ABSTRACT
Background
Carnitine deficiency is common in patients on hemodialysis. However, the efficacy of L-carnitine supplementation for improving lean body mass (LBM) and physical function has not yet been evaluated.
Methods
In this multicenter, prospective, parallel, randomized, controlled trial, 91 patients on hemodialysis who developed carnitine deficiency were randomly assigned to receive injections of 1,000 mg L-carnitine 3 times per week after each hemodialysis session (L-carnitine group) or no injections (control group) with monitoring for 12 months.
Results
The data for 84 of the 91 patients were available for analysis (L-carnitine group, n = 42; control group, n = 42). Dry weight and body mass index did not significantly change in the l-carnitine group, but significantly decreased in the control group. Arm muscle area (AMA) did not change significantly in the L-carnitine group but decreased significantly in the control group; the difference in mean AMA between the groups was 6.22% (95% confidence interval [CI] 1.90–10.5; P = 0.037). Hand grip strength did not change significantly in the L-carnitine group, but decreased significantly in the control group. The difference in change in hand grip strength between the groups was 4.27% (95% CI 0.42–8.12; P = 0.030). Furthermore, LBM did not change significantly in the l-carnitine group but decreased significantly in the control group; the difference in mean LBM between the groups was 2.92 % (95% CI 1.28–4.61; P = 0.0007).
Conclusions
l-carnitine supplementation is useful in patients who develop carnitine deficiency on hemodialysis because it maintains physical function and LBM.
Similar content being viewed by others
Introduction
Carnitine deficiency is known to develop in patients receiving hemodialysis (HD) [1, 2], and may contribute to a number of clinical conditions, including cachexia, erythropoiesis stimulating agent-resistant anemia, myasthenia, insulin resistance, and myopathy, as well as intradialytic symptoms, including muscle cramps, hypotension, and arrhythmias [3, 4].
Sarcopenia is part of an age-related biological phenomenon of diminished reserve and impaired resistance to stressors because of deterioration in several physiological systems. It is characterized by a decline in skeletal muscle mass resulting in decreased muscle strength and decreased physical function [5, 6]. Previous studies have confirmed the association of sarcopenia with adverse health outcomes, including disability, hospital admission, placement in long-term care, reduced quality of life, falls, and mortality [5, 6]. Sarcopenia is also common in patients with chronic kidney disease and is associated with adverse clinical outcomes, especially in individuals with end-stage kidney disease (ESKD) on maintenance dialysis [7,8,9]. A substantial number of factors affect nutritional and metabolic status in ESKD and have multiple adverse consequences [10]. Physical activity typically decreases with age in both the general population and in patients on maintenance HD [11], and reduced physical function is associated with higher mortality rate in patients on maintenance HD [12].
In spite of the clinical significance of sarcopenia, there are but a few comprehensive intervention programs. Amino acid deficiency, including carnitine deficiency, is thought to be associated with the pathophysiology of this syndrome. However, the effects of L-carnitine on physical function in patients on HD have not been documented. The aim of this study was to determine the effects of L-carnitine treatment for 12 months on the physical and nutritional status of patients undergoing HD.
Subjects and Methods
Patient characteristics
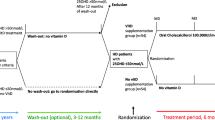
Ninety-one of 146 patients on HD who were screened for eligibility to participate in this multi-center, prospective, randomized, open-label, parallel controlled trial were assigned by random allocation to either an L-carnitine group or a control group. For the L-carnitine group, patients received injections of l-carnitine 1,000 mg (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) 3 times weekly after dialysis sessions for 12 months, while for the control group, patients received standard treatment without l-carnitine injections. An independent investigator with no prior knowledge of the subjects before the start of the trial monitored the order of randomized allocation of patients to the groups. Dynamic balancing randomization was carried out based on duration of HD, age, sex, body mass index (BMI), and presence or absence of diabetes mellitus. In this way, we ensured that baseline characteristics were not significantly different between the groups. The group assignment details were then provided to each of five independent investigators. All participants were treated with HD or hemodiafiltration treatment for 4-h sessions three times per week at any one of three Japanese blood purification centers.
The inclusion criteria were as follows: age ≥20 years and ≤85 years, duration of HD > 12 months at enrollment, carnitine deficiency (free plasma carnitine concentration <40 μmol/L), and patients for whom medical decisions were made at the participating hospitals. The following exclusion criteria were applied: a history of severe cardiac failure, myocardial infarction, angina pectoris, or stroke within the preceding 6 months; concurrent infectious disease, thyroid disorder, or malignancy; steroid or immunosuppressant therapy; current hospitalization; limb amputation; and l-carnitine treatment within the past 6 months. Participants were regularly provided with dietary guidance by a dietician, particularly those with dietary restrictions on their energy, protein, salt, and fluid intake. Participants were withdrawn from the study, when they developed l-carnitine intolerance during the study period, if medical events occurred leading to death, admission to hospital, substantial disability, or incapacity, or if there was a transfer to another hospital. In the control group, participants were withdrawn from the study when they developed apparent symptoms of L-carnitine deficiency and administration of l-carnitine was to be initiated or when the investigator deemed that withholding L-carnitine treatment would pose a safety problem. The study protocol was approved by the ethics committee of Keiai Hospital and all procedures fully adhered to the Declaration of Helsinki. The study was registered with the University Hospital Medical Information Network (UMIN000025327). All participants provided written informed consent. The study was conducted between January 2016 and December 2017.
Study evaluations
The primary efficacy endpoints were physical function-related parameters, including dry weight, BMI, mid-upper arm muscle area (AMA), hand grip strength, and lean body mass (LBM), which were measured as the magnitude of change from baseline and compared between the two groups. The secondary endpoints were the differences in magnitude of change from baseline in clinical parameters between the two groups.
Blood samples were collected before the beginning of each HD treatment. Serum urea nitrogen and serum albumin levels were measured using routine clinical chemistry methods with commercially available kits. C-reactive protein (CRP) levels were measured using the latex agglutination test. Cardiothoracic ratio was measured on chest radiographs. Normalized protein catabolic rate (nPCR) was calculated for each subject. These variables were assessed at baseline and at the end of the 12-month study period. Efficacy variables were also analyzed within the two groups according to age and diabetes status.
Sarcopenia-related and physical function parameters
All eligible participants were required to undergo dual-energy X-ray absorptiometry (DXA) scans to determine their LBM, fat mass, and skeletal muscle mass. DXA scanning was performed immediately after the mid-week or end-of-week HD session to minimize any influence of hydration status at the start or end of the study. The DXA scanning was performed using a Lunar iDXA® machine (GE Healthcare, Tokyo, Japan).
Hand grip strength was measured bilaterally in the standing position using a dynamometer adjusted to fit each subject’s hand size. The mean of the hand grip strength values in the two hands was used for the analysis. Arm circumference (AC) and triceps skinfold thickness (TSF) were measured by trained instructors. AMA was then calculated using the following formula:[13]
Muscle mass was assessed by performing DXA using height-adjusted skeletal muscle mass with the recommended cutoff values of 7.0 kg/m2 for men and 5.4 kg/m2 for women [14]. Muscle strength was assessed by measuring hand grip strength using the cutoff values of 26 kg for men and 18 kg for women as recommended by the Asian Working Group for Sarcopenia [14]. Thus, sarcopenia was defined as a muscle mass of <7.0 kg/m2 for men and <5.4 kg/m2 for women by DXA and hand grip strength of <26 kg for men and <18 kg for women [14]. Hand grip strength (kg/cm2) was assessed with adjustment for AMA.
Statistical analysis
The data are expressed as the mean ± standard deviation or median (interquartile range) as appropriate. Continuous variables were compared using Mann-Whitney U test or the Student’s t-test and categorical variables were compared using Fisher’s exact test or chi-square test based on the data distribution. Changes in physical function parameters, including dry weight, BMI, AMA, hand grip strength, and LBM, were defined as the differences between the baseline values and those at the study end; values are shown as the mean and 95% confidence interval (CI). Changes in these parameters between baseline and 12 months between the two groups were analyzed by using logistic regression. Spearman’s rank correlation coefficients were used to determine the associations between changes in plasma carnitine concentrations and the variables. Effect size was calculated using Cohen’s d. Sample size was calculated based on 80% power, with a presumed effect size of a 2.8% difference in change in LBM from baseline between the groups with a standard deviation of 4.5% according to previous studies [15, 16]. This resulted in a significance level of 0.05 (two-sided) and the estimated number of patients to be evaluated was 84 (42 per group). With an allowed dropout rate of 10% after randomization, we calculated that 90 randomized subjects (45 per group) would be required. A value of P < 0.05 was considered statistically significant. All analysis was carried out using JMP version 12 (SAS Institute Inc., NC, USA).
Results
Study characteristics at baseline
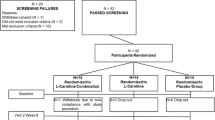
In total, 91 eligible participants were enrolled and randomly assigned the L-carnitine group (n = 45) or the control group (n = 46); 7 patients had incomplete assessments or did not complete treatment (3 in the L-carnitine group (6.7%) and 4 in the control group (8.7%)). The data for the remaining 42 participants in the l-carnitine group and 42 patients in the control group were entered into the final analysis (Supplementary Figure 1). None of the patients in the control group required initiation of l-carnitine administration. No significant differences were noted in baseline demographic, or anthropometric features, mode of dialysis, type of vascular access, history of cardiovascular disease, or medicines used between the groups (Table 1). The mean plasma free carnitine concentration at baseline was 26.0 ± 6.2 μmol/L in the L-carnitine group and 26.6 ± 6.9 μmol/L in the control group (P = 0.708). The prevalence of sarcopenia was 28.6% in the L-carnitine group and 26.2% in the control group (P = 0.806).
Effects of L-carnitine supplementation on physical function-related parameters
Table 2 shows the physical function-related and clinical parameters in the two groups at baseline and at the end of the study. There was no significant difference in dry weight between the two groups at baseline. Also, there was no significant change in dry weight in the L-carnitine group (from 57.4 ± 10.9 kg at baseline to 57.7 ± 11.1 kg at the end of the study; P = 0.419); however, there was a significant decrease in dry weight in the control group (from 58.4 ± 12.2 kg to 57.8 ± 12.9 kg; P = 0.035). The difference in the mean change in dry weight in the L-carnitine group versus the control group at the end of the study was 1.95% (95% CI: −0.36, −0.02; P = 0.021; Supplementary Figure 2a). There was no significant change in BMI in the L-carnitine group, but BMI decreased significantly from 21.5 ± 3.8 at baseline to 21.2 ± 3.9 at the end of the study in the control group (P = 0.044; Table 2). The difference in the mean change in BMI in the l-carnitine group versus the control group by the end of the study was 2.18% (95% CI: −0.51, −0.07; P = 0.004; Supplementary Figure 2b).
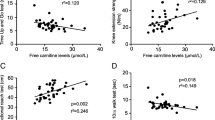
There was no significant change in AMA in the L-carnitine group but there was a significant decrease in AMA in the control group from 40.0 ± 10.7 cm2 at baseline to 38.3 ± 10.7 cm2 at 12 months (P = 0.001; Fig. 1a). The difference in the mean AMA between the groups at the study end was 6.22% (95% CI: 1.90–10.5; P = 0.037; Fig. 1b).
Hand grip strength adjusted for AMA showed a significant decrease in the control group from 0.57 ± 0.25 kg/cm2 at baseline to 0.55 ± 0.25 kg/cm2 at the study end (P = 0.041) with no significant change in the l-carnitine group (0.59 ± 0.21 kg/cm2 at baseline, 0.59 ± 0.21 kg/cm2 at 12 months; P = 0.408; Fig. 2a). The difference in mean change in hand grip strength at 12 months was 4.27% (95% CI 0.42–8.12; P = 0.030; Fig. 2b).
There was no significant change in LBM in the L-carnitine group (44.6 ± 7.5 kg at baseline, 44.9 ± 7.3 kg at 12 months; P = 0.224; Fig. 3); however, there was a significant decrease in LBM in the control group (from 43.3 ± 9.8 kg at baseline to 42.5 ± 9.7 kg at 12 months; P = 0.009). The difference in the mean change in LBM between the groups at 12 months was 2.92 % (95% CI: 1.28–4.61; P = 0.0007).
In the L-carnitine group, there was no association between changes in plasma concentrations of free carnitine and the measured variables, including dry weight (ρ = −0.19, P = 0.066), BMI (ρ = −0.17, P = 0.117), AMA (ρ = -0.08, P = 0.469), hand grip strength (ρ = 0.12, P = 0.495), and LBM (ρ = −0.13, P = 0.224).
Effects of L-carnitine supplementation on HD efficiency and clinical parameters
There were no significant changes in serum urea nitrogen and nPCR in either of the two groups during study period. Similarly, there was no significant difference in CRP levels or Kt/V between the two groups. Serum albumin level significantly decreased in the control group but not in the L-carnitine group.
Subgroup analysis
The efficacy of L-carnitine was also examined in subgroups of patients according to diabetes status and age (median cutoff 74 years) at baseline. No significant difference was found in sex distribution, age, duration of HD, dry weight, BMI, AMA, hand grip strength, or LBM at baseline between the subgroups with and without diabetes (Table 3). Also, no significant difference was noted in CRP levels between the L-carnitine and the control groups (0.27 [0.08–0.70] mg/dL vs. 0.10 [0.03–0.41] mg/dL; P = 0.272). Notably, there were significant differences in percent change in dry weight, BMI, AMA, hand grip strength, and LBM in the subgroup with diabetes but not in the subgroup without diabetes.
When patients were divided according to age (≥74 or <74 years), no significant difference was found in sex distribution, age, duration of HD, dry weight, BMI, AMA, hand grip strength, or LBM at baseline between the two groups (Table 4). However, in the older subgroups, there were significant differences in percent change in dry weight, BMI, AMA, and LBM between the two groups; in the younger subgroups, only the change in LBM was significant between the two groups.
Safety and tolerability
There were no significant adverse events during the study. None of the patients required interruption of treatment and l-carnitine was well tolerated.
Discussion
In this 12-month study of patients undergoing HD, there was a significant reduction in clinical dry weight, BMI, and serum levels of albumin in the control participants but not in the participants who received L-carnitine supplementation. Furthermore, the percentage mean changes in AMA, hand grip strength, and LBM differed significantly between the two groups, especially in diabetes patients and those who were older. L-carnitine supplementation is reported to have beneficial effects on physical, mental, and cognitive function in older adults [15,16,17]. Significant increases in lean arm and leg muscle mass without any significant change in arm or leg strength have been reported in healthy older adults receiving carnitine supplementation [18]. To the best of our knowledge, this study is the first to investigate the effect of L-carnitine supplementation on physical status in patients on HD.
Our findings suggest that l-carnitine supplementation has a significant effect on physical status as assessed with AMA, hand grip strength, and LBM. These findings suggest that L-carnitine may have favorable effects on mitochondrial function, including correction of a deficient cellular energy supply, facilitation of long-chain fatty acid transport, which are the main source of energy in skeletal muscle, into the mitochondria, facilitation of short- and medium-chain fatty acids removal following their accumulation during metabolism, and maintenance of optimal protein and lipid levels [19]. l-carnitine supplementation may result in increased rate of fatty acid oxidation, preservation of muscle glycogen stores, and increased production of ATP [17]. These putative mechanisms may improve and maintain muscle function by improving energy metabolism in skeletal muscle.
The most important risk factors for mortality and morbidity in dialysis patients are inflammation and protein-energy wasting (PEW) [20,21,22]. The reported prevalence of PEW and frailty in patients on dialysis is ~30%; one third of patients have mild to moderate PEW and about 6–8% of these patients also have severe malnutrition [23,24,25,26]. Sarcopenia and its component features are highly correlated with not only hospitalization but also mortality, even after adjustment for well-established risk factors across multiple domains. Some reports have described malnutrition and chronic inflammation in patients on maintenance HD [27, 28]. Also, high levels of proinflammatory cytokines have been shown to cause muscle wasting by stimulating protein catabolism via the ubiquitin-proteasome pathway [29]. In addition, l-carnitine can reduce the production of interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, all of those are associated with several pathogenic processes [30,31,32]. In the present study, there was no change in CRP levels or nPCR, which reflects dietary protein intake. Inflammatory cytokines and lipid peroxides were not evaluated in this study, so further research would be needed to confirm a link between L-carnitine supplementation and reduction in inflammation and prevention of muscle wasting.
Patients with ESKD often develop insulin resistance. Insulin resistance may be caused by malnutrition or sarcopenia. Therefore, inflammation is a common feature in insulin resistance, sarcopenia, and atherosclerosis. Lack of physical fitness might also be a factor in insulin resistance in patients with ESKD [33, 34]. Improvements in tissue oxygenation and exercise tolerance have been shown to reverse insulin resistance in patients with erythropoiesis stimulating agent-resistant anemia [34,35,36]. l-carnitine has been reported to ameliorate insulin resistance in ESKD patients, by regulating cellular energy metabolism or reducing free fatty acid levels and impaired carnitine metabolism [37,38,39]. L-carnitine is capable of promoting glucose use in peripheral tissues and cellular insulin sensitivity [40, 41] by increasing oxidative use of glucose, activating pyruvate dehydrogenase, and decreasing the acetyl-CoA/CoA ratio in the mitochondria [42]. These mechanisms may have led to the preserved physical function seen in our study. However, we could not evaluate the precise changes of insulin resistance or proinflammatory cytokine levels in response to L-carnitine supplementation. Therefore, further study is needed to clarify exactly how L-carnitine effects changes in LBM.
Our study has several limitations. First, it did not include monitoring of markers of oxidative stress or of inflammatory cytokines. Therefore, the mechanisms underlying the preserved physical function mediated by anti-oxidative or anti-inflammatory processes during L-carnitine supplementation remain unknown. Second, it is well known that changes in LBM of <1 kg cannot be measured confidently with DXA; given that we could not measure the tissue hydration, further analysis adjusted for tissue hydration is necessary to assess the changes in LBM. Third, this study was not performed in a double-blind manner. Therefore, an adequately powered, double-blind, randomized clinical trial would be necessary to determine whether improved physical function in patients who receive L-carnitine is associated with better prognosis.
In conclusion, our findings showed the utility of L-carnitine injection for prevention of muscle weakness in patients who develop carnitine deficiency while on HD. The benefits of L-carnitine supplementation in these patients include maintenance of both physical function and LBM. Further large-scale clinical studies would be needed to establish l-carnitine supplementation significantly influences mortality rates for dialysis patients.
References
Evans AM, Faull RJ, Nation RL, Prasad S, Elias T, Reuter SE, et al. Impact of hemodialysis on endogenous plasma and muscle carnitine levels in patients with end-stage renal disease. Kidney Int. 2004;66:1527–34.
Higuchi T, Abe M, Yamazaki T, Okawa E, Ando H, Hotta S, et al. Levocarnitine improves cardiac function in hemodialysis patients with left ventricular hypertrophy: a randomized controlled trial. Am J Kidney Dis. 2016;67:260–70.
Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20:218–43.
Matera M, Bellinghieri G, Costantino G, Santoro D, Calvani M, Savica V. History of L-carnitine: implications for renal disease. J Ren Nutr. 2003;13:2–14.
Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7.
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–56.
Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. International Society of Renal Nutrition and Metabolism. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84:1096–107.
Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24:337–51.
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23:77–90.
Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–70.
Stack AG, Martin DR. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am J Kidney Dis. 2005;45:730–42.
Bishop CW. Reference values for arm muscle area, arm fat area, subscapular skinfold thickness, and sum of skinfold thicknesses for American adults. J Parenter Enter Nutr. 1984;8:515–22.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
Badrasawi M, Shahar S, Zahara AM, Nor Fadilah R, Singh DK. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging. 2016;11:1675–86.
Evans M, Guthrie N, Pezzullo J, Sanli T, Fielding RA, Bellamine A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study. Nutr Metab (Lond). 2017;14:7.
Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007;86:1738–44.
Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, Koverech A, et al. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008;46:181–90.
Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. 2003;41(Suppl 4):S4–12.
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–63.
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8.
Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–13.
Johansen KL. The frail dialysis population: a growing burden for the dialysis community. Blood Purif. 2015;40:288–92.
Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–7.
Bellinghieri G, Santoro D, Calvani M, Savica V. Role of carnitine in modulating acute-phase protein synthesis in hemodialysis patients. J Ren Nutr. 2005;15:13–7.
Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–48.
Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationship between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15:953–60.
Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307.
Bistrian BR, Schwartz J, Istfan NW. Cytokines, muscle proteolysis, and the catabolic response to infection and inflammation. Proc Soc Exp Biol Med. 1992;200:220–3.
Duranay M, Akay H, Yilmaz FM, Senes M, Tekeli N, Yücel D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3211–4.
Suchitra MM, Ashalatha VL, Sailaja E, Rao AM, Reddy VS, Bitla AR, et al. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J Kidney Dis Transpl. 2011;22:1155–9.
Shakeri A, Tabibi H, Hedayati M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. 2010;14:498–504.
Siew ED, Ikizler TA. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial. 2010;23:378–82.
Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia. 1995;38:565–72.
Mak RHK. Correction of anemia by erythropoietin reverses insulin resistance and hyperinsulinemia in uremia. Am J Physiol. 1996;270:F839–94.
Abe M, Okada K, Soma M, Matsumoto K. Relationship between insulin resistance and erythropoietin responsiveness in hemodialysis patients. Clin Nephrol. 2011;75:49–58.
Rasmussen BB, Holmbäck UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest. 2002;110:1687–93.
Günal AI, Celiker H, Dönder E, Günal SY. The effect of L-carnitine on insulin resistance in hemodialysed patients with chronic renal failure. J Nephrol. 1999;12:38–40.
Savica V, Calvani M, Benatti P, Santoro D, Monardo P, Peluso G, et al. Carnitine system in uremic patients: molecular and clinical aspects. Semin Nephrol. 2004;24:464–8.
Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20:218–43.
Ferrannini E, Buzzigoli G, Bevilacqua S, Boni C, Del Chiaro D, Oleggini M, et al. Interaction of carnitine with insulin-stimulated glucose metabolism in humans. Am J Physiol. 1988;255:E946–952.
Capaldo B, Napoli R, Di Bonito P, Albano G, Saccà L. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14:191–5.
Author contributions
Research concept and study design: TH, TM, NM. Acquisition of data: CN, HT, MK, KU, RT, TF, TY, EO. Data analysis and interpretation: TM, NM. Statistical analysis: TH, MA. Supervision or mentorship: HA, FK. Each author contributed significant intellectual content during drafting or revision of the manuscript and takes accountability for the overall work by ensuring appropriate investigation and resolution of questions pertaining to the accuracy or integrity of any aspect of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MA has received honoraria from Otsuka Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Takashi Maruyama, Noriaki Maruyama
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Maruyama, T., Maruyama, N., Higuchi, T. et al. Efficacy of L-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: a randomized controlled trial. Eur J Clin Nutr 73, 293–301 (2019). https://doi.org/10.1038/s41430-018-0348-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0348-y
- Springer Nature Limited
This article is cited by
-
Was ist L-Carnitin, und sollten wir es bei CKD supplementieren?
Die Nephrologie (2024)
-
Mitigating the pro-oxidant state and melanogenesis of Retinitis pigmentosa: by counteracting mitochondrial dysfunction
Cellular and Molecular Life Sciences (2021)