Abstract
The immune system plays a crucial role in protecting the body from invading pathogens and maintaining tissue homoeostasis. Maintaining homoeostatic lipid metabolism is an important aspect of efficient immune cell function and when disrupted immune cell function is impaired. There are numerous metabolic diseases whereby systemic lipid metabolism and cellular function is impaired. In the context of metabolic disorders, chronic inflammation is suggested to be a major contributor to disease progression. A major contributor to tissue dysfunction in metabolic disease is ectopic lipid deposition, which is generally caused by diet and genetic factors. Thus, we propose the idea, that similar to tissue and organ damage in metabolic disorders, excessive accumulation of lipid in immune cells promotes a dysfunctional immune system (beyond the classical foam cell) and contributes to disease pathology. Herein, we review the evidence that lipid accumulation through diet can modulate the production and function of immune cells by altering cellular lipid content. This can impact immune cell signalling, activation, migration, and death, ultimately affecting key aspects of the immune system such as neutralising pathogens, antigen presentation, effector cell activation and resolving inflammation.
Similar content being viewed by others
Introduction
The immune system is comprised of innate and adaptive cell lineages which have specialised functions [1, 2]. Intimately linked to immune cell function is their metabolism. As such we have identified that the various immune cells have unique lipid signatures which are linked to their functions [3]. Disruption of lipid metabolism through ectopic lipid accumulation or a defect in metabolism in immune cells can promote inflammatory phenotypes [4], impair cell migration [5] and immune functions [6] as well as increasing cell proliferation [7, 8]. Therefore, maintaining homoeostatic lipid metabolism is crucial for efficient immune cell function.
One of the key functions of lipids in immune cells is their role as a fuel source for cellular maintenance, proliferation and modulating cellular signalling. For example, it is well established that classical and alternatively activated macrophages utilise lipids differently as sources of energy. Classical macrophages have reduced oxidative capacity and increased rates of de novo lipogenesis [9, 10], whereas alternatively activated macrophages exhibit increased rates of oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO) along with decreased glycolysis [9, 11]. These metabolic differences between classical and alternatively activated macrophages are tightly linked to their specific functions and are further highlighted by their distinct lipid compositions [12]. Metabolic diseases have highlighted that dysregulated systemic lipid metabolism, e.g., that caused by a high fat or western-type diet, influences immune cell function. In the setting of atherosclerosis, monocytes are recruited to the cholesterol rich atherosclerotic plaque core and differentiate into macrophages to clear the accumulated cholesterol. As macrophages accumulate more cholesterol than they have the capacity to efflux, intracellular cholesterol is converted into cholesteryl esters (CE) giving rise to macrophages with impaired lipid metabolism and cellular function. Functionally, due to the excessive accumulation of cholesterol, macrophages increase the release of inflammatory cytokines [13] and exhibit impaired migratory capabilities [14, 15].
Apart from serving as a source of energy, one of the most crucial roles of lipids is their capacity to create bilayers, facilitating the development of cellular membranes. Cellular membranes play a vital role in sustaining life by enabling cells to separate their internal components from the external surroundings [16]. Additionally, these membranes contribute to the formation of sub-cellular organelles; mitochondria and endoplasmic reticulum both of which are integral to fatty acid (FA) metabolism. The diverse chemical makeup of lipids permits the creation of membranes with unique biophysical and biochemical attributes, such as fluidity, shape, and thickness [17, 18]. These attributes are pivotal for essential cellular functions like migration, adhesion, signalling, and cell death [16]. The importance of maintaining systemic lipid homoeostasis is highlighted by the many diseases that stem from dysregulated lipid metabolism, perhaps best exemplified by obesity and its associated metabolic diseases, e.g., insulin resistance and type 2 diabetes mellitus (T2DM). A key feature of the dysregulated lipid metabolism that occurs in obesity is the over accumulation of lipids in critical metabo-regulatory tissues, notably the skeletal muscle, adipose tissue, and the liver. This ectopic lipid accumulation disrupts normal tissue function, most classically leading to insulin resistance, a key stage in the development of T2DM. While obesity leads to dysregulated lipid metabolism and lipid accumulation in many cell and tissue types, whether the lipid metabolism of adaptive immune cells is altered in metabolic disease states is unknown. Importantly, however, it is known that the function of innate immune cells: monocytes and macrophages are altered in metabolic diseases [19, 20]. Altered immune cell function can contribute to systemic inflammation via the release of inflammatory cytokines due to excessive accumulation of intracellular lipid within the adipose tissue and liver [21,22,23]. Inflammation persists due to the ongoing release of inflammatory cytokines and infiltration of inflammatory immune cells into metabolic tissues [24].
While we know that immune cells become dysfunctional in metabolic diseases, the cause of this dysfunction is yet to be fully elucidated. In this review we hypothesise that cellular defects in immune cells may arise from altered lipid metabolism, similarly to how dysfunction arises in the skeletal muscle, adipose tissue, and liver. It has been well established that innate immune cells, monocytes [25, 26] and macrophages [27, 28] undergo significant lipid remodelling within the adipose tissue and atherosclerotic plaques, but less is known about circulating and tissue resident lymphoid cells; T and B cells. This review highlights the importance of lipid metabolism in immune cells and when disturbed how this impacts immune cell function. We will also discuss how in the setting of metabolic disease immune cell function is impaired and propose the hypothesis that modulating the lipid composition of immune cells can improve their function.
Importance of lipid metabolism to immune cell function
T cells
The intrinsic metabolic preference of immune cells is precisely controlled and is broadly coupled to immune activity, function, differentiation, and activation status [29]. Naïve CD4+ and CD8+ T cells are quiescent and can be activated by cytokine, costimulatory signals and antigen-T cell receptor signalling to undergo a process of effector T cell differentiation and proliferation. Effector CD8+ T cells upregulate cytolytic (granzymes, perforin) and effector cytokine expression [type I interferon (IFN)-γ, tumour necrosis factor (TNF)], involved in direct killing of pathogen infected or malignant cells, while effector CD4+ T cells differentiate into diverse helper subsets (TH1, TH2, TH17, Tfh and Treg) which mediate diverse immune responses and have roles in maintaining self-tolerance [30].
Naïve T cells deriving their energy from lipids generally do so via their catabolism in the mitochondria. Through FAO, the breakdown of FAs fuels the electron transport chain to generate adenosine triphosphate (ATP). The other major metabolite is glucose, which can be metabolised through the citric acid cycle to generate several intermediates important for cellular processes such as gene regulation and ATP production through the electron transport chain [31]. Additionally, glucose metabolised through the TCA can provide citrate for de novo lipogenesis, providing FAs for other cellular functions. It is well documented that disruption to this finely tuned immune cell metabolism can impair their ability to function correctly [32, 33]. While this will not be a focus in this review, aerobic glycolysis is an important metabolic pathway for immune cells. In actively proliferating effector cells pyruvate generated from glucose metabolism is converted to lactate via lactate dehydrogenase (Ldh) under limiting oxygen conditions, instead of being utilized in the TCA cycle. This phenomenon of Warburg metabolism is a well-defined post-transcriptional and epigenetic modulator of effector genes and is reviewed in several articles [34, 35].
Lipid metabolism is essential for immune cell effector functions, but also plays wider roles in regulating immune cell development, maturation, survival, and longevity. Under homoeostatic conditions, FA synthesis (FAS) driven by ACC1 (acetyl coenzyme A carboxylase 1) is required for both CD4+ and CD8+ effector memory (CD44+CD62L+) T cell maintenance in peripheral tissues, homoeostatic proliferation and survival, without influencing normal thymic T cell development [36]. Following infection with Listeria monocytogenes expressing OVA (LmOVA), mice lacking ACC1 specifically with CD4+ T cells had a significantly reduced number of OVA+ specific and IFN-γ producing CD8+ T cells compared with WT controls. FAS also regulates CD4+ T helper cell differentiation. By inhibiting the activity of ACC1 and 2 in naïve CD4+, T cells differentiated under TH17-polarising conditions skewed towards a Treg phenotype [37]. This was evidenced by reduced expression of TH17 associated genes, Il7f, Stat3 and Hif1a, along with a downregulation of interleukin (IL)-23 receptor expression which is essential for TH17 differentiation. During induced experimental autoimmune encephalomyelitis, a TH17 cell mediate autoimmune disease, inhibition of ACC1 reduced the number of IL-17 producing cells in central nervous system, and resulted in milder disease severity compared to controls, further demonstrating the essential role of FAS during TH17 differentiation. Collectively, these studies highlight the importance of lipid metabolism in directing T cell differentiation, survival and cell fate decisions (TH17/Treg balance), which can differentially influence outcomes during pathogen infection and autoimmune diseases. For example, in the setting of metabolic disorders AMP-activated protein kinase (AMPK) is inactivated and can no longer phosphorylate and restrain ACC1 which would, as detailed above, favour TH17 cell maturation over Tregs, propagating an inflammatory environment [38,39,40].
Subsets of CD4+ and CD8+ effector T cells are poised to be long-lived and remain after the resolution of infection, and after contraction and apoptosis of short-lived effector T cells. Memory T cell differentiation has also been shown to rely upon FAO. CD8+ memory T cells employ lysosomal acid lipase to mobilise FA from internal stores fuelling FAO as they develop [41] (Fig. 1). IL-7, a memory promoting cytokine, induces Aquaporin 9 expression in memory T cells allowing glycerol transport fuelling triglyceride synthesis that is subsequently utilized for FAO [42], thus CD8+ memory T cells synthesise substrates for FAO from internal and external sources. In mice with a T-cell specific deletion of tumour necrosis factor receptor associated factor 6 (TRAF6), preventing negative regulation of antigen-specific T-cell activation, LmOVA induced a robust CD8+ T cell effector response, but failed to produce antigen specific memory CD8+ T cells [43]. This was not due to the loss of a negative regulator of antigen-specific T-cell activation as mice lacking Cbl-b5, still developed a robust CD8+ T memory response following LmOVA immunisation [43]. TRAF6 deficient T cells displayed reduced expression of genes involved in FA metabolism which was consistent with reduced FAO [43]. The reduced FAO was explained by reduced phosphorylated-AMPKα, a key regulator of FAO. However, what drives reduced AMPK in these cells is unclear, but could be linked to an inability to dampen glucose utilization when growth signals (i.e., IL-2) are removed. Importantly, when AMPK activity was promoted indirectly by Metformin, this too restored FAO in TRAF6 deficient T cells, which was accompanied by an increase in CD8+ T memory cell production [43], likely via the uptake of fatty acyl-CoA through carnitine palmitoyltransferase (CPT)-1a. As such, AMPK is a key regulator of CD8+ T cell contraction as they switch to FAO, allowing them to persist as long-lived memory T cells.
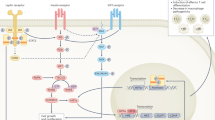
Fatty acid oxidation (FAO) and fatty acid synthesis (FAS) are essential components to immune cell differentiation and survival. A CD8+ memory T cell development relies upon lysosomal adipose lipase (LAL) activity to mobilise fatty acids (FA) from internal lipid droplet stores [41, 64]. CD8+ Memory T cells then generate ATP via FAO within mitochondria. B Germinal centre B cells (GCBC) proliferation is fuelled by the uptake of exogenous FAs whereby ATP is generated via FAO [45]. C The inhibition of acetyl coenzyme A carboxylase 1 (ACC1) via AMPK activation in naïve CD4+ T cells promotes regulatory T cell differentiation [37]. D When ACC1 is activated naïve CD4+ T cells differentiate towards a TH17 cell phenotype highlighted by the upregulation of TH17 cell associated genes [40]. E CD8+ T cell survival is dependent on FAS [36]. ATP citrate lyase (ACLY), carnitine palmitoyltransferase (CPT), electron transport chain (ETC), fatty acid synthase (FASN) and tricarboxylic acid (TCA). Schematic created with BioRender.com
B cells
Lipid metabolism also plays a critical role in differentiation and maintenance of B cells. Following activation, naïve B cells undergo differentiation, proliferation and form long-lasting antibody secreting plasma cells [44]. During germinal centre formation, follicular B cells are recruited to germinal centres by T-dependent antigens and become highly proliferative, as they undergo somatic hypermutation and clonal expansion on their way to providing long-term humoral immunity. Interestingly, unlike many lymphocyte classes that employ glycolysis to proliferate at high rates, germinal centre B cells (GCBCs) oxidise FAs to meet their demands. This was elegantly shown through a series of experiments employing genetic and pharmacological inhibition of CPT-2, a key enzyme involved in FA transport into the mitochondria [45] (Fig. 1). Furthermore, GCBC can be categorised into light zone (LZ) and dark zone (DZ) B cells [46]. LZ and DZ B cells express their own unique set of machinery to meet their metabolic requirements [46]. LZ B cells are enriched in genes that support glycolysis whereas DZ B cells are enriched in genes that facilitate FAO [47]. The genetic differences between these B cell subsets highlights how localisation of cells contributes to their metabolism, ensuring metabolic demands are met to function correctly. This poses the question whether the same genetic differences would be observed between other immune cell subsets such as follicular DCs or Tfh cells that interact with GCBCs or T cell subsets in the thymus during T cell development. Palmitic acid labelling studies revealed that these cells derive their FAs extracellularly as opposed to intrinsic lipolysis [45], a point of difference to CD8+ memory T cell development. Interestingly, the uptake of FAs, particularly mono-unsaturated fatty acids (MUFAs), can activate mammalian/mechanistic target of rapamycin complex 1 (mTORC1) [48], which is required to stimulate activation-induced cytidine deaminase for GCBCs to undergo somatic hypermutation, class switching and form antibody secreting plasma cells [49]. Why these cells favour FAO, particularly from extrinsic sources has not been fully determined but may be an efficient way to obtain FAs where surrounding cells undergo apoptosis, providing a rich source of membrane phospholipids which contain FAs. Whether the type of FAs used to achieve their proliferative and survival rates matters is also an open question (with the noted exception of MUFA oleic acid [48]), and if this process is perturbed (or enhanced) with different FAs would be interesting to explore.
Impact of AMPK signalling on immune cell function
The AMPK signalling pathway plays a pivotal role in coordinating lipid metabolism in metabolic tissues and its dysregulation is tightly associated with metabolic disease [50]. Reduced AMPK activity is associated with increased inflammation in adipose tissue and systemic insulin resistance in obese individuals [51]. Aside from coordinating lipid metabolism in metabolic tissues, AMPK signalling is also integral to B and T cell function [52, 53]. Genetic ablation and pharmacological inhibition have revealed the importance of AMPK-dependent signalling in maintaining OXPHOS capacity, mitochondrial quality and in reducing ROS production (Fig. 2). AMPK controls antibody synthesis in plasma cells as well as promoting the long-term survival and function of memory B cells by maintaining mitochondrial function and preventing lipid peroxidation [32], which may safeguard these cells from ferroptosis. We have reported that T cells are highly susceptible to ferroptosis due to their increased abundance of poly-unsaturated fatty acid (PUFA) phospholipids [3]. Accordingly, protecting Tfh cells from ferroptosis via the dietary supplementation of selenium (cofactor of GPX4) has been shown to promote their interactions with GCBCs and enhance humoral immunity following influenza vaccination [54]. Thus, we ponder how metabolic perturbations resulting in defective AMPK signalling would alter the formation of germinal centres. The clinical relevance of AMPK activation in B cell responses has been noted in individuals with T2DM, where those on metformin had significantly more memory B cells and more robust antibody responses to influenza vaccination [55]. Ablation of AMPK1α in T cells led to reduced homoeostatic proliferation of CD4+ and CD8+ T cells and impaired long-term maintenance of peripheral effector memory (CD44+CD62L-) T cells due to perturbed FAO, accelerated mitochondrial clearance, and increased ROS production [33]. Consequently, AMPK1α-deficient T cells mounted impaired antigen-specific CD8+ T cell responses to viral or bacterial challenge, and reduced CD4+ TH1 and TH17 differentiation during experimental transfer colitis [56]. Given that in metabolic disease AMPK signalling is dysregulated in key metabolic tissues, as well as in B and T memory cells, we speculate whether an explanation for immune cell dysfunction in metabolic disease is due to dysregulated AMPK signalling and the sequalae of lipid related consequences, including ferroptosis.
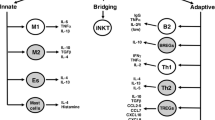
AMPK signalling regulates lymphoid cell function via fatty acid oxidation (FAO). A Intact AMPK signalling: Effector & memory T cell survival is maintained through regulated FAO. As T cells mature there is decreased reliance upon glycolysis for energy production and as such AMPK signalling is activated, indirectly promoting LAL activity generating fatty acids from internal lipid stores. These fatty acids along with some that are acquired exogenously then undergo FAO to sustain effector & memory T cell populations. B Dysfunctional AMPK signalling (i.e., in metabolic disorders): AMPK signalling becomes dysfunctional following sustained glycolysis/nutrient excess. Reduced AMPK signalling has been shown to cause mitochondrial dysfunction and increases ACC1 activity and increased production of malonyl-CoA. Malonyl-CoA, inhibits CPT-1a, preventing mitochondrial FAO, and is a key substrate for polyunsaturated fatty acid (PUFA) production and coupled with ACSL4 could result in increased phospholipids containing PUFAs (PUFA-PL). PUFA undergo lipid peroxidation due to increased production of reactive oxygen species (ROS) as a result of sustained glycolysis and mitochondrial dysfunction. Increased lipid peroxidation of membrane PUFA-PL causes ferroptotic cell death which could be contributing to decreased survival of effector & memory T cells that have impaired AMPK signalling. Schematic created with BioRender.com
Insights into the importance of intracellular lipid trafficking in immune cells
The above sections defined the importance of cellular metabolism to immune cell function. However, another important aspect of lipid metabolism is localization through intracellular trafficking as lipids pass through cellular compartments such as peroxisomes, endoplasmic reticulum and mitochondria. Trafficking of lipids in not only essential to cellular organization, but also to how a cell functions [57]. Indeed, some of the important regulators of lipid movement within cells has been explored in the setting of immune function. Fatty acid binding protein 5 (FABP5) is a small cytoplasmic protein that facilitates FA uptake, transport, and metabolism. In Tregs, inhibition of FABP5 (genetic or pharmacologic) had a profound effect on the restrained suppressive regulation of these cells, causing a massive induction of IL-10. This was achieved through mitochondrial dysfunction highlighted by decreased OXPHOS, impaired lipid metabolism and altered mitochondrial structure [58]. Following the knockdown of Fabp5, genes encoding enzymes that elongate and desaturate FAs were decreased in Tregs. In conjunction with decreased expression of genes controlling FA elongation and saturation, Tregs had reduced cardiolipin synthesis. Consequently this resulted in defective mitochondria, characterized by smaller structure with increased cristate width, which promoted the release of mitochondrial DNA, engagement of the cGAS-STING-dependent IFN and subsequently induction of IL-10 expression [58]. Consequently, this signalling cascade resulted in CD4+ T cell suppression [58]. The increased suppression of CD4+ T cells can lead to reduced activation of the innate immune system, B cells and cytotoxic T cells which would be detrimental to the host’s ability to elicit an effective immune response against pathogens, increasing their likelihood of developing infection and disease. Indeed, tumour Tregs, while having dramatically increased expression of Fabp5 compared to splenic Tregs, reside in tumour microenvironment that lacks lipid substrate. This also results in defective FABP5 function and thus the ensuing mitochondrial dysfunction and induction of copious amounts of IL-10, contributing to a cold tumour microenvironment [58]. Isoforms of Fabp, including Fabp1, 2, 4 and 6 also play essential roles in the survival and maintenance of resident memory T cells found in skin [59], and other non-lymphoid tissues such as the liver and small intestine [60] following pathogen infection. Frizzell et al., also demonstrated that expression of Fabp was dependent on the tissue microenvironment, and that T cells could modulate their FABP expression upon relocation to new microenvironments [60]. Trafficking of lipids in immune cells is also influenced by the mitochondrial enzyme CPT-1a (the main isoform in leucocytes), which together with CPT-2 transports FAs from the cytosol into the mitochondria to facilitate FAO [61,62,63]. Inhibiting CPT-1a in CD8+ T cells reduced memory T cell survival compared to CD8+ effector T cells highlighting the importance of CPT-1a and FAO to specifically CD8+ memory T cell survival [64]. Furthermore, activated CPT-1a transduced OT-I T cells adoptively transferred into congenic mice following L. monocytogenes immunisation displayed a significant increase in CPT-1a transduced memory T cells compared to control-transduced memory T cells, further demonstrating the role of CPT-1a in memory T cell development and survival. The genetic knockdown of Cpt1a in tissue-resident memory T cells impairs their expansion and survival compared to effector T cells [59] further demonstrating that exogenous FA uptake and metabolism is essential for long-term survival of memory T cells.
These studies highlight that intrinsic metabolic pathways can be influenced by extrinsic substrates and that there is a complex interplay between the cell and the tissue microenvironment which could be targeted for effective immunotherapy. We suggest that detailed lipidomic analysis of various tumour microenvironments, along with tumour-infiltrating immune cell populations, would be critical to improving current immunotherapy regimes by understanding the effects of specific lipid substrates in the regulation of immune cell function and fate.
The role of phospholipids on immune cell function
To understand the role of phospholipids in immune cell function, it is first important to appreciate the three key components to phospholipids that dictate their function. These are, 1) the biochemical composition of the head group, 2) the type of chemical bond between the head group and acyl chain at the sn-1 position 3) the length and saturation status of the two fatty acyl chains [65]. Active transport of phosphatidylinositol (PI) plays a crucial role in thymic T cell development and the maintenance of peripheral T cells as loss of phosphatidylinositol-transfer protein Nir3 resulted in lymphopenia, loss of TCR-induced calcium flux and peripheral T cell maintenance [66]. Another study show that the saturation status of phosphoinositide (PIPn) FAs drives CD8+ effector T cell signalling and function [67]. Inhibiting the final step of de novo PI synthesis resulted in a significant reduction in the number of saturated PI species in T cells, which was accompanied by a reduction in CD8+ T cell viability, proliferation and IFN-γ production. Deletion of CDP-diacylglycerol-inositol 3-phosphatidyltransferase, an enzyme that produces saturated PIPs, revealed that saturated PIPn synthesis is essential for effector T cell fitness and function during infection, in tumours, and potentiates the therapeutic effects of checkpoint blockade in mouse and human T cells [67]. From an intracellular signalling perspective, the researchers showed that a reduction in saturated phosphatidylinositol biphosphate (PIP2) causes a decrease in DAGs, subsequently reducing the phosphorylation of downstream signalling proteins; rapidly accelerated fibrosarcoma, mitogen-activated protein kinase-1/2 and extracellular signal-regulated protein kinase (ERK) 1/2, ultimately hindering T cell function [67]. This finding suggests that phospholipase C (PLC)-γ1 preferentially binds with saturated PIPn compared with polyunsaturated PIPn and further highlights the importance of specific lipid species in coupling cell signalling with nutrient uptake. By skewing the composition of phospholipids this ultimately alters the intracellular signalling mechanics in T cells. PIPs also play an important role in the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway. PI3K converts PIP2 to PIP3 leading to the activation of Akt which coordinates, immune cell metabolism, survival, proliferation and differentiation [68, 69]. In metabolic disease, the PI3K/AKT pathway is indispensable to the insulin signalling pathway and dysregulation of this signalling is associated with insulin resistance [70]. Given this and the importance of PI3K to immune cell function, there is potential that a consequence of metabolic disease is impaired PI3K/AKT signalling pathways in immune cells leading to immune cell dysfunction. For example, this dysfunction could be presented through expansion of inflammatory immune cell subsets or reduced inability to combat pathogens during infection as lipid metabolism regulates these functions.
The role of ceramides on immune cell function
Ceramides are a class of bioactive sphingolipids that play a role in cell signalling and regulate important functions such as cell differentiation, proliferation and death [71]. The high-fat diet-induced accumulation of ceramides leads to cellular dysfunction and is suggested as a central driver of dysregulated whole-body metabolism, insulin resistance and T2DM. While the mechanisms that account for this are multifactorial, one of the key basis by which increased intracellular ceramide levels leads to cellular dysfunction is through the activation of protein phosphatase 2A (PP2A), a highly conserved serine-threonine phosphatase or by modulating T cell receptor signalling [72]. It was observed that ceramides accumulate at T cell receptor synapses following T cell activation, localizing with CD3, and influencing the phosphorylation of zeta-chain-associated protein kinase (ZAP)-70 and PLCγ. Accordingly, T-cell specific deletion of acid sphingomyelinase, which reduced total ceramide levels in activated T cells, or deletion of acid ceramidase, which blocked ceramide to sphingosine conversion leading to ceramide accumulation, impaired or enhanced T cell receptor signalling, effector function and cytotoxicity respectively, thereby influencing tumour control [72]. Intriguingly, ceramide levels have been shown to influence PP2A activity in Treg cells. Specifically, Apostolidis and colleagues investigated the activity of PP2A, its relationship with ceramide abundance, and how this impacts Treg function [73]. PP2A controls the activity of the mTORC1 complex in Treg cells, thereby influencing key cellular processes such as apoptosis, cell metabolism and cell migration. PP2A activity is controlled by the phosphorylation of subunit PP2Ac at Tyr307, which in turn is mediated by the ceramide-SET pathway. Through mass spectrometry, pathway enrichment analysis and intracellular signalling analysis, it was shown that PP2A activity in Treg is controlled by ceramide abundance. Of note, Tregs have increased ceramide levels compared with conventional T cells, which helps to restrain the mTOR pathway. This appears to be important as uncontrolled activation of the mTOR pathway in Tregs is associated with cellular dysfunction. The findings from this study demonstrate the importance of regulating the abundance of ceramides in T cells and highlights the detrimental effects that dysregulated ceramide metabolism could potentially have on Tregs and the function of conventional immune cells. Certainly, mTOR activity is essential to preserving cellular metabolism of T cells to maintain long-term T cell responses in chronic infection as dysregulated mTOR signalling contributes to T cell exhaustion [74], suggesting this could be impacted by ceramide levels. Efficient anti-tumour or bacterial immune responses require Tregs to reduce the abundance of intracellular ceramides to inhibit their immunosuppressive function [73]. If they are unable to control the abundance of intracellular ceramides, for example due to ceramide accumulation in response to a high fat diet, this could promote immunosuppressive functions and be detrimental to anti-tumour or bacterial immune responses. It is currently unknown if high fat diets increase ceramide levels within T cells or other immune cells; however, it is worth noting that increased ceramide levels have been observed in many tissues (e.g. skeletal muscle, heart, adipose tissue, liver, regions of the brain, and kidney) following a high fat diet [75,76,77], suggesting that many cell types are vulnerable to excessive ceramide accumulation.
The impact of metabolic disease on immune cell function
Research into metabolic disorders has primarily focussed on metabolic tissues, such as the liver, adipose tissue, and skeletal muscle [78]. An altered immune system is often observed in the setting of metabolic diseases. A textbook example of this is the formation of macrophage foam cells, due to excessive phagocytosis of lipids, coupled with an inability to efflux cholesterol, resulting in the progression of atherosclerotic plaques. However, metabolic disorders can also negatively influence other immune cells that are not professional phagocytes. Herein we have highlighted how T and B cell function is impaired in metabolic disease and the ensuing immune related consequences.
T cell function in obesity
T cells coordinate multiple aspects of immunity, including anti-viral [79] and anti-tumour [80] immune responses. In lean healthy mice, white adipose tissue depots are enriched with a significant reservoir of CD4+ and CD8+ memory T cells with distinct tissue-specific functional attributes [81]. During mucosal pathogen infection, mesenteric and gonadal adipose residing T cells were able to confer enhanced protection compared with lymphoid tissue memory T cells, in part by their modulation of lipid metabolism gene expression in neighbouring adipocytes [81]. Numerous studies have examined how obesity increases innate and adaptive immune cell trafficking and residency into adipose and non-lymphoid tissue sites and how these aspects influence essential T cell functions [82,83,84]. Chronic low-grade inflammation impairs CD8+ T cell effector functionality and fitness, while skewing CD4+ T helper differentiation to favour CXCR3 + TH1 and TH17 development due to increased cell intrinsic PI3K p100d/AKT signalling and ACC1 expression, respectively [40, 85]. Consequently, obese mice exhibit impaired survival during pathogen infection and exacerbated pathology in models of TH17-mediated autoimmune disease [21]. Compared with healthy controls, obese mice exhibited impaired control of influenza infection due to reductions in IFN-γ mRNA expression, decreased influenza specific and IFN-γ producing effector cells, and diminished functional lung resident memory CD8+ T cells [86]. Increased pathogen susceptibility could also contribute to impairments in antigen presentation and cytotoxic T lymphocyte (CTL) differentiation as IFN-γ also regulates dendritic cell antigen presentation and CTL differentiation [87, 88]. During secondary chronic viral rechallenge, obese mice had reduced survival due to increased CD8+ T cell mediated pancreatitis and adipose tissue necrosis [89]. Given the worldwide prevalence of obesity it is necessary to elucidate the mechanism(s) driving this defective T cell phenotype to improve anti-tumour and viral immune responses in obese individuals.
In a mouse model exploring breast cancer in lean and obese mice, obesity increased the proportion of tumour-derived T cells expressing programmed cell death protein (PD)-1, indicative of T cell exhaustion, while expression of T cell effector molecules Ifng and Gzmb is also reduced [90]. Notably, these changes were associated with higher numbers of tumours as well as significantly increased tumour size in obese mice compared with control mice. Insights into the mechanism could be obtained in T cells from cancer free obese humans, which revealed a significant increase in PD-1 expression and decreased proliferative capacity compared with nonobese individuals, suggesting that the increase in PD-1 expression is driven by pathways present in obesity [91] (Fig. 3). The upregulation of PD-1 in obesity could be driven by the underlying inflammation through TNF upregulating ERK1/2, a known inducer of PD-1 [92]. PD-1 dependent signalling supresses T cell glycolysis via the inhibition of Akt coupled with the subsequent inhibition of hexokinase 2 (HK2) and in turn upregulates adipocyte triglyceride lipase promoting lipolysis and FAO. As such this control over T cell metabolism prevents the switch to aerobic glycolysis required for effector T cell differentiation and leading to the loss of anti-tumour activity [93]. These mechanisms could underpin the clinical scenario where obese individuals with elevated levels of PD-1 have a poorer cancer prognosis compared to healthy individuals due to increased T cell exhaustion resulting in reduced anti-tumour T cell response. As the upregulation of PD-1 expression is linked to inhibiting effector cell function and a switch in T cell metabolism to FAO, therapeutic intervention should aim to reduce the rate of FAO in T cells to reduce PD-1 expression and T cell exhaustion resulting in improve anti-tumour responses and clinical outcomes for people with obesity (Fig. 4A). However, it should be noted that increased expression of markers of T cell exhaustion are not always found in obesity, yet the tumour infiltrating CD8+ T cells are still defective [94].
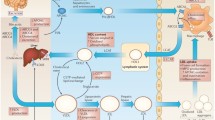
Obesity induced metabolic reprogramming promotes T cell exhaustion via the upregulation of PD-1 and glycolysis. A Obesity driven expression of TNF binds to TNFR-1/2 upregulating ERK1/2 which induces the expression of PD-1. B The engagement of PD-1 with PD-L1/2 results in metabolic reprogramming in T cells highlighted by the upregulated of lipolysis and ATGL, and CPT-1a promoting FAO. C The inhibition of Akt causes reduced expression of HK2 and consequently reduces glycolysis [93]. D The lowered rate of glycolysis prevents T cells from undergoing a’metabolic switch’ that is necessary for effector cell maturation which results in T cell exhaustion. As the T cells cannot maturate to effector cells, they are unable to elicit anti-tumour responses resulting in increased tumour growth. Adipose triglyceride lipase (ATGL) and programmed cell death protein (PD)-1. Schematic created with BioRender.com
Metabolic disease alters immune function of lymphoid immune cells. T and B cells from individuals with obesity have altered expression of cell surface markers and cytokine secretion impairing immune responses. A T cells from individuals with obesity have increased PD-1 expression, reduced IFNγ mRNA production reducing anti-tumour responses. B B cells have increased production of IgM antibodies and increased proliferation of DN B cells both of which are associated with metabolic disease. Schematic created with BioRender.com
Notably, not all studies show that changes in weight are associated with alterations in tumour development. Thus, while on the one hand a recent study exploring tumour infiltrating CD8+ T cells showed that tumour development is increased in a mouse model of diet induced obesity, this could be reversed by switching mice back to a chow diet, where weight loss and other positive changes in metabolic parameters were observed [94]. However, preforming a clinically significant experiment to determine if the blockbuster anti-obesity drug semaglutide [glucagon-like peptide(GLP)-1R agonist] could also improve tumour outcome, proved unsuccessful, even though the mice lost a significant amount of weight. This suggests that while improvement of whole-body metabolism and weight loss can improve tumour outcomes, this isn’t extended to all forms of weight loss. Another important point to note is that many studies are conducted with transplantable tumours, and this doesn’t reflect the ability of the intrinsic immune surveillance response to negate tumorigenesis. Interestingly, in a model of de novo tumorigenesis, it was found that obese mice had poor tumour surveillance, resulting in a significantly higher susceptibility to tumour transformation [94]. However, tumours in obese mice were more immunogenic and when transplanted into lean mice had a significantly delayed growth compared with transplanted tumours from lean mice, but ultimately grew to the same size over the experimental period [94]. Importantly, anti-PD-1 resulted in a significant improvement in survival in the mice that received tumours from obese mice, suggesting that immune checkpoint therapy could be effective in obese individuals with tumours that are subjected to rapid weight loss programs. While this study did not show an improvement with GLP-1R therapy, this was in one transplantable tumour model (B16 melanoma) and not in a de novo model. Thus, we wait with anticipation the epidemiological evidence for tumour incidence and outcomes from this class of weight loss drugs.
B cell function in obesity
Several studies have examined how obesity impacts B cell function demonstrating that B cell activity is impaired in obese mice and humans through the influence of essential FAs on humoral immunity [95]. B cells from non-obese and obese male subjects were stimulated via the toll like receptor-9 agonist CpG oligodeoxynucleotide (CpG-ODN). A decrease in IL-6 secretion but elevated levels of IgM from obese B cells, relative to lean controls, indicate dysfunctional B cell activity in obese individuals [95] (Fig. 4B). Furthermore, obese C57BL/6 mice had reduced haemagglutination inhibition antibody titres following influenza infection compared with lean mice [95]; an effect that was reversed by docosahexaenoic acid (DHA) dietary supplementation, an omega-3 (ω-3) essential FA with reduced levels in obese individuals [95]. The composition and function of the B cell pool influences an individual’s humoral immunity responses. B cells collected from obese individuals exhibited increased production of inflammatory cytokines IL-6 and TNF, decreased IL-10, increased mRNA expression of senescence associated genes, and consequently, reduced production of vaccine specific antibodies [96]. Profiling of B cell composition in obese individuals revealed a reduced frequency of class-switched memory B cells and an increase in double negative B cells which are associated with chronic autoimmune diseases [97] and inhibition of humoral immunity [96, 98]. Double negative B cells exhibit characteristics of exhaustion due to increased expression of PD-1 [99] and hence have impaired immune function. Therefore, it can be expected that obese individuals will have impaired preservation of memory and plasma B cells, impaired immune response to infection, as well as deficient vaccine-induced antibody production. While it is evident that obesity and metabolic diseases are accompanied by impaired adaptive and humoral immune responses are due to altered T and B cell differentiation and function, mechanisms are incompletely understood. Further understanding of how metabolic disease impacts intracellular signalling pathways that are involved in cellular lipid metabolism are required. Furthermore, connecting lipid metabolism to cell function and differentiation could help reveal novel targets to modulate PD-1 expression for example, to circumvent aspects of T cell exhaustion and improve the development of efficient immunotherapies in the context of chronic viral infection or cancer [100, 101].
Future perspectives: how can lipids be used to enhance immune cell function?
A hallmark of metabolic disease is the excessive accumulation of specific lipids in key metabolically regulated tissues. While we know that excessive lipid accumulation induces dysfunction in skeletal muscle [102, 103], liver [104, 105], adipose tissue [106] and tissue resident immune cells (such as macrophages [107]), we are only just beginning to understand how circulating and resident lymphoid cell function are affected by ectopic lipid accumulation and changes in cellular lipid composition in metabolic disease. While there is no direct evidence in the literature indicating a direct link between excessive lipid accumulation and cellular dysfunction of T and B cells, we propose that similarly to metabolic tissues and resident macrophages, T and B cell dysfunction arises because of excessive lipid accumulation and, in particular, dysregulated lipid metabolism during metabolic disease. Accordingly, numerous studies have assessed how FA treatment affects immune cells, revealing that particular FAs can enhance or supress immune activity. B cells isolated from mice fed a diet enriched in ω-3 PUFA, which are reduced in obesity [108,109,110], had increased IL-6 and IFN-γ secretion ex vivo, which was accompanied by an upregulation of CD69 expression [111]. B cell lymphoblasts treated with PUFAs had improved survival when incubated with alloreactive cytotoxic CD8+ T cells due to reduced MHC class I expression [112]. T cell lymphoblasts treated with ω-3 PUFAs in vitro increased MHC class I expression [113], while another study showed that treating T cells with PUFAs reduces CD69 expression reducing T cell activation [111]. Mass spectrometry confirmed that treatment of PUFAs elevated the abundance of PUFAs in T and B cells, highlighting that lymphoid cells can acquire exogenous FAs [111,112,113]. Given that immune cells uptake FAs from the extracellular environment, it would be highly beneficial for the immunometabolism field to investigate how the lipid composition of microenvironments affects immune cell lipid composition and function to enhance the current therapeutic landscape. It is even foreseeable that metabolic profiling of tumour biopsies could form part of routine screening to stratify patients to the most efficient therapy. Individuals diagnosed with metabolic disease face multiple health challenges, including poor cancer prognosis and an increased risk of severe disease from pathogen infection. It is proposed that deficient anti-tumour T cell responses are due to solid tumour microenvironments (TME) promoting FAO in T cells, consequently leading to the upregulation of PD-1 [93, 114]. Another avenue by which PD-1 is upregulated on CD8+ T cells is through the accumulation of cholesterol in the TME [115]. Given that excess cholesterol accumulation is a feature of metabolic disease, it is possible that a similar mechanism could promote PD-1 expression and T cell exhaustion in this context. To overcome these brakes on T cell function, the use of FA dietary supplementation or lipid nanoparticles with cell fusion technology to deliver FAs to immune cells could improve anti-tumour function by rewiring lipid metabolism. The use of FAs to enhance T cell anti-tumour responses can indeed be achieved, as preconditioning CD8+ T cells with linoleic acid prior to adoptive transfer into tumour bearing mice dramatically slowed the growth of the tumour [116]. Perhaps these preconditioning strategies could be combined with next generation cancer immunotherapies to improve clinical outcomes independent of the metabolic status of the patient. Obesity also impairs anti-viral immune responses [117] and hence is associated with increased risk of severe influenza and Coronavirus Disease 2019 (COVID-19) [118] infection and mortality. In a mouse model of obesity, the impaired immune response has been shown to be related to impaired B cell maturation from bone marrow progenitors and reduced plasma B cell antibody production [95]. However, this was ameliorated by supplementation of the essential FA DHA [95]. Mechanistically, DHA was found to have an indirect effect, inducing the synthesis of specialised pro-resolving lipid mediators 14-hydroxyDHA and 17-hydroxyDHA which promoted the production of bone marrow plasma cells.
In summary, as studies tackle the interplay between diet, whole body, and local metabolism, it is becoming apparent that it is not just the professional phagocytes (i.e., macrophages) that are susceptible to adverse lipid environments. As discussed herein, the metabolism and interaction of immune cells with the lipid microenvironment dictates their fate and function. We have moved into the era of rediscovering, but now at a detailed molecular level, how changes to diet or supplementation with lipids can have profound impact on the immune response. To translate these emerging concepts and improve immune outcomes, it will be important to gain a better understanding of how the lipid composition of diets and tissue microenvironments along with local signalling modulates the metabolism of immune cells. This will inform how we can intervene with custom diets or supplements to achieve the most efficient immune outcomes. The scope could range from immune responses to infection, vaccination or improving the efficacy of immunotherapies for cancer.
References
Medzhitov R, Janeway C. Innate immune recognition: Mechanisms and pathways. Immunol Rev. 2000;173:89–97.
Abbas AK, Janeway CA. Immunology: Improving on nature in the twenty-first century. Cell. 2000;100:129–38.
Morgan PK, Pernes G, Huynh K, Giles C, Paul S, Smith A, et al. A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility. Nat Cell Biol. 2024;26:645–59. https://doi.org/10.1038/s41556-024-01377-z
Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS One. 2012;7:1–13.
Nguyen DH, Espinoza JC, Taub DD. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mech Ageing Dev. 2004;125:641–50.
Nojima I, Eikawa S, Tomonobu N, Hada Y, Kajitani N, Teshigawara S, et al. Dysfunction of CD8 + PD-1 + T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci Rep. 2020;10:1–12.
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population. Sci Rep. 2017;7:1–10.
Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, et al. LXR Signaling Couples Sterol Metabolism to Proliferation in the Acquired Immune Response. Cell. 2008;134:97–111.
Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–55.
Lauterbach MA, Hanke JE, Serefidou M, Mangan M, Kolbe CC, Hess T, et al. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity. 2019;51:997–1011.e7.
Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016;46:13–21.
Morgan PK, Huynh K, Pernes G, Miotto PM, Mellett NA, Giles C, et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J Biol Chem. 2021;297:101341.
Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 2017;47:621–34.
Young MP, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–45.
Van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–43.
Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;19:281–96.
Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24.
Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18:361–74.
Dossett LA, Dageforde LA, Swenson BR, Metzger R, Bonatti H, Sawyer RG, et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect. 2009;10:137–42.
Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36:1072–7.
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Jovinge S, Ares MPS, Kallin B, Nilsson J. Human monocytes/macrophages release TNF-α in response to Ox-LDL. Arterioscler Thromb Vasc Biol. 1996;16:1573–9.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808.
Kapinsky M, Torzewski M, Büchler C, Duong CQ, Rothe G, Schmitz G. Enzymatically degraded LDL preferentially binds to CD14high CD16+ monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler Thromb Vasc Biol. 2001;21:1004–10.
Aqel NM, Ball RY, Waldmann H, Mitchinson MJ. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 1984;53:265–71.
Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116:789–96.
Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–21.
O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65.
Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12.
Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11:1–11.
Lim SA, Su W, Chapman NM, Chi H. Lipid metabolism in T cell signaling and function. Nat Chem Biol. 2022;18:470–81.
Howie D, Bokum A, Ten, Necula AS, Cobbold SP, Waldmann H. The role of lipid metabolism in T lymphocyte differentiation and survival. Front Immunol. 2018;8:1949.
Man K, Kallies A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol. 2015;15:574–84.
Chapman NM, Chi H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity. 2022;55:14–30.
Lee J, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. Regulator of Fatty Acid Metabolism, Acetyl Coenzyme A Carboxylase 1, Controls T Cell Immunity. J Immunol. 2014;192:3190–9.
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–33.
Endo Y, Yokote K, Nakayama T. The obesity-related pathology and Th17 cells. Cell Mol Life Sci. 2017;74:1231–45.
Artemniak-Wojtowicz D, Pyrżak B, Kucharska AM. Obesity and chronic inflammation crosslinking. Cent Eur J Immunol. 2020;45:461–8.
Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, et al. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 2015;12:1042–55.
O'sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, et al. Memory CD8 + T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity. 2014;41:75–88.
Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, et al. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8 + T cell longevity. Cell. 2015;161:750–61.
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7.
Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: From B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58.
Weisel FJ, Mullett SJ, Elsner RA, Menk AV, Trivedi N, Luo W, et al. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat Immunol. 2020;21:331–42.
Kennedy DE, Okoreeh MK, Maienschein-Cline M, Ai J, Veselits M, McLean KC, et al. Novel specialized cell state and spatial compartments within the germinal center. Nat Immunol. 2020;21:660–70.
Chen D, Wang Y, Manakkat Vijay GK, Fu S, Nash CW, Xu D, et al. Coupled analysis of transcriptome and BCR mutations reveals role of OXPHOS in affinity maturation. Nat Immunol. 2021;22:904–13.
Zhou X, Zhu X, Li C, Li Y, Ye Z, Shapiro VS, et al. Stearoyl-CoA Desaturase-Mediated Monounsaturated Fatty Acid Availability Supports Humoral Immunity. Cell Rep. 2021;34:108601.
Boothby MR, Brookens SK, Raybuck AL, Cho SH. Supplying the trip to antibody production—nutrients, signaling, and the programming of cellular metabolism in the mature B lineage. Cell Mol Immunol. 2022;19:352–69.
Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:1–13.
Gauthier MS, O'Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–7.
Brookens SK, Cho SH, Basso PJ, Boothby MR. AMPKα1 in B Cells Dampens Primary Antibody Responses yet Promotes Mitochondrial Homeostasis and Persistence of B Cell Memory. J Immunol. 2020;205:3011–22.
Lepez A, Pirnay T, Denanglaire S, Perez-Morga D, Vermeersch M, Leo O, et al. Long-term T cell fitness and proliferation is driven by AMPK-dependent regulation of reactive oxygen species. Sci Rep. 2020;10:1–14.
Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, et al. Selenium–GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol. 2021;22:1127–39.
Diaz A, Romero M, Vazquez T, Lechner S, Blomberg BB, Frasca D. Metformin improves in vivo and in vitro B cell function in individuals with obesity and Type-2 Diabetes. Vaccine. 2017;35:2694–2700.
Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vázquez G, Yurchenko E, et al. The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses InVivo. Immunity. 2015;42:41–54.
Jackson CL, Walch L, Verbavatz JM. Lipids and Their Trafficking: An Integral Part of Cellular Organization. Dev Cell. 2016;39:139–53.
Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2020;31:422–37.e5.
Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–6.
Frizzell H, Fonseca R, Christo SN, Evrard M, Cruz-Gomez S, Zanluqui NG, et al. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci Immunol. 2020;5:1–10.
DeBerardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–80.
Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Asp Med. 2004;25:475–93.
Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–51.
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8 + T Cell Memory Development. Immunity. 2012;36:68–78.
Romanauska A, Köhler A. Lipid saturation controls nuclear envelope function. Nat Cell Biol. 2023;25:32–34.
Lu W, Helou YA, Shrinivas K, Liou J, Au-Yeung BB, Weiss A. The phosphatidylinositol-transfer protein Nir3 promotes PI(4,5)P2 replenishment in response to TCR signaling during T cell development and survival. Nat Immunol. 2023;24:136–47.
Edwards-Hicks J, Apostolova P, Buescher JM, Maib H, Stanczak MA, Corrado M, et al. Phosphoinositide acyl chain saturation drives CD8+ effector T cell signaling and function. Nat Immunol. 2023;24:516–30.
Essig K, Hu D, Guimaraes JC, Alterauge D, Edelmann S, Raj T, et al. Roquin Suppresses the PI3K-mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity. 2017;47:1067–82.e12.
Uche UU, Piccirillo AR, Kataoka S, Grebinoski SJ, D'Cruz LM, Kane LP. PIK3IP1/TrIP restricts activation of T cells through inhibition of PI3K/Akt. J Exp Med. 2018;215:3165–79.
Kahn ARS, Ronald C. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806.
Lee M, Lee SY, Bae YS. Functional roles of sphingolipids in immunity and their implication in disease. Exp Mol Med. 2023;55:1110–30.
Hose M, Günther A, Naser E, Schumacher F, Schönberger T, Falkenstein J, et al. Cell-intrinsic ceramides determine T cell function during melanoma progression. Elife. 2022;11:1–21.
Apostolidis SA, Rodríguez-Rodríguez N, Suárez-Fueyo A, Dioufa N, Ozcan E, Crispín JC, et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat Immunol. 2016;17:556–64.
Gabriel SS, Tsui C, Chisanga D, Weber F, Llano-León M, Gubser PM, et al. Transforming growth factor-β-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity. 2021;54:1698–714.e5.
Turinsky J, O’Sullivan DM, Bayly BP. 1,2-diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–5.
Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, et al. Increased Ceramide in Brains with Alzheimer’s and Other Neurodegenerative Diseases. J Alzheimers Dis. 2012;29:537–47.
Liang L, Li D, Zeng R, Zhang H, Lv L, Wei W, et al. Long- and very long-chain ceramides are predictors of acute kidney injury in patients with acute coronary syndrome: the PEACP study. Cardiovasc Diabetol. 2023;22:92.
Samuel VT, Shulman GI. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22.
Le Bert N, Tan AT, Kunasegaran K, Tham C, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62.
Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martínez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8 + T cells. Nat Commun. 2017;8:1–11.
Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, et al. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity. 2017;47:1154–68.e6.
Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity. 2017;47:406–20.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20.
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7.
Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, et al. Obesity-Induced Metabolic Stress Leads to Biased Effector Memory CD4 + T Cell Differentiation via PI3K p110δ-Akt-Mediated Signals. Cell Metab. 2017;25:593–609.
Karlsson EA, Sheridan PA, Beck MA. Diet-Induced Obesity Impairs the T Cell Memory Response to Influenza Virus Infection. J Immunol. 2010;184:3127–33.
Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8 + T cell homeostasis by perforin and interferon-γ. Science. 2000;290:1354–7.
Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8:1–11.
Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, Whitmire JK. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep. 2019;27:514–24.e5.
Kado T, Nawaz A, Takikawa A, Usui I, Tobe K. Linkage of CD8 + T cell exhaustion with high-fat diet-induced tumourigenesis. Sci Rep. 2019;9:1–8.
Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–51.
Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:1–17.
Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692.
Piening A, Ebert E, Gottlieb C, Khojandi N, Kuehm LM, Hoft SG, et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat Commun. 2024;15:2835.
Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, et al. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J Immunol. 2017;198:4738–52.
Frasca D, Romero M, Diaz A, Blomberg BB. Obesity accelerates age defects in B cells, and weight loss improves B cell function. Immun Ageing. 2023;20:1–11.
Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity. 2016;24:615–25.
Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49:725–39.e6.
Frasca D, Diaz A, Romero M, Blomberg BB. Phenotypic and Functional Characterization of Double Negative B Cells in the Blood of Individuals With Obesity. Front Immunol. 2021;12:1–9.
Kahan SM, Wherry EJ, Zajac AJ. T cell exhaustion during persistent viral infections. Virology. 2015;479–480:180–93.
Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:1–9.
Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88:960–6.
Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98.
Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–42.
Wiesenthal SR, Sandhu H, McCall RH, Tchipashvili V, Yoshii H, Polonsky K, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes. 1999;48:766–74.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25.
Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–4.
Yu Y, Cai Z, Zheng J, Chen J, Zhang X, Huang XF, et al. Serum levels of polyunsaturated fatty acids are low in Chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr Res. 2012;32:71–77.
Albert BB, Derraik JG, Brennan CM, Biggs JB, Smith GC, Garg ML, et al. Higher omega-3 index is associated with increased insulin sensitivity and more favourable metabolic profile in middle-aged overweight men. Sci Rep. 2014;4:6697.
Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–97.
Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J Lipid Res. 2007;48:127–38.
Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9.
Jin HR, Wang J, Wang ZJ, Xi MJ, Xia BH, Deng K, et al. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. 2023;16:1–33.
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol Induces CD8 + T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143–56.e5.
Nava Lauson CB, Tiberti S, Corsetto PA, Conte F, Tyagi P, Machwirth M, et al. Linoleic acid potentiates CD8 + T cell metabolic fitness and antitumor immunity. Cell Metab. 2023;35:633–50.e9.
Rebeles J, Green WD, Alwarawrah Y, Nichols AG, Eisner W, Danzaki K, et al. Obesity-induced changes in T-cell metabolism are associated with impaired memory T-cell response to influenza and are not reversed with weight loss. J Infect Dis. 2019;219:1652–61.
van der Klaauw AA, Horner EC, Pereyra-Gerber P, Agrawal U, Foster WS, Spencer S, et al. Accelerated waning of the humoral response to COVID-19 vaccines in obesity. Nat Med. 2023;29:1146–54.
Acknowledgements
We are grateful to the following funding sources: National Health and Medical Research Council of Australia grants GNT2027074 & GNT2012119 to KM, GNT1189012 to GIL and GNT1194329 to AJM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Collins, T.J.C., Morgan, P.K., Man, K. et al. The influence of metabolic disorders on adaptive immunity. Cell Mol Immunol (2024). https://doi.org/10.1038/s41423-024-01206-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41423-024-01206-1
- Springer Nature Limited