Abstract
Background
Colorectal cancer is associated with lifestyle characteristics such as diet, physical inactivity, obesity, and smoking, but these are not incorporated in screening recommendations. Moreover, the joint association of these factors with various colorectal polyps is not established.
Methods
A case–control study, among consecutive subjects aged 40–70 years, undergoing colonoscopy. Cases with colorectal polyps were compared with controls. Detailed information was gathered regarding polyp histology and anatomic location, demographics, medical history, anthropometrics, and lifestyle. The healthy lifestyle index was estimated as the sum of: non-smoking, maintaining a healthy weight, healthy diet, and physical activity.
Results
A total of 788 participants were included (cases n = 403, controls n = 385). The healthy lifestyle index had a negative association with colorectal polyps (OR = 0.72, 95% CI 0.62–0.85, P < 0.001), both adenomas and serrated polyps (OR = 0.75, 0.64–0.89, and OR = 0.59, 0.44–0.79, respectively), and both proximal and distal adenomas (OR = 0.77, 0.62–0.95, and OR = 0.73, 0.59–0.90, respectively). Adherence to ≥ 2 healthy lifestyle components was strongly related with colorectal polyps (OR = 0.50, 0.34–0.75, P = 0.001). Abstinence from smoking, and a healthy diet were the factors most strongly associated with lower odds of colorectal polyps (OR = 0.58, 0.42–0.79, and OR = 0.61, 0.44–0.85, respectively).
Conclusions
Adherence to a healthy lifestyle (≥2 healthy lifestyle components) is inversely associated with colorectal polyps, especially serrated and distal polyps, with no dose–response association. Components most strongly associated with lower odds of colorectal polyps were maintaining a healthy diet and abstinence from smoking. Lifestyle-related characteristics may assist in risk stratification and are potential goals for colorectal neoplasia prevention.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is a multifactorial disease, resulting from genetic and environmental factors. The malignant transformation of colonic epithelial cells is hypothesized to occur as a result of both prolonged exposure to environmental modifiable risk factors, and an inherited genetic predisposition, which can lead to the development of adenomas and carcinomas [1]. Unhealthy eating habits [2, 3], lack of physical activity (PA) [4, 5], obesity [6], abdominal obesity [7], and cigarette smoking [8, 9] are among the modifiable risk factors identified. In recent studies, smoking has been established as a risk factor for CRC among various populations [10,11,12], and has been suggested to be the strongest predictor of CRC in a western, average risk population [13]. Lack of PA has been linked with reduced risk of CRC, by potentially increasing secretion of anti-carcinogenic myokins such as interleukin-6, interleukin-8, and tumor necrosis factor-α [14, 15], and by reducing obesity. Obesity and abdominal obesity in turn, are both acknowledged risk factors for many cancer types and CRC [16, 17]. The dietary factors most strongly associated with colorectal neoplasia are high intake of red and processed meat, and low intake of dietary fiber [18,19,20]. Plant-based diets such as the Vegetarian and Mediterranean diets, which are characterized by a high fiber and low heme-iron, N-nitroso compounds and lipid peroxidation content [21, 22], have been associated with lower risk of CRC [2, 3, 23]. Physical inactivity [5], diet [24, 25], excess body weight, and smoking [26] have been found in previous studies to be more strongly associated with CRC of the distal colon, rather than the proximal colon [27, 28]. This may be explained by the exposure to the content and variety of microbiota, bile acids, and particular dietary factors, which differs between the proximal and distal colorectum [29]. Sporadic proximal CRC differ from distal tumors in their molecular profiling [30] that may suggests different interaction with these exposure factors.
Although the individual role of these lifestyle factors has been investigated in CRC, little is known about their joint effect on colorectal neoplastic occurrence. “Real-life” behavior typically combines several of these exposures, making lifestyle-related risk factors difficult to assess, and personalized risk-stratification complex. Therefore, four lifestyle characteristics (healthy diet, abstinence from smoking, performance of PA and maintaining a normal weight) have recently been combined by the American Heart Association (AHA) to a healthy lifestyle index, which has been shown to be associated with a lower risk for the metabolic syndrome [31], cardiovascular disease [32], and cancer [33], including CRC [34]. This association is hypothesized to be mediated by insulin resistance (IR) and low grade inflammation [35, 36]. As lifestyle characteristics are associated with risk for IR and Diabetes mellitus (DM) [37], and DM is an established risk factor for colorectal neoplasia [38], it is unclear if a beneficial lifestyle could potentially be associated with lower odds for colorectal polyps within this high risk population. Also, the association between the healthy lifestyle index and colorectal polyps, and polyp anatomical location has not been studied yet. As colorectal polyps are the precursors of CRC, and as this pathology may be progressive, establishing risk factors for this early stage in the carcinogenic pathway could add to the understanding of the potential means for efficient CRC prevention and contribute to personalized colonoscopy screening intervals. Therefore, we aimed to (a) evaluate the association between overall adherence to a healthy lifestyle index and colorectal polyps of various types and anatomic locations; (b) examine the independent association between healthy lifestyle index components and colorectal polyps; (c) examine the modifying effect of pre-diabetes or diabetes in the relationship between overall adherence to the healthy lifestyle index, and colorectal polyps.
Materials and methods
A case–control study, among consecutive subjects aged 40–70 years, undergoing colonoscopy at the Department of Gastroenterology and Hepatology at the Tel-Aviv Medical center (TLVMC) during 2010–2015. The source population for this study included all subjects undergoing colonoscopy at a large tertiary referral center. We aimed to select a population with minimal risk of genetic predisposition for colorectal neoplasia so the impact of environmental risk factors for CRC could be assessed. Exclusion criteria for both cases and controls were: familial hereditary CRC syndromes (such as Lynch and Familial polyposis syndromes), personal history of CRC, first-degree family history of CRC below the age of 70, inflammatory bowel disease, celiac disease, solid malignancy, hyperthyroidism, past colectomy, recent hospitalization or surgery, pregnancy, chronic liver disease, or grade 4–5 chronic kidney disease. Cases were further excluded for personal history of colorectal polyps before the age of 40, or diagnosis of > 5 colorectal polyps (ever). In addition, controls were excluded for any past colonic polyps. Participants with excessive alcohol intake (≥ 30 g/day in men or ≥ 20 g/day in women), positive hepatitis serology, or an unreasonable food frequency questionnaire (FFQ) (total calories did not reach 500 or 800 kcal, or exceeded 3500 or 4000 kcal for women/men, respectively) were also excluded.
The study protocol was approved by the Institutional Review Board of the TLVMC, and all participants provided informed consent prior to the study enrollment.
Definition of cases and controls
Polyp histology was reviewed by a gastrointestinal (GI) pathologist, and classified as adenomatous or serrated polyps (hyperplastic polyps and serrated adenomas). Regarding cases with more than one polyp, polyp type, and location were defined according to that with the highest neoplastic potential. Polyp location was defined based on International Statistical Classification of Diseases 10th edition for CRC as proximal colon polyps (cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure) and distal colon polyps (descending and sigmoid colon and rectum) [39]. Due to a small sample size, serrated polyps were not sub-grouped according to location.
Data collection
Within 2 months after their colonoscopy, participants were requested to undergo a medical interview, anthropometric measurements, and blood tests, and answer questionnaires on lifestyle and diet. Participant’s blood tests were obtained following a 12-hour fast, and were analyzed at a single lab. Blood pressure, weight, height, hip, and waist circumference were measured using a uniform protocol. Body mass index (BMI) was calculated as weight (kilograms)/high (meters)2 [2]. Participants were face-to-face interviewed for their medical history, demographic characteristics, lifestyle, and dietary intake. Evaluation of dietary intake was performed using a structured detailed semi-quantitative FFQ, assembled by the Food and Nutrition Administration of the Israeli Ministry of Health, validated for the Israeli population [40], and composed of 116 food items with specified serving sizes. Participants were asked to report their average diet during the past year. Mean daily intake was calculated for each food group and micro-nutrient including sodium intake (mg/day).
Medical background was documented and positive DM status (pre DM or DM) was defined as HbA1c > 5.7%/Glucose ≥ 100 mg/dl/use of antidiabetic medication [41].
Definition of a healthy lifestyle and calculation of the healthy lifestyle index
Four healthy lifestyle components from the strategic goals of AHA [42, 43] were defined as follows: (1) lifetime abstinence from smoking, (2) no current obesity (BMI < 30 kg/m2), (3) regular performance of strenuous physical activity (running, biking, dancing, ball games etc.) at least once weekly or moderate physical activity (walking on a treadmill, body shaping, yoga, Pilates, resistance training etc.) at least five times weekly. (4) A healthy dietary pattern based on: consumption of an increased amount of fruits (fresh whole fruit/1 cup fruit salad ≥ 3 portions/day), nuts (0.5 cup of natural unsalted nuts and seeds/1 tablespoon of nut or seed paste products ≥ 1 portions/day), vegetables (fresh or cooked whole vegetables/1 cup of chopped vegetables ≥ 3 portions/day), whole grains (one slice of whole wheat bread/0.5 cup cooked whole grains or whole-grain products ≥ 3 portions/day), fish (one serving fresh fish cooked, baked, or grilled ≥ 2 portions/week), and dairy products (one cup of milk or yogurt/two slices of hard cheese/two tablespoons of soft spreadable cheese ≥ 2.5 portions/day), and a reduced amount of refined grains (one slice of bread/half cup cooked grains or grain products ≤ 1.5 portions/day), processed meat and fish (one serving of any processed chicken, beef or fish/smoked meat or fish/canned fish ≤ 1 portions/week), unprocessed red meats (one serving fresh beef, veal, pork products, and internal organs cooked, baked, and grilled ≤ 1.5 portions/week), sugar-sweetened beverages (one cup fruit juice/iced tea/non-diet soft drinks ≤ 1 portions/day), and sodium (≤ 2000 mg/day).
A healthy diet score was calculated as the sum of all components to which participants adhered, ranging from 0 to 11, and a value of ≥5 defined as a healthy diet pattern.
A healthy lifestyle index was calculated as the sum of all four AHA lifestyle components to which participants adhered and ranged from 0 to 4 points. Healthy lifestyle index categories were defined as an unfavorable, intermediate, and a favorable lifestyle; adherence to 0–1, 2, or 3–4 healthy lifestyle components respectively as previously described [43].
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as means ± SD and dichotomous variables as proportions. Pearson Chi-Square test was used to test the association between categorical variables. As all continuous variables distributed normally, these were compared between study groups using the independent samples t test. Multivariate logistic regression analysis was used to test the association between lifestyle characteristics and colorectal polyps, controlling for potential confounders (variables that distributed differently between cases and controls and may be related with lifestyle characteristics). Adjustments were made also for potential mediators, metabolic, and inflammatory markers, which are likely to be influenced by lifestyle and are related with the outcomes.
P < 0.05 was considered statistically significant for all analyses.
Results
Description of the study population and univariate comparison between cases with colorectal polyps and controls
The study population included 788 eligible participants (mean age 58.8 ± 6.5 years, 52.7% men). A flowchart of the included participants and study groups is described in Fig. 1.
Among the total study population, the mean healthy lifestyle score was 1.8 ± 1.04, whereas 37.2% were categorized as having an unfavorable lifestyle, 35.3% intermediate lifestyle, and 27.5% favorable lifestyle.
A significant portion was treated with Statins (45.9%) and Aspirin (32.5%), 61.9% were defined as pre-diabetic/diabetic, and 73.2% had hypertension.
Comparison between cases with colorectal polyps and controls is described in Table 1. Cases with colorectal polyps were older, had a higher proportion of men and as expected, had a higher proportion of surveillance colonoscopies. Though caloric intake and proportion of PA performance were similar between cases and controls, cases had a higher BMI, metabolic parameters such as fasting serum HbA1C%, and TG/HDL ratio. Cases with colorectal polyps had a significantly lower proportions of participants who never smoked, were non-obese, and maintained a healthy diet. The proportions of healthy lifestyle parameters were specifically lower among cases with distal polyps, distal adenomas and serrated polyps as compared with controls (Table 1).
The proportion of participants with an unfavorable lifestyle index was significantly higher among cases with proximal polyps/adenomas, distal polyps/adenomas, and serrated polyps as compared with controls (Fig. 2).
Multivariate association between the healthy lifestyle index and colorectal polyps
In a multivariate analysis, adjusting for: age, gender, low SES, colonoscopy indication, use of statins, aspirin, antihypertensive, and antidiabetic medication, the healthy lifestyle index was inversely associated with colorectal polyps, including proximal and distal polyps and adenomas, and serrated polyps. Additional adjustment for potential mediators: fasting serum HbA1C%, TG/HDL ratio, and C-reactive protein (CRP), attenuated the association only with proximal polyps or adenomas (Table 2).
Stratification by pre DM/DM status revealed negative association between the healthy lifestyle index and colorectal polyps among both groups, although the associations were stronger among participants without DM (Table 2).
Multivariate association between the number of healthy lifestyle components and colorectal polyps
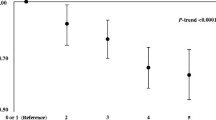
Adjusting for potential confounders, strong negative associations were seen between adherence to ≥2 healthy lifestyle components and colorectal polyps, compared with an unfavorable lifestyle (Fig. 3). Additional adjustments for potential mediating factors: fasting serum HbA1C, TG/HDL and CRP levels, did not attenuate the association (OR = 0.50, 95% CI 0.34–0.75, P = 0.001). There was no apparent dose–response association between the number of adhered healthy lifestyle index components and colorectal polyps. Strong associations were seen between adherence to ≥2 healthy lifestyle components and lower odds of both adenomas and serrated polyps.
Adjusted association between the number of healthy lifestyle index characteristics and colorectal polyps, compared with controls. Unfavorable lifestyle—0–1 healthy lifestyle categories, intermediate lifestyle—2 healthy lifestyle categories, Favorable lifestyle—3–4 healthy lifestyle categories. ORs are adjusted for: age, gender, low SES, colonoscopy indication (screening, diagnostic, and surveillance), use of statins, aspirin, antihypertensive, and antidiabetic medications
Association between individual healthy lifestyle components and colorectal polyps
Adjusting for various confounders and for each other, the individual component of the healthy lifestyle index, which were consistently associated with lower odds of colorectal polyps were: lifetime abstinence from smoking and maintaining a healthy diet. Strong negative associations were seen with lower odds of both proximal and distal adenomas and serrated polyps (Table 3).
Discussion
Lifestyle is a major modifiable exposure, extensively studied in the context of chronic disease prevention, including CRC [44]. As opposed to the evidence of the protective association between healthy lifestyle and CRC, evidence regarding an association with colorectal polyps is scarce [45, 46]. Our study elaborates on this association by testing the role of a healthy lifestyle as a whole, and its four individual components, in colorectal neoplasia. As colorectal polyps are the precursors of CRC, they are an independent target for endoscopic screening practices. Our results show that a healthy lifestyle pattern is inversely associated with both adenomas and serrated polyps, and with both distal and proximal adenomas. The association seems to be stronger with serrated polyps and distal colorectal adenomas. These associations are independent of many confounding factors and some of the potential metabolic and inflammatory mediators.
Proximal and distal colorectal polyps have been shown to be independent of one another [47], and to differ in their association with CRC [27]. Although traditionally distal polyps have been considered more strongly linked to environmental carcinogens [48], proximal polyps have been linked to genetic background [49]. Conversely, our results show that both distal and proximal polyps are associated with lifestyle, even with adjustment for various potential confounders. If these results will be confirmed in prospective studies, it may imply that individuals with an unfavorable lifestyle who have greater risk for colorectal neoplasia, may benefit from screening colonoscopy instead of flexible sigmoidoscopy, as some clinical guidelines suggest [50].
Interestingly, a strong protective association with colorectal polyps, particularly distal colorectal adenomas, was seen with adherence to merely two healthy lifestyle components, which is a realistic goal for lifestyle modifications as means of colorectal neoplasia prevention. If these results are confirmed by large prospective studies, adherence to at least two healthy lifestyle components may be set as a target for prevention of the early stages of the colorectal carcinogenic pathway.
Of all four components of the healthy lifestyle index, maintaining a healthy diet and abstinence from smoking were most strongly associated with colorectal neoplasia, with adjustment for many potential confounding factors and for other lifestyle characteristics. Therefore, this study supports an independent association of these lifestyle behaviors with colorectal polyps, implying that they should be in the focus of colorectal carcinogenesis prevention. Association between the healthy lifestyle index components, and colorectal polyps was strong for both adenomas and serrated polyps, though to a greater extent with the latter, similarly to previous studies [27, 28]. Indeed, smoking has been demonstrated to be a strong risk factor for serrated polyps [51], and neoplasia [52]. Furthermore, some studies have shown that smoking may interact with red meat, an important component of the AHA unhealthy diet, synergistically increasing the risk for CRC [53]. Surprisingly, there was no independent association between being non-obese and performance of PA and colorectal polyps in this study, though these have been shown by others to be independently protective of colorectal neoplasia [5, 54]. A possible explanation for this discrepancy may be the fact that these previous studies did not adjust for smoking and healthy eating.
As DM is an established risk factor for colorectal neoplasia [38], these associations were analyzed while stratifying by DM status. Our data suggest that adhering to a healthy lifestyle can be protective from distal and serrated colorectal polyps in patients with and without pre DM or DM. Further adjustment for metabolic parameters, which may act as mediators, attenuated the association among participants with pre DM/DM, but not among participants without pre DM/DM. This may imply that among patients with pre DM/DM, the impact of healthy lifestyle is mainly exerted through improved metabolic profile. Thus, this study adds to the existing body of evidence and supports the notion that maintaining a healthy lifestyle may be an effective strategy for colorectal neoplasia prevention even among metabolically high-risk populations.
The limitations of this study include the lack of temporal sequence, which does not permit a causal inference. Reverse causality should also be considered, though exposures were evaluated as long-term habits. Cases and controls were recruited from the same population and had comparable socioeconomic characteristics, thus minimizing a potential selection bias.
In terms of external validity, this study population was intentionally selected to represent a population with low to medium risk for colorectal polyp, thus our findings cannot be generalized to high-risk populations. We had a relatively high prevalence of chronic disease in this study population, which may be attributed to the nature of a hospital-based colonoscopy setting. We attempted to minimize a potential confounding effect and elaborate on effect modification by stratification across DM status, as this chronic condition is highly associated with CRC. Nutritional data have been collected within a single country, which may have impact on the generalizability across populations with different diets. Information bias, and particularly recall bias, on lifestyle characteristics may exist, owing to the retrospective nature of the study. This was minimized by a uniform structured lifestyle and dietary questionnaire, which was assessed in the same manner in cases and controls, to prevent differential bias in participant’s reports owing to interview or seasonal changes. Participants were blinded to the study hypothesis, and owing to the fact that the association under investigation is not common knowledge, we assume a potential report bias is most probably non-differential, and may only weaken the strength of the observed associations. Finally, regression dilution bias may exist as data were gathered from a single measurement, which may weaken the strength of the observed associations.
In conclusion, adherence to a healthy lifestyle pattern is inversely associated with colorectal polyps, especially serrated polyps and distal adenomas, among people with or without pre DM/DM. Strong associations were seen with adherence to at least two healthy lifestyle components, with no apparent dose–response association. Adhering to as little as two healthy lifestyle components may be a sufficient and realistic goal for prevention of colorectal neoplasia, even in populations with established risk factors such as obesity and DM. The individual components most strongly associated with lower odds of colorectal polyps were maintenance of a healthy diet and abstinence from smoking, both are established major goals for chronic disease prevention. In the era of precision medicine, where individual risk stratification is of high importance, the healthy lifestyle index can be easily obtained and perhaps aid in the construction of personalized CRC screening and surveillance recommendations.
References
Smith RA, Andrews K, Brooks D, DeSantis CE, Fedewa SA, Lortet-Tieulent J, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2016;66:95–114.
Park S-Y, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L. High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the multiethnic cohort. Gastroenterology. 2017;153:386–394.e2.
Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 2017;152:1944-1953.e1.
Mehta M, Shike M. Diet and physical activity in the prevention of colorectal cancer. J Natl Compr Canc Netw. 2014;12:1721–6.
Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22:492–505.
Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177–90.
Ortega LS, Bradbury KE, Cross AJ, Morris JS, Gunter MJ, Murphy N. A prospective investigation of body size, body fat composition and colorectal cancer risk in the UK biobank. Sci Rep. 2017;7:17807.
Ordóñez-Mena JM, Schöttker B, Mons U, Jenab M, Freisling H, Bueno-de-Mesquita B, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016;14:62.
Lee PN, Thornton AJ, Hamling JS. Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul Toxicol Pharm. 2016;80:134–63.
Kaminski MF, Polkowski M, Kraszewska E, Rupinski M, Butruk E, Regula J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut. 2014;63:1112–9.
Corte C, Zhang L, Chen J, Westbury S, Shaw J, Yeoh KG, et al. Validation of the Asia Pacific Colorectal Screening (APCS) score in a Western population: an alternative screening tool. J Gastroenterol Hepatol. 2016;31:370–5.
IJspeert JEG, Bossuyt PM, Kuipers EJ, Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Smoking status informs about the risk of advanced serrated polyps in a screening population. Endosc Int Open. 2016;4:E73–8.
Schroy PC, Wong JB, O’Brien MJ, Chen CA, Griffith JL. A risk prediction index for advanced colorectal neoplasia at screening colonoscopy. Am J Gastroenterol. 2015;110:1062–71.
Devin JL, Hill MM, Mourtzakis M, Quadrilatero J, Jenkins DG, Skinner TL. Acute high intensity interval exercise reduces colon cancer cell growth. J Physiol (Lond). 2019;597:2177–84.
Roy P, Chowdhury S, Roy HK. Exercise-induced myokines as emerging therapeutic agents in colorectal cancer prevention and treatment. Future Oncol. 2018;14:309–12.
Martinez-Useros J, Garcia-Foncillas. J obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med 2016;14:21.
Hu H, Cai Y, Huang J, Zhang J, Deng Y. Visceral adipose tissue and the risk of colorectal adenomas: a meta-analysis of observational studies. Eur J Cancer Prev. 2015;24:462–9.
Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, Castaño-Vinyals G et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr. 2018;58:1495–1505.
Yao Y, Suo T, Andersson R, Cao Y, Wang C, Lu J, et al. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2017;1:CD003430.
Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: a systematic review of experimental results. Exp Biol Med (Maywood). 2017;242:813–39.
Demeyer D, Mertens B, De Smet S, Ulens M. Mechanisms linking colorectal cancer to the consumption of (Processed) red meat: areview. Crit Rev Food Sci Nutr. 2016;56:2747–66.
Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–60.e16.
Cottet V, Bonithon-Kopp C, Kronborg O, Santos L, Andreatta R, Boutron-Ruault M-C, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14:21–9.
Abe SK, Inoue M, Sawada N, Iwasaki M, Ishihara J, Sasazuki S, et al. Rice, bread, noodle and cereal intake and colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective Study (JPHC Study). Br J Cancer. 2014;110:1316–21.
Bernstein AM, Song M, Zhang X, Pan A, Wang M, Fuchs CS, et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS ONE. 2015;10:e0135959.
Martínez F, Fernández-Martos C, Quintana MJ, Castells A, Llombart A, Ińiguez F, et al. APC and KRAS mutations in distal colorectal polyps are related to smoking habits in men: results of a cross-sectional study. Clin Transl Oncol. 2011;13:664–71.
He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. 2018;155:355–73.e18.
Davenport JR, Su T, Zhao Z, Coleman HG, Smalley WE, Ness RM, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. 2018;67:456–65.
Gianfredi V, Salvatori T, Villarini M, Moretti M, Nucci D, Realdon S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int J Food Sci Nutr. 2018;69:904–15.
Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz H-J. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69–80.
Lucini D, Zanuso S, Blair S, Pagani M. A simple healthy lifestyle index as a proxy of wellness: a proof of concept. Acta Diabetol. 2015;52:81–9.
Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, et al. Adherence to healthy lifestyle and cardiovascular diseases in the chinese population. J Am Coll Cardiol. 2017;69:1116–25.
Orenstein L, Chetrit A, Dankner R. Healthy lifestyle pattern is protective against 30-yr cancer incidence in men and women: a cohort study. Nutr Cancer. 2016;68:410–9.
Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168.
Tilg H, Moschen AR. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract Res Clin Gastroenterol. 2014;28:599–610.
Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357–73.
Levesque C. Therapeutic lifestyle changes for diabetes mellitus. Nurs Clin North Am. 2017;52:679–92.
Luo S, Li J-Y, Zhao L-N, Yu T, Zhong W, Xia Z-S, et al. Diabetes mellitus increases the risk of colorectal neoplasia: an updated meta-analysis. Clin Res Hepatol Gastroenterol. 2016;40:110–23.
ICD-10-CM. Chapters List. https://icd.codes/icd10cm (Accessed 11 Apr 2018).
Kaluski DN, Goldsmith R, Arie OM, Mayer C, Green M. The first Israeli national health and nutrition survey (MABAT) as a policy maker. Public Health Rev. 2000;28:23–6.
Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the american heart association and the american diabetes association. Diabetes Care. 2015;38:1777–803.
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Horn LV, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613.
Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–58.
Medina-Remón A, Kirwan R, Lamuela-Raventós RM, Estruch R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2018;58:262–96.
Tabung FK, Steck SE, Burch JB, Chen C-F, Zhang H, Hurley TG, et al. A healthy lifestyle index is associated with reduced risk of colorectal adenomatous polyps among non-users of non-steroidal anti-inflammatory drugs. J Prim Prev. 2015;36:21–31.
Erben Vanessa, Carr PrudenceR, Holleczek Bernd, Stegmaier Christa, Hoffmeister Michael, Brenner Hermann. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: results from a large cohort of participants of screening c…—PubMed—NCBI. Int J Cancer. 2019;144:2135–43.
Huang JLW, Wang YH, Jiang JY, Yu CP, Wu YL, Chen P, et al. The association between distal findings and proximal colorectal neoplasia: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1234–45.
Lindblom A. Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol. 2001;13:63–9.
Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001.
Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2964–73.
Fliss-Isakov N, Zelber-Sagi S, Webb M, Halpern Z, Kariv R. Smoking habits are strongly associated with colorectal polyps in a population-based case-control study. J Clin Gastroenterol. 2018;52:805–11.
Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–22.
Ognjanovic S, Yamamoto J, Maskarinec G, Le Marchand L. NAT2, meat consumption and colorectal cancer incidence: an ecological study among 27 countries. Cancer Causes Control. 2006;17:1175–82.
Nimptsch K, Pischon T. Body fatness, related biomarkers and cancer risk: an epidemiological perspective. Horm Mol Biol Clin Invest. 2015;22:39–51.
Author information
Authors and Affiliations
Contributions
Fliss-Isakov N. conceived the study; designed the study; collected the data; and preformed statistical analysis, interpreted the results, and wrote the manuscript. Kariv R. conceived the study; designed the study; interpreted the results; and wrote the manuscript. Zaslavsky O. critically reviewed the manuscript. Webb M. performed medical data collection. Ivancovsky-Wajcman D. performed the data collection. Margalit D. performed the data collection. Shibolet O. critically reviewed the manuscript. Zelber-Sagi S. conceived the study; designed the study; interpreted the results; and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fliss-Isakov, N., Kariv, R., Webb, M. et al. A healthy lifestyle pattern has a protective association with colorectal polyps. Eur J Clin Nutr 74, 328–337 (2020). https://doi.org/10.1038/s41430-019-0481-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0481-2
- Springer Nature Limited