a cut-off for adipose tissue for several reasons. First, VAT did not have a normal distribution in the female cohort. Second, metabolic derangements are known to start with a VAT area >100 cm2 and become generalised at a VAT area >130 cm2 [25, 26]. Consequently, these cut-offs would be more appropriate than the thresholds derived from our normative data.
There are several limitations to this study. First, our sample size is small, and could not be considered as reference population. Second, skeletal muscle data were derived only using the most common technique for estimating muscle mass on CT; alternative approaches have not been investigated [3]. This includes the HU threshold values used to define muscle on CT imaging, which have varied in prior publications [3]. It is also important to recognise that HU can vary depending on CT hardware calibration introducing another source of variation, although the significance of this it yet to be determined. Third, our young reference population included patients up to 50 years of age compared with an upper limit of 40 years of age used by Baumgartner et al. [27]. Finally, our cohort is ethnically limited to Caucasian subjects. Further studies are required to further build on the normative data presented here, both in Caucasian populations as well as other ethnic groups.
To conclude, our data provide an insight into the distribution of skeletal muscle and adipose tissue surface area values on CT, in a healthy Caucasian population at the level of the third lumbar vertebrae. The cut-offs for SMM depletion, which represent two SDs below the sex-specific healthy population mean values, are much lower than those frequently used in the literature. We suggest the use of a multi-level staging system for CT defined SMM loss in future studies.
References
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PloS One. 2017;12:e0169548.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J cancer. 2016;57:58–67.
van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, JN IJ. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–92.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Buentzel J, Heinz J, Bleckmann A, Bauer C, Rover C, Bohnenberger H, et al. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. 2019;39:4603–12.
Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB. 2019;21:1603–12.
Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PloS One. 2017;12:e0186990.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–7.
Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol. 2015;50:1206–13.
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101:565–74.
Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circulation J. 2002;66:987–92.
Campins L, Camps M, Riera A, Pleguezuelos E, Yebenes JC, Serra-Prat M. Oral drugs related with muscle wasting and sarcopenia. A Rev Pharmacol. 2017;99:1–8.
Gomez-Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: a step-by-step guide for clinicians using National Institutes of Health ImageJ. J Parenter Enter Nutr. 2016;40:308–18.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol, Nutr, Metab. 2008;33:997–1006.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum Associates; 1988 567.
Heymsfield S. Human body composition. 2nd ed. Champaign, IL: Human Kinetics; 2005. 523.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol . 2000;89:81–8.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatrics Soc. 2002;50:889–96.
Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–20.
Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6:137–59.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63.
Acknowledgements
The authors would like to acknowledge the statistical advice provided by Dr Anne Bernard.
Funding
AJW received a project grant from the Royal Australasian College of Physicians and is a recipient of the Princess Alexandra Hospital Research Support Scheme Postgraduate Scholarship. SEK is supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship.
Author information
Authors and Affiliations
Contributions
AJW, LCW, JCW and GAM were responsible for the study design; AJW was responsible for data collection and image analysis; AA provided radiological expertise and assisted with protocol design; SEK provided assistance with image analysis; AJW, LCW, JSC and GAM contributed to the analysis and interpretation of data. AJW, AA, SEK, LCW, JSC and GAM contributed to the manuscript preparation and revisions.
Corresponding author
Ethics declarations
Conflict of interest
AA and SEK have no personal or funding interests to disclose. AJW has received funding to speak on behalf of Janssen-Cilag for unrelated work. LCW consults to ImpediMed Ltd. JSC has received an unrestricted research grant from Coca Cola and funding from Renew Corp, Pfizer, Cyanotech, Terumo, Gatorade, Numico, Northfields and Baxter for unrelated work. JSC has also received honorariums to present at meetings from Novartis, Amgen and Roche. GAM is on an advisory board for AbbVie and has received funding to speak on behalf of MSD and Gilead for unrelated work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Woodward, A.J., Avery, A., Keating, S.E. et al. Computerised tomography skeletal muscle and adipose surface area values in a healthy Caucasian population. Eur J Clin Nutr 74, 1276–1281 (2020). https://doi.org/10.1038/s41430-020-0628-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-0628-1
- Springer Nature Limited