Abstract
Background/objective
To investigate the impact of preoperative immunological and nutritional status, using the prognostic nutritional index (PNI), on completion of planned adjuvant chemotherapy (AC), and the potential additive effects of low PNI and incomplete AC on gastric cancer-specific survival (CSS) after curative resection of stage II/III gastric cancer (GC).
Methods
Medical records of 1288 consecutive stage II/III GC patients who underwent curative resection and planned to receive AC between November 2010 and December 2017 were retrospectively reviewed. The optimal cut-off value of PNI for CSS was determined by X-tile. The independent predictive factors for incomplete AC were identified using univariate and multivariate analyses. Cox regression analyses assessed the association of low PNI, incomplete AC and CSS.
Results
Of the 1288 patients, 406 (31.5%) completed at least six cycles of AC within 6 months following initial of AC (complete AC). Low PNI (<43.9, n = 386) was identified to be an independent risk factor for incomplete AC (<6 cycles). Both low PNI and incomplete AC independently predicted poor CSS (hazard ratio (HR): 1.287, 95% confidence interval (CI): 1.058–1.565; HR: 1.667, 95% CI: 1.342–2.071). Further analyses confirmed an additive effect with those with both low PNI and incomplete AC having an even worse CSS.
Conclusions
Low preoperative PNI significantly affects completion of AC. Low PNI and incomplete AC has an additive effect and is associated with even worse outcomes. Further prospective studies are needed to clarify whether perioperative nutrition intervention could improve completion of AC and improve prognosis of GC patients.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is ranked as one of the most prevalent malignancies worldwide with about 50% of cases occurring in China and surgical resection offering the only possible curative treatment at present [1, 2]. Unfortunately, the majority of patients in China and western countries are diagnosed at an advanced stage. For these patients, prognosis remains dismal even after radical gastrectomy with D2 lymphadenectomy alone, with about 40% of tumor recurrence occurring within 2 years of the initial surgery [3]. In order to delay and/or decrease postsurgical recurrence, adjuvant chemotherapy (AC) is recommended as a standard treatment following curative resection of stage II/III GC in guidelines adopted in both eastern and western countries [4, 5]. However, it is common to encounter patients who refuse to receive AC, or cannot complete the full course of planned AC for various reasons including delayed recovery due to severe postoperative complications, poor patient condition and adverse events during chemotherapy. In fact, almost 50% of patients could not complete the allocated postoperative treatment as planned even in recent prospective large-scale randomized controlled studies [6,7,8]. Furthermore, incomplete AC has been identified to independently predict unfavorable outcomes for patients with various types of malignancies [9,10,11].
It has been well established that immunological and nutritional status is significantly related not only to postoperative complications but also to prognosis of patients with different malignancies. Serum albumin levels and lymphocyte counts are the most commonly used indicators to define nutritional and immunological status, based on which several indices have been explored, for example, the widely used prognostic nutritional index (PNI) [12, 13]. Findings from a meta-analysis have supported the association between low PNI and postoperative complications and prognosis in GC [12]. Given that malnutrition and postoperative complications also significantly affect compliance with AC [11, 14, 15], we hypothesized that low PNI would be a reliable indicator for incompleteness of AC, and there may be an additive detrimental effect observed for prognosis of advanced stage GC patients both with low PNI and incomplete AC. In this retrospective study, for the first time, we investigated the impact of PNI on completion of planned AC, and the association among PNI, completeness of AC and cancer-specific survival (CSS) of stage II/III GC patients after radical gastrectomy, using the database from a high volume center in China.
Methods
Design and patients
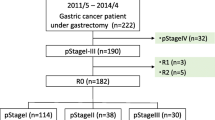
We retrospectively reviewed the medical records of all patients with GC who underwent surgery in the Hunan Cancer Hospital from November 2010 to December 2017. Adult patients (≥18 years old) with pathologically confirmed stage II/III gastric adenocarcinoma who underwent curative resection (R0 resection and D2 lymphadenectomy) were eligible for inclusion in our study, which complied with the standards of the Declaration of Helsinki and was approved by the ethics committee of the Hunan Cancer Hospital. Every patient provided written informed consent for surgery and the use of their clinical data. The exclusion criteria and flow diagram of the study is shown in Supplementary Fig. 1.
Perioperative management and follow-up
Surgeons with sufficient experience of curative gastrectomy and D2 lymphadenectomy performed or supervised all operations. Lymphadenectomy and digestive tract reconstruction were performed according to the Japanese GC treatment guidelines [4]. The TNM stage was classified based on the eighth edition of the American Joint Committee on Cancer TNM staging system [16]. Our previous studies described the main surgical procedures, perioperative management and follow-up [17, 18]. Briefly, postoperative complications were identified within 30 days after surgery and classified according to the Clavien-Dindo classification system [19]. AC was usually started about 4 (±2) weeks after surgery and included fluorouracil and platinum based regimens (generally 3 week cycles of capecitabine/S-1 and oxaliplatin) for 6 months following surgery [9, 20].
Every patient was followed up at an outpatient visit or by telephone 1 month after their initial surgery, every 3 months during the first 2 years, at 6-month intervals between years 3 and 5, and yearly thereafter. Physical examination, routine blood tests, carcinoembryonic antigen, and carbohydrate antigen 199 were examined at each follow-up. An ultrasonography and/or computed tomography scan was performed every 6 months during the 5 years following surgery and endoscopy was performed every 2 years. The latest follow-up date was December 2018.
Evaluation
Clinicopathological variables that included demographic, operative details, pathological and follow-up data were obtained from medical records. Routine laboratory measurements including lymphocyte counts and the serum albumin levels were measured in all patients within 1 week before surgery. As previously reported, the PNI was calculated thus: serum albumin level (g/L) + 0.005 × total lymphocyte count in the peripheral blood (per mm3). The cut-off value of PNI was determined by X-tile, as reported in our previous studies [21, 22]. Whereas for other commonly used variables such as age, body mass index (BMI), albumin levels or anemia, standard clinical, or widely accepted thresholds were used.
The assessed primary outcome was CSS, which was calculated from the time of surgery until death form GC or the last follow-up. The secondary outcome was compliance with AC. Complete AC was defined as a patient receiving at least 6 cycles of fluorouracil and platinum based regimens within 6 months following surgery, considering that patients who received <6 cycles of AC had significant poorer outcomes than those receiving at least 6 cycles [9]. Incomplete AC was defined as patients who received no AC or <6 cycles of AC within 6 months after the initial AC.
To investigate the relative effects of completeness of AC and/or low PNI on prognosis, patients were divided into complete AC and high PNI (complete AC/high PNI), complete AC and low PNI (complete AC/low PNI), incomplete AC and high PNI (incomplete AC/high PNI), incomplete AC and low PNI (incomplete AC/low PNI) groups, respectively. To assess whether an additive effect of both low PNI and incomplete AC was present for outcomes, additional survival analysis was performed using the incomplete AC/high PNI group as a reference [23].
Statistical analysis
Continuous data are presented as the mean ± standard deviation or mean (range), and comparisons between groups were made using a Student’s t-test or a Mann–Whitney U test. Categorical variables are presented as numbers (%) and comparisons made by a χ2 or Fisher exact test. Independent predictors for incomplete AC were identified by univariate and multivariate regression analyses. The optimal cut-off value of PNI for CSS was determined by X-tile when the maximum χ2 log-rank values were reached. CSS was calculated using the Kaplan–Meier method and any differences were assessed by the log-rank test. Multivariate Cox proportional hazard regression analysis was performed to confirm the prognostic factors that may affect CSS. Statistical significance was set at P < 0.05 as two-sided. Statistical analyses were performed using SPSS software (ver. 24.0, IBM Corporation, New York, US).
Results
Characteristics of patients
A total of 1288 consecutive patients were eligible for inclusion in the present study and the baseline demographics of the entire cohort are described in Table 1. The mean age of patients was 55.40 years (range, 19–82), mean BMI 21.76 kg/m2 (range 13.84–33.76) and the mean PNI was 46.68 (25.70–73.90), respectively. The majority of patients were male (66.1%), underwent open surgery (84.5%) and received a subtotal gastrectomy (73.5%). According to the eighth edition of the TNM classification, there were 101 (7.8%), 321 (24.9%), 481 (37.3%), 290 (22.5%), and 95 (7.4%) patients in stages IIA, IIB, IIIA, IIIB, and IIIC, respectively. The mean duration of surgery was 200 min (range 70–584) and the mean estimated intraoperative bleeding was 207 mL (range 50–2300). One hundred and eighteen (9.2%) patients suffered from postoperative complications within 30 days after surgery, which were defined as Clavien-Dindo grade II or greater.
Risk factors for incomplete AC
Of 1288 patients, only 113 patients (8.8%) received at least eight cycles of AC within 6 months after initial AC and another 293 (22.7%) patients received 6–7 cycles. Whereas 245 (19.0%), 344 (26.7%), and 293 (22.7%) of patients received 4–5, 1–3, and 0 cycles of AC, respectively (Supplementary Fig. 1). The 5-year CSS rate of patients who received 6–7 cycles of AC was 63.2%, which was similar to those who received at least eight cycles (59.5%, hazard ratio (HR): 1.130, confidence interval (CI): 0.762–1.674, P = 0.543), but was significantly higher than in patients who received 4–5, 1–3, or 0 cycles of AC (56.4, 48.1 and 50.4%, respectively, P < 0.01) (Table 2 and Fig. 1). Therefore, patients (n = 406, 31.5%) who received at least six cycles of AC were considered to have good compliance with AC and were enrolled into the complete AC group. The remaining 882 (68.5%) patients who received none or 1–5 cycles of AC were enrolled in the incomplete AC group.
Each clinicopathological variable was categorized (Table 3a) and analyzed for a potential impact on completion of AC. The cut-off value of the PNI for CSS was set at 43.9 by X-tile (Supplementary Fig. 2). Univariate analyses found that age (≥65 years), an American Society of Anesthesiologist (ASA) score ≥ 3, low albumin concentration (<35 g/L), open surgery, low PNI (<43.9), and TNM stage II adversely affected the completeness of AC. After multivariate regression analysis, except for age, an ASA score ≥ 3 and TNM stage II, low PNI (odds ratio (OR): 1.380, 95% CI: 1.045–1.822, P = 0.023) was also identified to be significantly associated with incomplete AC (Table 3b). In fact, 34.4% (310/902) of patients with high PNI completed at least six cycles of AC, which was a significantly greater number than that in the low PNI group (24.9%, 96/386, P = 0.001).
Risk factors for poor CSS
A total of 470 deaths (36.5%) occurred among the 1288 patients during the median follow-up time of 29 months (range, 7–98), with a median survival time of 73 months. There were also 476 cases of tumor recurrences and 436 GC-specific deaths.
In the univariate analyses, low PNI, incomplete AC, total gastrectomy, intraoperative blood loss ≥300 mL, TNM stage III and perioperative blood transfusion were related to poor CSS (all P < 0.05). After adjusting for potential confounders in the multivariate Cox regression model, both incomplete AC (HR: 1.667, 95% CI: 1.342–2.071, P < 0.001) and low PNI (HR: 1.287, 95% CI: 1.058–1.565, P = 0.011) were identified to be independent predictors for poor CSS (Table 4).
Association among PNI, AC, and CSS
After risk adjustment, compared with the complete AC/high PNI group (n = 310), although the complete AC/low PNI group had comparable outcomes (n = 96, HR: 1.227, 95% CI: 0.806–1.868, P = 0.339), the incomplete AC/high PNI group was associated with worse outcomes (n = 592, HR: 1.638, 95% CI: 1.261–2.126, P < 0.001) and the incomplete AC/low PNI group had the worst prognosis (n = 290, HR: 1.980, 95% CI: 1.498–2.617, P < 0.001) among the four groups (Fig. 2). Moreover, an additive effect was confirmed for the incomplete AC/low PNI group, compared with the incomplete AC/high PNI group, which was used as a reference (HR: 1.292, 95% CI: 1.035–1.614, P = 0.024); as were the overall and disease free survival (OS and DFS) times (Supplementary Fig. 3).
Cancer-specific survival curves in 1288 patients who underwent curative resection for stage II/III gastric cancer stratified by the prognostic nutritional index (PNI) and completeness of planed adjuvant chemotherapy (AC). (+) defined as receiving less than six cycles of AC or PNI < 43.9, (−) defined as at least six cycles of AC or PNI ≥ 43.9
Discussion
Although AC has become a standard treatment after radical resection of stage II/III GC both in eastern and western countries, only 406 (31.5%) in our cohort of patients received at least six cycles of AC. In contrast, 67% of patients in the chemotherapy group received eight cycles of capecitabine and oxaliplatin as planned in the CLASSIC study and 70.2% continued adjuvant S-1 for 1 year in a multicenter retrospective study in Japan [9, 15]. Although the exact reason was difficult to determine, possible explanations include the retrospective nature of our study, differences in the process of healthcare during hospitalization and post discharge, insurance coverage, and the economic burden given that China is a developing country nowadays.
To investigate whether the dose intensity influenced the outcomes, we divided patients into 0, 1–3, 4–5, 6–7, and ≥8 cycles (some patients received orally S-1 or capecitabine as maintenance treatment after eight cycles of combination chemotherapy) of AC. The 5-year CSS rate of patients who received 6–7 cycles of AC was similar to those who received at least eight cycles, but was significant greater than those patients who received 4–5, 1–3, or 0 cycles of AC (P < 0.01, Fig. 1). The result was echoed by Noh and colleagues who carried out a post hoc analysis of outcomes in the CLASSIC study and revealed that patients who received fewer than six cycles of capecitabine and oxaliplatin had significantly poorer prognosis than those who received at least six cycles of either of the study drugs [9]. Thus, patients who received at least six cycles of AC were considered to have completed AC in the present study. Further multivariate analysis confirmed that incomplete AC (<6 cycles) was significantly associated with poor outcomes. Therefore, besides a higher proportion of patients with stage III GC (also including IIIC disease), poorer compliance with AC, to a certain extent, also contributed to a poorer prognosis in our study, as compared with the well-known CLASSIC study [9]. In some ways, there may be room for consideration of reducing the number of AC cycles to six, especially in those patients at a relatively lower risk of relapse [24]. Although four courses of S-1 was demonstrated to be associated with poorer relapse-free survival for stage II GC than eight courses, whether the results would be the same in those patients who underwent six courses of S-1, or combination chemotherapy needs further prospective large-scale studies [25].
Several studies have investigated the risk factors that may influence compliance with adjuvant S-1 therapy in GC patients. An age >65 years, postoperative infection complications, body weight loss especially loss of lean body mass were clarified to be independent predictors for poor compliance [14, 15, 26,27,28]. But these conclusions were generally based on limited number of patients and only a few perioperative variables were included in the prognostic analysis, which may have hampered the statistical power. Moreover, subgroup analysis of 5-year OS in the ACTS-GC study revealed insufficient efficacy of S-1 alone for patients at a high risk of relapse (stage IIIB) and other studies have demonstrated that combination chemotherapy, e.g., with capecitabine and oxaliplatin, was superior to S-1 alone for survival in patients with stage IIIB and IIIC disease [29, 30]. Given that patients were usually diagnosed at a more advanced stage in China and western countries than in Japan and South Korea, where therapeutic trials with S-1 have generally shown less promising findings, and combined chemotherapy was considered as standard chemotherapy, it seemed inappropriate to copy their experiences verbatim. To our knowledge, the current study is the largest to investigate the risk factors for incomplete AC with combination chemotherapy after curative resection of locally advanced GC. Our results confirmed that poor patient condition (including an older age and high ASA score) adversely affected compliance with AC and there was a strong tendency towards statistical significance that laparoscopic surgery could improve compliance (P = 0.050) [15, 31]. Patients with stage II disease had poorer compliance to AC, probably due to its relatively lower risk of relapse and some patients may receive S-1 alone for their chemotherapy regime.
Malnutrition is common in GC patients due to the malignant disease process and its attendant anorexia and in some cases pyloric obstruction. Recently, emerging evidence has demonstrated the prognostic value of the nutritional status of patients in various malignancies [12, 13, 21, 22]. Possible explanations include that malnutrition cripples the immune system, suppresses cell-mediated immunity, which is essential in defending cancer and thus facilitating metastasis. Malnutrition also increases the risk of postoperative infection, thus activating systemic inflammatory responses and reducing the therapeutic efficacy of treatment regimens [12, 32].
It is well known that systemic inflammatory responses play a critical role in the prognosis of cancer patients. Several reasons lay behind this association, such as impairment of immune functions and the production of various inflammatory cytokines and as a result tumor proliferation and progression is stimulated [33]. The number of CD4+ T cells, which recognize tumor antigens and activate CD8+ T cells leading to cytotoxic effects on tumor cells, was decreased in the tumor microenvironment in low PNI GC patients and was associated with poor prognosis [34]. Our previous study found that both low albumin levels (<33 g/L) and lymphocyte counts (<1.5 × 109/L) were independent predictors for OS in stage II/III GC [22]. PNI, which was simply calculated according to the albumin level and lymphocyte count, reflecting both malnutrition and systemic inflammation, has been identified as a reliable indicator for postoperative complications and the prognosis of various malignancies [12, 13]. But, the potential influence of PNI on AC has never been thoroughly investigated.
The present study has demonstrated for the first time that a low PNI, indicating malnutrition and inflammatory suppression, is an independent predictor for incomplete AC after gastrectomy. Possible explanations for this association include malnutrition leading to sarcopenia, physical frailty, poor quality of life, postoperative infection complications and an increased risk of chemotherapy-induced toxicity [12, 32]. Several studies have confirmed that perioperative nutrition support, either enteral immunonutrition or n-3 fatty acid-based parenteral nutrition, could significantly improve postoperative nutritional status and immunity, resulting in a decrease in postoperative complications and infections in cancer patients [32, 35]. To the best of our knowledge, whether nutritional intervention could improve completeness of AC has never been fully investigated.
With respect to outcomes, both a low PNI and incomplete AC were identified to be independent risk factors for prognosis in our study, a finding consistent with previous reports [10,11,12,13, 26]. Further analysis revealed that patients with both a low PNI and incomplete AC had the worst outcomes compared with those with either or none of these two risk factors as shown in Fig. 2. Thus, for the first time, an additive effect was confirmed in patients with both low PNI and incomplete AC. Our results emphasize the importance of nutrition support for potential malnutrition in GC patients undergoing surgery and chemotherapy and it seems reasonable to assume that appropriate nutrition intervention could improve the prognosis of these patients. Immunonutrition intervention may reverse the status of malnutrition and inflammatory suppression for example by raising the CD4/CD8 ratio, thus improving cytotoxic effects on tumor cells leading to an improvement in the prognosis [32, 34]. In addition, nutrition intervention may reduce postoperative infection complications and toxicity induced by chemotherapy [12, 32]. This intervention may improve compliance with AC and finally improve the outcomes for patients. Further large-scale prospective studies are needed to confirm this hypothesis.
Although the present study has revealed some important findings, it has a number of limitations, first and foremost being that it was a retrospective and single-institution study. Second, the median follow-up time (29 months) was relatively short, thus later tumor recurrence and death of patients could not be recorded. Third, although patients undergoing AC was usually treated with fluorouracil and platinum based regimens (oxaliplatin and capecitabine/S-1), several other combinations of anticancer drugs have been used in our hospital including epirubicin, cisplatin plus fluorouracil [36] and oxaliplatin plus fluorouracil/leucovorin [37]. The incidence and severity of chemotherapy-induced toxicity and the treatment interval (tri-weekly or bi-weekly) might also affect the completeness of AC. Fourth, considering only 148 patients (8.6%) who received neoadjuvant chemotherapy during the study process, and the pathological tumor stage after neoadjuvant chemotherapy (yp TNM stage) may be different from those patients who underwent surgery first, thus we excluded these patients from the present study. However, more and more prospective large-scale studies have demonstrated that neoadjuvant chemotherapy can improve OS in patients with advanced GC. As a result, this may impact on the generalizability of our conclusions. Last, but by no means least, the exact reason for discontinuing AC was not investigated in the present study. In China as a developing country, except for malnutrition and adverse events caused by chemotherapy, the type of medical insurance held and the economic burden might also significantly impact on compliance with AC, which might serve as a confounding factor in our study. Although the follow-up period was short in our cohort, the majority of GC patients will relapse within 2 years after curative resection [3]. Notwithstanding these limitations, this is the first study to investigate the impact of preoperative PNI on the completeness of AC and the potential additive effects of PNI and completeness of AC on the prognosis of stage II/III GC after curative resection, based on a large cohort of patients.
In conclusion, we found that preoperative low PNI was an independent risk factor for incompleteness of planned AC after curative resection of stage II/III GC. Both low PNI and incomplete AC were significantly associated with a worse CSS, and there was a synergistic adverse effect of both low PNI and incomplete AC. As poor immunological and nutritional status had an independent and also additive effect with incomplete AC on outcomes after gastrectomy for GC, further prospective studies are needed to clarify whether nutrition intervention could improve compliance with AC and as a result improve the prognosis of GC patients.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–312.
Sakuramoto S, Sasako M, Yamaquchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:10–1820.
Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiquchi N, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886–93.
Cats A, Jansen EPM, van Grieken NCT, Skorsha K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616–28.
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96.
Morris M, Platell C, Fritschi L, lacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96:701–7.
Matsumoto I, Tanaka M, Shirakawa S, Shinzeki M, Toyama H, Asari S, et al. Postoperative serum albumin level is a marker of incomplete adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2015;22:2408–24.
Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. 2016;42:1176–82.
Chan AW, Chan SL, Wong GL, Wong WV, Chong CC, Lai PB, et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015;22:4138–48.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24:2639–45.
Amin MB, Edge SB, Greene FL, Brierley JD. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Xiao H, Xiao Y, Quan H, Liu W, Pan S, Ouyang Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: incidence, pathogens, risk factors and outcomes. Int J Surg. 2017;48:195–200.
Xiao H, Liu W, Quan H, Ouyang Y. Peri-operative blood transfusion does not influence overall and disease-free survival after radical gastrectomy for stage II/III gastric cancer: a propensity score matching analysis. J Gastrointest Surg. 2018;22:1489–500.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175–81.
Onodera T, Goseki N, Kosakim G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Zasshi. 1984;85:1001–5.
Shen Q, Liu W, Quan H, Pan S, Li S, Zhou T, et al. Prealbumin and lymphocyte-based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Br J Nutr. 2018;120:1359–69.
Aquina CT, Blumberg N, Becerra AZ, Boscoe FP, Schymura MJ, Noyes K, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg. 2017;266:311–7.
Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Eng J Med. 2018;378:1177–88.
Yoshikawa T, Terashima M, Mizusawa J, Nunobe S, Nishida Y, Yamada T. et al. Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomised trial. Lancet Gastroenterol Hepatol.2019;4:208–16.
Jang SH, Jung YJ, Kim MG, Kwon SJ. The prognostic significance of compliance with postoperative adjuvant chemotherapy in patients with stage III gastric cancer: an observational study. J Gastric Cancer. 2018;18:48–57.
Aoyama T, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015;22:2560–6.
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Oqata T, et al. Risk factors for 6-months continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013;16:133–9.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaquchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, et al. Efficacy of adjuvant S-1 versus XELOX chemotherapy for patients with gastric cancer after D2 lymph node dissection: a retrospective, multi-center observational study. Ann Surg Oncol. 2018;25:1176–83.
Bao H, Xu N, Li Z, Ren H, Xia H, Li N, et al. Effect of laparoscopic gastrectomy on compliance with adjuvant chemotherapy in patients with gastric cancer. Medicine. 2017;96:e6839.
Xu J, Zhong Y, Jing D, Wu D. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30:1284–9.
Sun X, Wang J, Liu J, Chen S, Liu X. Albumin concentrations plus neutrophil lymphocyte ratios for predicting overall survival after curative resection for gastric cancer. Onco Targets Ther. 2016;9:4661–9.
Choi Y, Kim JW, Nam KH, Han SH, Kim JW, Ahn SH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. 2017;20:602–11.
Zhang B, Wei G, Li R, Wang Y, Yu J, Wang R, et al. n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: a randomized controlled clinical trial. Clin Nutr. 2017;36:1239–44.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Cho YH, Kim SY, Hong Lee M, Yoo MW, Bang HY, Lee KY, et al. Comparative analysis of the efficacy and safety of chemotherapy with oxaliplatin plus fluorouracil/leucovorin between elderly patients over 65 years and younger patients with advanced gastric cancer. Gastric Cancer. 2012;15:389–95.
Acknowledgements
The authors gratefully thank all of the participants in this study and Hunan Cancer Hospital for supporting this study.
Funding
This study was supported by grants from the Natural Science Foundation of Hunan Province (no. 2018JJ6108) and the 2017 Annual Research Project of Health and Family Planning Commission of Hunan Province (no. B2017101).
Author information
Authors and Affiliations
Contributions
HX, XLY, and YZOY contributed to the conception and the design of the study; HX, HJZ, PZ, HFX, KL, XYC, HQ, BY, RRL, and GH collected, analyzed, and interpreted the data; HX grafted the manuscript. XLY and YZOY critically revised the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approve the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiao, H., Zhou, H., Zhang, P. et al. Association among the prognostic nutritional index, completion of adjuvant chemotherapy, and cancer-specific survival after curative resection of stage II/III gastric cancer. Eur J Clin Nutr 74, 555–564 (2020). https://doi.org/10.1038/s41430-019-0502-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0502-1
- Springer Nature Limited
This article is cited by
-
Nomogram to predict overall survival of patients receiving radical gastrectomy and incomplete peri-operative adjuvant chemotherapy for stage II/III gastric cancer: a retrospective bi-center cohort study
BMC Cancer (2024)
-
Prognostic value of post-operative serum procalcitonin in gastric adenocarcinoma patients undergoing radical gastrectomy: propensity score matching analysis of extended cohort from a prospective bi-center study
Gastric Cancer (2023)
-
Association among prognostic nutritional index, post-operative infection and prognosis of stage II/III gastric cancer patients following radical gastrectomy
European Journal of Clinical Nutrition (2022)