Abstract
Background/objectives
Numerous clinical trials have confirmed that supplementation with purified anthocyanins has favorable effects on metabolic diseases, but the dose–response of dyslipidemia to anthocyanin supplementation remains unclear. This study aimed to investigate the effect of anthocyanin supplementation in different doses on lipid profile.
Subjects/methods
We randomly assigned 176 dyslipidemic subjects aged 35–70 to three purified anthocyanin groups (40 mg/day, n = 45; 80 mg/day, n = 42; 320 mg/day, n = 43) and a placebo group (n = 46). Anthropometric parameters, serum lipid profiles, and cholesterol efflux capacity (CEC) were measured at baseline, and at the end of 6 and 12 weeks.
Results
After 12 weeks of supplementation, significant differences in CEC (P = 0.033), high-density lipoprotein cholesterol (HDL-C) (P = 0.043), and apolipoprotein A-I (ApoA-I) (P = 0.022) were observed between four groups. Compared with placebo, 320 mg/day anthocyanin significantly increased CEC (35.8%, 95% CI: 11.5–60.2%; P = 0.004), HDL-C (0.07 mmol/L, 95% CI: 0.01–0.14; P = 0.003), and ApoA-I (0.07 g/L, 95% CI: 0.01–0.12; P = 0.008). Linear trend analysis showed that anthocyanin supplementation has a strong dose–response relationship with CEC (P = 0.002), HDL-C (P = 0.038), and ApoA-I (P = 0.023). Moreover, the enhancement of CEC showed positive correlations with the increase in HDL-C (r = 0.215, P < 0.01) and APOA-I (r = 0.327, P < 0.01).
Conclusions
Anthocyanin supplementation at 0–320 mg/day for 12 weeks enhances CEC in a dose–response manner in dyslipidemic subjects. Anthocyanin supplementation doses of 80–320 mg/day can improve serum HDL-C levels and HDL-induced CEC.
Similar content being viewed by others
Introduction
Dyslipidemia, a major risk factor of cardiovascular disease (CVD), is closely related to the pathological development of atherosclerosis [1, 2]. It is reported that death from CVD accounts for 30% of all deaths per year worldwide [3]. Without effective intervention, the incidence of CVD caused by dyslipidemia in China will increase by about 9.2 million in the near future [4]. Therefore, the prevention of dyslipidemia is of great significance.
Numerous studies have confirmed a negative correlation between the level of circulating high-density lipoprotein cholesterol (HDL-C) in the serum and the risk of cardiovascular events [5, 6]. However, traditional strategies of CVD risk reduction that merely increase the HDL-C level have not always yielded consistent outcomes [7, 8]. Independent of serum HDL-C level, HDL particles have been shown to have atheroprotective effects through various functions, such as reverse cholesterol transport (RCT), anti-inflammatory, and antioxidant functions [9]. Since cholesterol efflux from macrophages is the initial and rate-limiting step of RCT, cholesterol efflux capacity (CEC) is considered a significant indicator in the assessment of HDL-C functions. Moreover, several studies have shown a negative correlation between HDL-induced CEC and both the incidence and prevalence of CVD [10,11,12]. Therefore, improving HDL function, especially in improving CEC, is expected to effectively reduce CVD risk.
According to the 2018 American College of Cardiology/American Heart Association guidelines, maintaining a healthy diet is an appropriate approach for CVD risk reduction [13]. Epidemiological studies have shown a negative correlation between the intake of plant-based foods that contain a variety of flavonoids and the risk of chronic metabolic diseases [14]. Anthocyanins are the largest subclass of flavonoids, and they occur abundantly in commonly consumed food, such as grapes, blueberries, black rice, and red wine [15]. In our earlier studies, we found that supplementation with purified anthocyanins had positive effects on serum lipid profile, RCT, inflammatory disorders, and endothelial function [16,17,18].
However, the dose–response relationship between anthocyanin supplementation and dyslipidemia had not yet been investigated. This made dietary recommendations to the general public difficult. Therefore, we designed this randomized controlled trial to investigate the dose–response relationship between supplementation with purified anthocyanins over a 12-week period and serum lipid profile, particularly the CEC, in subjects with dyslipidemia.
Materials and methods
Study design and subjects’ recruitment
All the subjects were Chinese men and women aged 35–70 years with dyslipidemia. Methods of recruitment and details of inclusion/exclusion criteria are given in Supplementary information. All the study protocols were in compliance with the Helsinki Declaration and approved by the ethics committee of Sun Yat-sen University. All subjects provided written informed consent. This trial has been registered at clinicaltrials.gov as NCT03415503.
A statistician who did not participate in data collection and analysis was in charge of randomization and management of the packaged supplements. According to the random numbers generated by computer, 176 subjects were stratified by gender and allocated into four groups at recruitment: three groups who received 40 mg/day (n = 45), 80 mg/day (n = 42), and 320 mg/day (n = 43) of anthocyanin supplementation, respectively, and a placebo group (n = 46). The serial numbers and the supplements were assigned to the subjects in the order of enrollment into the trial. The subjects, researchers, and data analysts were all blinded to the allocation information.
Supplement preparation
Anthocyanin capsules (Medox®) and placebo capsules of identical weight and appearance were obtained from Medpalett AS (Sandnes, Norway). The Medox capsules were of two kinds, containing 40 or 80 mg of anthocyanin purified from bilberry (Vaccinium myrtillus) and blackcurrant (Ribes nigrum). The participants in each group ingested two capsules twice a day after meals. They were also instructed to maintain their normal lifestyle, and to avoid intake of anthocyanin-containing supplements during the 12-week trial. Capsules for 2 weeks were provided at baseline, and on the second, fifth, and tenth weeks, the subjects were instructed to visit the follow-up center and to return the remaining capsules, which were counted to evaluate compliance, and they were given new capsules for the next phase.
Outcome measures
The primary outcomes of the trial were changes in CEC and lipid profile. The secondary outcomes were changes in anthropometric characteristics, liver and renal functions, and serum-fasting glucose and insulin levels. The above-mentioned data were collated at baseline, in the 6th week, and at the end of the trial.
Basic information collection and anthropometric assessments
At baseline, basic information, such as date of birth, sex, marital status, level of education, occupation, smoking status and alcohol consumption, and medical history were collected. Anthropometric characteristics, including height, weight, hip and waist circumferences, and blood pressure, were recorded at baseline, in the sixth week, and at the end of intervention. We repeated the measurement of anthropometric characteristics twice and recorded the mean values. To monitor their dietary habits and physical activity during the trial, a 3-day 24-h dietary record was kept, and the International Physical Activity Questionnaire was completed three times. Data on physical activity were then converted into metabolic equivalent [19], and dietary intake of nutrients and anthocyanins was calculated based on the Chinese Food Composition Table [20].
Blood sample collection and laboratory measurements
After overnight fasting of 10–12 h, venous blood was collected between 8:00 and 9:00 a.m. on the following day at baseline, in the 6th week, and at the end of the trial. The blood samples were centrifuged at 3000 × g for 15 mins to isolate serum, which was divided into aliquots and stored at −80 °C until analysis.
Enzymatic methods were used to determine the concentrations of HDL-C, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG). The concentrations of ApoA-I and apolipoprotein B were determined by immunonephelometry. Blood glucose level was determined using the hexokinase method. Insulin concentration was ascertained using chemiluminescence. HbA1c was determined by cation-exchange high-pressure liquid chromatography (Bio-Rad Laboratories, CA, USA). Serum concentrations of aspartate aminotransferase and alanine aminotransferase were determined using the rate method, and creatinine concentration was determined using an enzymatic method. Total bilirubin was determined using a diazotization method. We estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease Study equation for standardized serum creatinine [21]: eGFR = 175 × (standardized serum creatinine in mg/dl)−1.154 × age−0.203 × 0.742 (if female). eGFR is reported in ml/minute per 1.73 m2 of body surface area.
Assessment of CEC
CEC was assessed by measuring the efflux of fluorescence-labeled cholesterol from J774 macrophages to apolipoprotein B-depleted plasma of study subjects with the use of a previously published protocol [22] (details of the protocol are provided in the Supplementary information).
Sample size estimation
Sample size estimation was conducted using the PASS software (version 11.0, NCSS Inc.). In our previous clinical trial, supplementation with 320 mg/day of anthocyanin resulted in a 0.34 mmol/L (13.1 mg/dL) decrease in LDL-C relative to treatment with placebo [17]. Based on the conventional assumption of a two-tailed α level of 0.05 and β level of 0.10, it was determined that 38 subjects should be recruited per group. Allowing for a 10% dropout rate, at least 42 subjects were needed in each group.
Statistical analysis
All statistical analyses were performed using SPSS (version 22.0, SPSS Inc.) and strictly followed the intention-to-treat (ITT) principle. The method of the last observation carried forward was used to process the missing values in the ITT analysis. For categorical variables, analysis was performed using the chi-square test. For continuous variables, data were presented as mean ± SD for normally distributed variables. Logarithmic transformation was performed if the variables were not normally distributed, and the data were presented as median and interquartile range.
Repeated-measures analysis of variance was used to analyze the main effect on groups over time. When there was no interaction effect, the main effect showed statistical inference of significance between the groups. Analysis of covariance adjusting for covariates (baseline value, age, sex, and body mass index) was performed to compare the means of normally distributed variables between the four groups, and the Student Newman–Keuls method was used for post hoc multiple comparisons if equal variances were assumed, while the Games–Howell method was used if equal variances were not assumed. The paired t-test was used to compare the differences in data values within each group at baseline and at 6 or 12 weeks. Bivariate correlation analysis was performed to assess the relationship between variables. Dose–response relationship was investigated using linear trend analysis. P < 0.05 was considered significant.
Results
Subject characteristics at baseline and follow-up
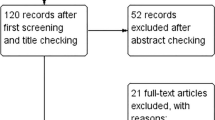
As shown in Fig. 1, 176 eligible subjects were recruited and randomized to four intervention groups at baseline (46 subjects in the placebo group, 45 in the 40 mg/day anthocyanin group, 42 in the 80 mg/day anthocyanin group, and 43 in the 320 mg/day anthocyanin group). The subjects recruited in this study were around 57 years old (57.41 ± 7.95), and 26.1% of them were men. Baseline data, including sociodemographic data, medical history, and medication use, were collected before allocation, and the data were comparable between the four groups. Moreover, there were no significant differences in anthropometric characteristics, dietary intake of nutrients and anthocyanins, physical activity, and markers of liver and renal function, glycemic control at baseline, or at follow-up between the four groups (Table 1, Supplementary Tables 2–4).
In the follow-up period, seven participants (4.0%) withdrew from the study and one had poor compliance (consumed <80% of the provided capsules, and failed to complete all assessments and sample collections [23]). The number of subjects who dropped out was comparable between the four groups (χ2 = 1.518, P = 0.678), and a total of 169 subjects completed the trial. The distribution of adverse events in four groups is shown in Supplementary Table 1.
The effect of different doses of anthocyanin on lipid profile and CEC
Baseline lipid profile and CEC were comparable between the four groups. After 6 weeks of intervention, no significant differences in CEC and lipid profile were observed between the four groups. After 12 weeks of intervention, significant differences in CEC (P = 0.033), HDL-C level (P = 0.043), and ApoA-I level (P = 0.022) were observed between the four groups (Table 2). Compared with placebo, supplementation with 320 mg/day of anthocyanin significantly increases CEC (35.8%, 95% CI: 11.5–60.2%; P = 0.004), HDL-C level (0.07 mmol/L, 95% CI: 0.01–0.14; P = 0.003), and ApoA-I level (0.07 g/L, 95% CI: 0.01–0.12; P = 0.008). However, compared with placebo, anthocyanin supplementation of 80 mg/day only improved ApoA-I level (0.05 g/L, 95% CI: 0.00–0.11; P = 0.036).
In subjects who received anthocyanin supplementation of 320 mg/day, compared with baseline, CEC (25.1%, 95% CI: 7.7–42.4%; P = 0.006) and ApoA-I (0.06 g/L, 95% CI: 0.03–0.08; P < 0.001) levels increased significantly, while LDL-C (−0.31 mmol/L, 95% CI: −0.46 to −0.17; P < 0.001), ApoB (−0.07 g/L, 95% CI: −0.11 to −0.03; P = 0.001), and TC (−0.34 mmol/L, 95% CI: −0.50 to −0.19; P < 0.001) levels decreased significantly after 12 weeks of supplementation. In addition, when compared with 40 mg/day anthocyanin group, the increase in CEC (29.8%, 95% CI: 5.4–54.3%; P = 0.017) after 12-week anthocyanin supplementation of 320 mg/day was also significant.
Moreover, compared with placebo, 12-week anthocyanin supplementation of 40 mg/day, 80 mg/day, and 320 mg/day improved CEC by 6.02, 19.10, and 35.84%, respectively (P trend = 0.002). In the three groups of subjects who received 12-week anthocyanin supplementation, a similar dose–response relationship was found with improvements in HDL-C (P trend = 0.038) and ApoA-I levels (P trend = 0.023) (Fig. 2) with reductions in LDL-C level (P trend = 0.050).
The changes from baseline to 12 weeks in CEC (a), HDL-C (b), and ApoA-I (c). Compared with placebo, the increase of CEC, HDL-C, and ApoA-I levels in 320mg/day anthocyanin group was significant. Anthocyanin supplementation improves CEC, HDL-C, and ApoA-I in a dose–response manner. *P < 0.05; **P < 0.01. ApoA-I apolipoprotein A-I, CEC cholesterol efflux capacity, HDL-C high-density lipoprotein cholesterol.
Correlations between CEC and lipid profile by anthocyanin supplementation
At baseline and at 6 weeks, no correlations were found between CEC and lipid profile (Table 3). After 12 weeks of anthocyanin supplementation, CEC showed significant and positive correlations with both HDL-C (r = 0.203, P < 0.01) and ApoA-I levels (r = 0.211, P < 0.01).
Correlations between changes over time (Δ) in CEC and lipid profile are shown in Table 3. At 12 weeks, a positive correlation was observed between enhancement of CEC and increase in both HDL-C (r = 0.215, P < 0.01) and ApoA-I levels (r = 0.327, P < 0.01) (Fig. 3). Partial correlation analysis showed that the relationship between Δ CEC and Δ ApoA-I persisted even after adjustment for changes in other lipid parameters (r = 0.244, P = 0.002). Interestingly, significant correlations were also found between Δ CEC and Δ ApoA-I in all three groups that received anthocyanin supplementation (r = 0.338, P = 0.025 in the 40 mg/day group; r = 0.403, P = 0.01 in the 80 mg/day group; r = 0.468, P = 0.002 in the 320 mg/day group), and the correlation coefficients of each of these three groups showed a dose–response pattern.
Correlation between changes in CEC and ApoA-I (a) and HDL-C (b). After 12-week supplementation, changes of CEC showed positive association with those of ApoA-I and HDL-C. Pearson correlation coefficients are presented. ApoA-I apolipoprotein A-I, CEC cholesterol efflux capacity, HDL-C high-density lipoprotein cholesterol.
Subgroup analysis
Before the subgroup analyses, we tested the effect modified by adding an interaction term of treatment and subgroup variables (sex, age, and BMI) to the univariate models. There was no significant interaction between treatment and subgroup variables in CEC. However, for HDL-C, LDL-C, and TG, significant interaction was observed between age and treatment (all P interaction < 0.05). Moreover, significant interaction was also observed between sex and treatment in TG (P interaction < 0.01).
Stratified analyses (Supplementary Table 5–7) showed that among subjects with age ≥58, CEC, HDL-C, and ApoA-I have robust dose–response relationship among four groups, and were notably improved under 320 mg/day supplementation when compared with placebo. Moreover, after 12 weeks of anthocyanin supplementation, ApoA-I levels were notably improved in men, while the enhancement in CEC was more remarkable in women.
Discussion
To the best of our knowledge, this is the first randomized controlled trial on human subjects with dyslipidemia to investigate the dose–response relationship between supplementation with purified anthocyanins and improvements in lipid profile, especially in CEC. Our results show that 12 weeks of supplementation with anthocyanin increases CEC and serum ApoA-I and HDL-C levels in a dose–response manner. It is important to note that supplementation with 320 mg/day of anthocyanin was most efficient in improving CEC and ApoA-I and HDL-C levels in the three anthocyanin groups. Subgroup analysis further suggested that older patients may obtain more beneficial improvements in lipid profiles from the supplementation of anthocyanin.
In this trial, we confirmed that, compared with placebo, anthocyanins can have positive effects on CEC and serum levels of HDL-C and ApoA-I. It has been recognized that ABCA1 plays a key role in the regulation of macrophage cholesterol efflux and ApoA-I-mediated RCT. By upregulating the expression of ABCA1 in macrophages, the process of excess free cholesterol efflux from peripheral tissues, and transfer to the liver for biliary excretion, will be greatly promoted [24]. Xia et al. [25] reported that anthocyanin increased ABCA1-mediated cholesterol efflux in macrophages by the regulation of peroxisome proliferator-activated receptor γ-liver X receptor α-ABCA1 signaling pathway activation, which shows that anthocyanin activated ABCA1 and induced cholesterol efflux to ApoA-I in macrophages. This may further explain the correlation between Δ CEC and Δ ApoA-I observed in this trial.
However, the positive effect of anthocyanin on CEC and lipid profile was obvious with 12 weeks of supplementation, but not with 6 weeks of supplementation. This means that the duration of supplementation is a crucial factor for the improvement of lipid metabolism using anthocyanins. Similarly, in other randomized clinical trials in healthy adults [26], patients with metabolic syndrome [27, 28], and patients after myocardial infarction [29], it was shown that anthocyanin supplementation for a duration no longer than 6 weeks does not have any effect on the HDL-C level or CEC. This was consistent with our findings and showed that 6 weeks of anthocyanin supplementation may not be long enough to produce favorable results. Since an increased length of treatment may achieve statistical and clinical relevance, especially for the modest efficacy of treatment achieved with phytochemicals, it is feasible to extend the intervention period to attain the results expected.
Furthermore, the dose–response relationship was found between elevations in CEC and levels of HDL-C and ApoA-I, as well as with the correlation coefficients between Δ CEC and Δ ApoA-I after 12 weeks of anthocyanin supplementation. Enhanced cholesterol efflux with increased dose of anthocyanin supplementation has also been reported in different experimental models. Millar et al. [30] found that anthocyanin intake of 35 and 150 mg/kg·bw for 24 weeks increased CEC by 64 and 85%, respectively, in ApoE−/− mice. Moreover, treatment with 1, 10, and 100 μM of C3G (cyanidin-3-O-β-glucoside, a monomer form of anthocyanins) caused a dose-dependent increase in cholesterol efflux in mouse peritoneal macrophages [25]. More importantly, we found that supplementation with 320 mg/day of anthocyanin had obvious positive effects on the serum lipid profiles and CEC of subjects with dyslipidemia. Several studies have reported that supplementation with 320–360 mg/day of anthocyanin for 12–24 weeks significantly increased CEC [16, 31], HDL-C level [16,17,18, 28, 29], and ApoA-I level [23, 32] in patients with metabolic syndrome. Given that anthocyanins are a class of natural phytochemicals associated with few complications, subjects with dyslipidemia can consider receiving supplementation with 320 mg/day of anthocyanin.
It should be noted that anthocyanin supplementation of 80 mg/day significantly improved ApoA-I level, but did not significantly increase HDL-C level or CEC, which still has important implications as ApoA-I is the most important structural protein of HDL [33]. The China Kadoorie Biobank prospective study showed that a 1-SD increase in ApoA-I level reduced the risk of myocardial infarction by 11% [34]. This study finding shows that moderate doses of anthocyanin can potentially be used to treat lipid dysfunction. A few earlier studies reported findings similar to ours. A 1-month consumption of red-peeled grapefruits (containing 51.5 mg/day of anthocyanins) improved TG, TC, and LDL-C levels in patients with hyperlipidemia [35], and red wine consumption (containing 71 mg/day of anthocyanins) increased the HDL-C level of healthy subjects [36]. These findings may add weight to the evidence that supplementation with 80 mg/day of anthocyanin can deter several of risk factors of CVD. Hence, we reckon that supplementation with 80 mg/day of anthocyanin may moderately improve lipid metabolism in healthy subjects.
The doses of anthocyanin used in the aforementioned trials were high compared with the average anthocyanin intake of 3.1 mg/day by Americans [37] and 12.87 mg/day in this trial. However, it can easily be achieved in daily life by selectively consuming foods rich in anthocyanins. For example, every 100 g of violet cabbage (Capitata rubra) contains about 163.7 mg of anthocyanin, and a small bowl (100 g) of black rice contains up to 397.9 mg of anthocyanins [38]. Besides, the safety and nontoxicity of anthocyanins have been demonstrated in several human trials in which higher doses such as 640 mg/day [39] and 500 mg/day [40] of anthocyanin were used.
This study still has several limitations that should be noted. First, like in most other anthocyanin supplementation trials, the circulating concentrations of anthocyanins or their metabolites were not measured in the blood samples because of the short half-life (4 h) of anthocyanins [41]. However, no significant changes in markers of liver and renal function was observed in this study, indicating that circulating anthocyanins had no undesirable effects on liver and kidney functions. Second, our results for CEC showed the in vivo process of free cholesterol movement from macrophages to plasma during anthocyanin supplementation, but variations in several crucial factors that assemble and remodel HDL particles in the RCT pathway, such as lecithin cholesterol acyltransferase and cholesteryl ester transfer protein, were not observed [42]. Further studies are needed to confirm our findings. Third, although several studies found that anthocyanins could improve glycemic metabolism [43], null effects on glucose and insulin were observed in this trial. The subjects in the present trial having a relatively low level of fasting blood glucose and HbA1c (average 5.37 mmol/L and 5.76% at baseline, respectively) may account for the phenomenon. Another potential limitation is that the dosage range of purified anthocyanin was relatively small; the extensionality of such dose–response relationship was limited. Future studies with a broader dosage range are warranted to expand the database and model an accurate curve.
In conclusion, this trial showed that supplementation with purified anthocyanin at 0–320 mg/day has beneficial effects on the lipid profile and CEC in a dose–response manner. Supplementation with 80 and 320 mg/day of anthocyanin can produce moderate and strong improvements, respectively.
References
Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16.
Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007;40:S118–22.
Moran A, Gu D, Zhao D, Coxson P, Wang YC, Chen CS, et al. Future cardiovascular disease in China: markov model and risk factor scenario projections from the coronary heart disease policy model-China. Circ Cardiovasc Qual Outcomes. 2010;3:243–52.
Gordon DJ, Knoke J, Probstfield JL, Superko R, Tyroler HA. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation. 1986;74:1217–25.
Gordon DJ, Rifkind BM. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989;321:1311–6.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–63.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93.
Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, et al. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arterioscler Thromb Vasc Biol. 2019;39:89–96.
Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381:1557–67.
Del RD, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–92.
Giampieri F, Forbes-Hernandez TY, Gasparrini M, Alvarez-Suarez JM, Afrin S, Bompadre S, et al. Strawberry as a health promoter: an evidence based review. Food Funct. 2015;6:1386–98.
Zhu Y, Huang X, Zhang Y, Wang Y, Liu Y, Sun R, et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab. 2014;99:561–9.
Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, et al. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011;57:1524–33.
Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013;23:843–9.
Bassett DJ. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1396.
Yang YX. Chinese Food Composition Table. Beijing, China: Peking University Medical Press; 2009.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54.
Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, et al. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res. 2011;52:2332–40.
Yang L, Ling W, Yang Y, Chen Y, Tian Z, Du Z, et al. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes—a randomized controlled trial. Nutrients. 2017;9:1104.
Hu YW, Ma X, Li XX, Liu XH, Xiao J, Mo ZC, et al. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis. 2009;204:e35–43.
Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem. 2005;280:36792–801.
Karlsen A, Retterstol L, Laake P, Paur I, Bohn SK, Sandvik L, et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137:1951–4.
Soltani R, Hakimi M, Asgary S, Ghanadian SM, Keshvari M, Sarrafzadegan N. Evaluation of the effects of vaccinium arctostaphylos L. fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evid Based Complement Altern Med. 2014;2014:217451.
Marin-Echeverri C, Blesso CN, Fernandez ML, Galvis-Perez Y, Ciro-Gomez G, Nunez-Rangel V, et al. Effect of Agraz (Vaccinium meridionale Swartz) on high-density lipoprotein function and inflammation in women with metabolic syndrome. Antioxidants. 2018;7:185.
Naruszewicz M, Laniewska I, Millo B, Dluzniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis. 2007;194:e179–84.
Millar CL, Norris GH, Jiang C, Kry J, Vitols A, Garcia C, et al. Long-term supplementation of black elderberries promotes hyperlipidemia, but reduces liver inflammation and improves HDL function and atherosclerotic plaque stability in apolipoprotein e-knockout mice. Mol Nutr Food Res. 2018;62:e1800404.
Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–92.
Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. 2019;109:1535–45.
Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–8.
Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–32.
Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem. 2006;54:1887–92.
Hansen AS, Marckmann P, Dragsted LO, Finne NI, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr. 2005;59:449–55.
Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–52.
Li G, Ling W, Lang J, Chen Y. The anthocyanidins contents of common vegetables and fruits in China. Acta Nutrimenta Sinica. 2010;32:592–7.
Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. 2013;27:100–6.
Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. 2009;139:2266–71.
McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51:702–13.
Tosheska TK, Topuzovska S. High-density lipoprotein metabolism and reverse cholesterol transport: strategies for raising HDL cholesterol. Anatol J Cardiol. 2017;18:149–54.
Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of Anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2017;8:684–93.
Acknowledgements
The authors are grateful to all the volunteers for their participation.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81730090 and 81973022) and the Guangzhou Science, Technology, and Innovation Commission (grant number 201804020045).
Author information
Authors and Affiliations
Contributions
ZX, JX, and WL developed the overall research plan and had study oversight; YY and WL provided the research guidance; ZX, HZ, JP, QL, and XW participated in collecting the data and the biological samples; ZX, HX, XS, and HZ performed the measurements of CEC and analyzed the data; ZX, JX, and WL wrote the paper and had the primary responsibility for the final content. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, Z., Xie, J., Zhang, H. et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—a randomized controlled trial. Eur J Clin Nutr 75, 345–354 (2021). https://doi.org/10.1038/s41430-020-0609-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-0609-4
- Springer Nature Limited
This article is cited by
-
How effective are anthocyanins on healthy modification of cardiometabolic risk factors: a systematic review and meta-analysis
Diabetology & Metabolic Syndrome (2023)