Abstract
Inhibitory immune receptors set thresholds for immune cell activation, and their deficiency predisposes a person to autoimmune responses. However, the agonists of inhibitory immune receptors remain largely unknown, representing untapped sources of treatments for autoimmune diseases. Here, we show that V-set and transmembrane domain-containing 1 (VSTM1) is an inhibitory receptor and that its binding by the competent ligand soluble galectin-1 (Gal1) is essential for maintaining neutrophil viability mediated by downregulated reactive oxygen species production. However, in patients with systemic lupus erythematosus (SLE), circulating Gal1 is oxidized and cannot be recognized by VSTM1, leading to increased intracellular reactive oxygen species levels and reduced neutrophil viability. Dysregulated neutrophil function or death contributes significantly to the pathogenesis of SLE by providing danger molecules and autoantigens that drive the production of inflammatory cytokines and the activation of autoreactive lymphocytes. Interestingly, serum levels of glutathione, an antioxidant able to convert oxidized Gal1 to its reduced form, were negatively correlated with SLE disease activity. Taken together, our findings reveal failed inhibitory Gal1/VSTM1 pathway activation in patients with SLE and provide important insights for the development of effective targeted therapies.
Similar content being viewed by others
Introduction
Inhibitory signaling is essential for the control of immune responses, and deficient or impaired signaling may result in overactivation of the immune system and autoimmune disease [1]. Inhibitory signals are delivered by various inhibitory receptors expressed primarily on immune cells to attenuate or terminate immune responses. Compelling evidence showing a role for inhibitory receptors in the development of autoimmunity is based on animal studies in which inhibitory receptors were abrogated genetically or blocked with antibodies [2,3,4,5]. Although the functional aspects of inhibitory receptors have been clarified, we still do not have enough information about the ligands that bind them.
Systemic lupus erythematosus (SLE) is a chronic, multisystem autoimmune disease characterized by antibodies against a wide range of autoantigens derived from uncleared dead cell debris [6, 7]. As the most abundant cell type in the blood, neutrophils have been suggested to be the main sources of autoantigens in patients with SLE [8,9,10]. It has been hypothesized that the absence of inhibitory signaling is the key driver of neutrophil abnormalities in the SLE context, as it leads to impaired phagocytosis, increased molecule aggregation and accelerated cell death [8, 9]. However, the expression and function of inhibitory receptors on neutrophils have not been characterized.
V-set and transmembrane domain containing 1 (VSTM1), a Type I transmembrane receptor with an extracellular immunoglobulin V-like domain and two cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs), is highly expressed on normal human peripheral blood leukocytes, including granulocytes and monocytes [11]. Overexpression of VSTM1 on monocytes has been shown to inhibit cell proliferation, chemotaxis, and proinflammatory cytokine production [12]. In this study, we first identified Galectin-1 (Gal1) as a ligand able to inhibit reactive oxygen species (ROS) production by neutrophils by binding the inhibitory receptor VSTM1. Oxidative modification of a series of molecules, such as DNA, HDL cholesterol and β2 glycoprotein I, has been reported in patients with SLE due to increased oxidative stress, and the metabolic imbalances caused by these modifications trigger autoimmune responses [13,14,15,16,17,18]. Notably, we found that circulating Gal1 in the serum of SLE patients is oxidized and unable to bind VSTM1, leading to increased intracellular ROS levels in neutrophils. Dysregulated ROS production accelerates programmed cell death, including apoptosis and NETosis, both of which have been shown to contribute to the immunopathogenesis of SLE. The increased oxidation of Gal1 can be explained by the reduction in the serum levels of the antioxidant glutathione, which can convert oxidized Gal1 back to its reduced form. Taken together, our results reveal a novel central mechanism involved in the pathogenesis of SLE involving the dysfunction of an inhibitory receptor and have important implications for the development of targeted therapies.
Materials and methods
Patients and controls
This study was approved by the ethics committee of Peking Union Medical College (PUMC) Hospital. Informed consent was obtained from each SLE patient and healthy control (HC). SLE was diagnosed according to American College of Rheumatology criteria. All potential SLE patient candidates were treatment naïve at their first visit, and those with the following conditions were excluded from enrollment: history of severe chronic infection; a current infection; a serious underlying physical disorder such as acute coronary syndrome, chronic renal failure, or any type of tumor; and pregnancy or lactation. Because ROS levels correlate significantly with the aging process [19], we restricted the age of the participants to 18–45 years to exclude patients whose ROS levels may be high and eliminate the impact of aging on the results. The clinical characteristics of all SLE patients are presented in Supplementary Table 1.
Chemicals, proteins and antibodies
The following chemicals and proteins were used: human recombinant protein Gal1 (10290-HNAE, Sino Biological), human recombinant protein VSTM1 (13171-H08H, Sino Biological), GSK484 (S7803, Selleck), NEC1 (S8037, Selleck), VX765 (S2228, Selleck), z-VAD (S7023, Selleck), N-acetyl cysteine (NAC) (S1623, Selleck), reduced glutathione (GSH) (G4251, Merck), 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) (S0033S, Beyotime), β 1-4 galactosidase (P0745S, NEB) and PNGase F (P0704S, NEB).
The following antibodies were used: anti-VSTM1 agonist mAb (HM2309, HycultBiotech), anti-VSTM1 (24382-1-AP, Proteintech), anti-Gal1 (11858-1-AP, Proteintech), anti-GAPDH (10494-1-AP, Proteintech), anti-Na-K-ATP (3010, Cell Signaling Technology), anti-p-p42 (4370, Cell Signaling Technology), anti-p42 (4695, Cell Signaling Technology), anti-p-p38 (4511, Cell Signaling Technology), anti-p38 (8690, Cell Signaling Technology), anti-tubulin (2146, Cell Signaling Technology), PE-conjugated anti-human CD11b (301305, BioLegend), FITC-conjugated anti-human CD11b (982614, BioLegend), APC-Cy7conjugated anti-human CD66b (305126, BioLegend), BV650-conjugated anti-human CD62L (304832, BioLegend), BV421-conjugated anti-human CD16 (302037, BioLegend), APC-conjugated anti-human CD181 (CXCR1) (320612, BioLegend), PerCP-Cy5.5-conjugated anti-human CD182 (CXCR2) (320718, BioLegend), APC-conjugated anti-human CD184 (CXCR4) (306509, BioLegend), BV605-conjugated anti-human CD10 (312221, BioLegend), PerCP-Cy5.5-conjugated anti-human CD54 (353120, BioLegend), FITC-labeled donkey anti-rabbit IgG antibody (406403, BioLegend), PE-labeled donkey anti-rabbit IgG antibody (406421, BioLegend).
Cell isolation and culture
Neutrophils were isolated from the peripheral blood of SLE patients and HCs using Ficoll–Hypaque density gradient centrifugation following the instructions of the centrifuge manufacturer. The remaining erythrocytes were lysed with lysing buffer (BD Biosciences). Monocytes were isolated with anti-CD14 microbeads (130050201, Miltenyi). Cells were cultured in complete RPMI 1640 medium with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% serum mixed with samples from ten randomly selected SLE patients or age- and sex-matched HCs. Anti-VSTM1 antibody (5 μg/mL), human recombinant protein Gal1 (1–50 μg/mL), oxidized Gal1 (25 μg/mL), GSK484 (10 μM), NEC1 (1 μM), VX765 (10 μM), z-VAD (10 μM) or NAC (1 μM) was added to the cultures in different experiments.
Differentiation of HL60 cells and cell deglycosylation
HL60 cells were cultured in complete RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (Gibco), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C with 5% CO2. To induce their differentiation, HL60 cells were passaged every 3 days in culture with DMSO (1.25%) to the sixth day. The rate of HL60 differentiation was evaluated by the expression of CD11b via flow cytometry (Supplementary Fig. 1A). For deglycosylation, dHL60 cells were fixed in 4% paraformaldehyde, and then, β 1-4 galactosidase or PNGase F was added to the cells following the manufacturer’s instructions. β 1-4Galactosidase was used to remove β-galactose from oligosaccharides, and PNGase F was used to remove N-linked oligosaccharides from glycoproteins.
Measurement of intracellular ROS levels
The intracellular ROS levels in peripheral blood neutrophils, dHL60 or THP-1 cells were determined by DCFH-DA assay. Cells were preloaded with 10 μmol/L DCFH-DA probe for 20 min at 37 °C, and then, DCF fluorescence was measured using a BD FACSCelesta flow cytometer (BD Biosciences). To detect the change in ROS levels after simulation, cells preloaded with the DCFH-DA probe were seeded in 96-well plates and treated with serum, anti-VSTM1 antibody or Gal1 protein. ROS levels were measured in real-time with a fluorescence microplate reader immediately after the initial stimulation and at 5 min intervals for 2 h or 16 h.
Cell viability quantification
The viability of freshly isolated or cultured neutrophils or dHL60 cells was determined by staining with PE-conjugated Annexin V and 7AAD following the manufacturer’s protocol (BD Pharmingen). The percentage of Annexin V-PE/7-AAD double-negative cells was considered to be the percentage of viable cells. Representative flow cytometry data are shown in Supplementary Fig. 1B.
RNA interference and plasmids
dHL60 cells were seeded in 24-well plates and transfected with 50 nM siRNA oligos using RNAiMAX reagent (Life Technology). After 72 h of transfection, the cells were collected, and the knockdown efficiency and the effect of ROS on cell viability were measured. The details of the siRNA sequences are shown in Supplementary Table 2.
Full-length sequences of VSTM1 were obtained from Sangon Biotech, and then, Myc-tagged VSTM1 was cloned and inserted into a pcDNA3.1 or pLenti-puro vector. The plasmids were transiently transfected into HEK 293 T cells with Lipo-2000 reagent (Invitrogen). For stable transfection, THP-1 cells were infected with packaged lentiviruses and selected after 1 μg/ml puromycin treatment.
RNA isolation and quantitative PCR (qPCR)
Total RNA was extracted with TRIzol (Invitrogen) following the manufacturer’s guidelines and then reverse transcribed to cDNA using PrimeScript RT Master Mix (Takara). QPCR was performed with a 7900HT Fast Real-Time PCR System (Applied Biosystems). The RNA expression level was calculated using the –ΔΔCT method and normalized to GAPDH. The sequences of the primers are presented in Supplementary Table 3.
Protein extraction and Western blotting
Total cellular proteins were extracted with RIPA buffer, and membrane proteins were extracted with a membrane and cytosol protein extraction kit (Beyotime). Protein concentration was determined with a BCA protein assay kit (Thermo Scientific). For serum protein level measurements, serum samples were depleted of highly abundant proteins using TOP14 Abundant Protein Depletion Mini Spin Columns (A36370, Pierce). Western blot assays were performed as described previously [20]. Under reducing conditions, the protein samples were boiled with SDS loading buffer and dithiothreitol (DTT), while under nonreducing conditions, the samples were suspended in loading buffer without DTT. Gray value analysis was performed with ImageJ software.
Flow cytometry
To measure the expression of VSTM1 or Gal1 on the cell surface, neutrophils suspended in FACS buffer were incubated with primary-targeting antibodies for 30 min at 4 °C, washed with FACS buffer and then incubated with the appropriate secondary antibodies for an additional 30 min. The relative mean fluorescence intensity (MFI) was measured with a BD FACSCelesta flow cytometer (BD Biosciences).
GST pull down
The purified GST-VSTM1 domain was incubated with GST agarose beads for 2 h at 4 °C and then incubated with neutrophil cell lysates in vitro for 2 h at 4 °C in lysis buffer (150 mM NaCl and 50 mM Tris-HCl, pH 8.0). Then, the beads were washed three times followed by elution with elution buffer (150 mM NaCl; 50 mM Tris-HCl, pH 8.0; and 15 mM GSH). The reactions were then analyzed by mass spectrometry (MS).
Immunoprecipitation
The indicated antibodies were incubated with AminoLink Plus Coupling Resin for 2 h, followed by further incubation with cell extracts for 4 h at 4 °C using a co-immunoprecipitation kit (26149, Thermo Scientific) according to the manufacturer’s instructions. The immune complexes were washed and eluted and then analyzed by immunoblot assay or MS.
LC‒MS/MS analysis
For IP-MS analysis, the eluted products were separated by SDS‒PAGE. Gel lanes were excised and the gel pieces digested following Coomassie blue staining and subjected to LC‒MS/MS analysis with LTQ Orbitrap Velos (Thermo Scientific). Database searching was carried out using Proteome Discoverer Version 2.1 (Thermo Scientific) against the UniProt human reference proteome database. For PRM analysis of Gal1, an equal volume of serum sample was prepared by depletion of highly abundant proteins using TOP14 Abundant Protein Depletion Mini Spin Columns (A36370, Pierce). Nonoxidative and oxidative standard proteins and preprepared serum samples were directly alkylated using 50 mM iodoacetamide (IAA) for 30 min at room temperature in the dark. In-solution digestion was conducted using Mass Spec Grade Trypsin (Promega) with an enzyme:protein ratio of 1:100. The digested samples were dried and redissolved in 50 μl of 0.1% formic acid. Data were acquired from an Orbitrap Fusion mass spectrometer (Thermo Fisher). Individual samples were injected at a flow rate of 300 nL min−1. The parameters used for the PRM analysis were set as follows: for MS1, the Orbitrap resolution was 120,000, AGC target value was 2 × 105, and injection time was 50 ms. For MS2, the Orbitrap resolution was 15,000, AGC target value was 5 × 104, injection time was 45 ms, isolation window was 1.6 m/z, and high energy collision dissociation normalized collision energy was 30%.
Immunofluorescence staining
Freshly isolated neutrophils (105 cells) from HCs and patients with SLE were fixed with 4% paraformaldehyde and treated with blocking buffer (100 mM Tris-HCl, pH 8.0; 2% BSA; and 50 μg/ml goat nonspecific IgG) for 1 h. Cells were stained using anti-VSTM1 and anti-Gal1 primary antibodies for 2 h and then with the corresponding anti-mouse IgG Alexa Fluor 488 and anti-rabbit IgG Alexa Fluor 594 secondary antibodies for 1 h. The slides were mounted with anti-quenching agent containing DAPI and examined with Leica microscopes.
Enzyme-linked immunosorbent assay (ELISA)
Gal1 levels in serum were measured with the Human Galectin-1 ELISA Kit (ab260053, Abcam). The reduced glutathione (GSH) and oxidized glutathione (GSSG) in serum were determined by fluorometry assay following the manufacturer’s protocol (ab138881, Abcam).
Gal1 oxidation
Recombinant Gal1 was purchased from Sino Biological. To generate oxidized Gal1, reduced Gal1 was diluted in 20 mM Tris-HCl at pH 8.0 supplemented with 0.001% (w/v) CuSO4 at 4 °C overnight according to methods previously described [21].
Sulfhydryl group labeling and immunoprecipitation
Reduced sulfhydryl groups were labeled by BIAM using similar methods previously reported [22]. In brief, serum samples were first depleted of highly abundant proteins using TOP14 Abundant Protein Depletion Mini Spin Columns (A36370, Pierce) and then incubated with catalase (200 unit/ml), 20 μM BIAM and 1% Triton X-100 at room temperature on a rotary shaker for 1 h. Free BIAM was removed by ultrafiltration. The supernatant was incubated with 50 μl of streptavidin-magnetic beads for 4 h at 4 °C. The beads were washed with PBS containing 1% Triton X-100 three times and boiled in loading buffer, and the eluted protein levels were measured by immunoblotting.
Biolayer interferometry (BLI)
For protein affinity measurement, His-VSTM1 was loaded onto anti-His biosensors for 4 min. Then, the loaded biosensors were exposed to a series of Gal1 and oxidized Gal1 concentrations (0.125–2 μM). The association and dissociation steps were recorded by Octet Red96e systems, and the KD values were calculated using Octet software with a 1:1 fit model.
Binding assay and Gal1 depletion
Gal1 and oxidized Gal1 protein were labeled with an Alexa Fluor 488 (AF488) Conjugation Lightning-Link Kit (ab236553, Abcam) according to the manufacturer’s guidelines. Then, the cells were incubated with AF488-labeled Gal1 or oxidized Gal1 for 20 min at 4 °C. Samples were analyzed using a BD FACSCelesta flow cytometer (BD Biosciences). For the competition experiments, cells were simultaneously incubated in HC, SLE serum, or Gal1-depleted serum at a concentration of 50%. Gal1 in serum was depleted by Gal1 antibody-conjugated AminoLink Plus Coupling Resin (20501, Thermo Scientific). Briefly, the anti-Gal1 antibody was incubated with AminoLink Plus Coupling Resin for 2 h, followed by further incubation with serum overnight at 4 °C. The supernatant contained serum with Gal1 depleted. The efficacy of Gal1 depletion is shown in Supplementary Fig. 2.
Statistical analysis
GraphPad Prism was employed for the statistical analysis performed in this study. To compare the differences between the two groups, two-tailed Student’s t test was performed. The Spearman method was used to calculate correlations. P values < 0.05 were considered significant.
Results
SLE serum increases the levels of ROS in neutrophils
To investigate the redox status of neutrophils in SLE, peripheral blood neutrophils were isolated from SLE patients, and the intracellular ROS levels were measured using DCFH-DA fluorescence probes. As shown in Fig. 1A, the intracellular ROS levels were significantly higher in SLE neutrophils than in those from healthy controls (HCs). Notably, the intracellular ROS levels in neutrophils from SLE patients correlated positively with disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (Fig. 1B) but correlated negatively with circulating neutrophil cell counts (Fig. 1C). Furthermore, neutrophil cell viability was assessed by 7AAD-Annexin V staining (Fig. 1D). As expected, SLE neutrophils with higher ROS levels were less viable (Fig. 1E). These results suggest that ROS levels in neutrophils from SLE patients correlated positively with both neutropenia and disease activity.
SLE serum increases ROS levels in neutrophils. A Flow cytometry quantification of ROS levels in neutrophils immediately after samples were isolated from HC and SLE patients (HC: n = 24, SLE: n = 20). The ROS MFI ratio was the MFI of ROS normalized to that of control cells. B The ROS levels of neutrophils in patients with SLE correlated with disease activity as measured using the SLEDAI (n = 20). C, D The ROS levels of neutrophils in patients with SLE correlated negatively with (C) the number of peripheral neutrophils (n = 20) and (D) the cell viability of neutrophils (n = 11). E Flow cytometry quantification of cell viability of neutrophils immediately after samples were isolated from HC and SLE patients with low ROS (ROS MFI ratio < 1.5) and high ROS (ROS MFI ratio ≥ 1.5) levels in their neutrophils (HC: n = 10, SLE with low ROS: n = 6, SLE with high ROS: n = 5). F The ROS production of neutrophils from HCs (n = 16) or SLE patients (n = 16) cultured in vitro with 10% pooled HC or SLE serum was measured with a fluorescence microplate reader. Quantification data are depicted as the fold change in peak fluorescence units compared with the level in 0-time point control cells. G Flow cytometry quantification of the viability of neutrophils from HCs (n = 5) cultured in vitro with 10% mixed HC serum or SLE serum for 4 h. HC neutrophils were cultured in the presence of HC or SLE serum with or without the addition of NAC (1 μM). H ROS production in neutrophils was measured with a fluorescence microplate reader (n = 8). I Neutrophil viability was measured by flow cytometry (n = 5). The data are shown as the mean ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; two-tailed unpaired or paired Student’s t test. MFI mean fluorescence intensity, NAC N-acetyl cysteine
Neutrophil fate can be regulated by several soluble factors in serum [10, 20, 23]. To determine whether SLE serum promotes ROS production leading to cell death, we examined ROS levels in neutrophils from HC or SLE patients after incubation with serum from either HC or SLE patients. The presence of SLE serum caused a significant increase in the ROS levels of neutrophils compared to HC serum within the first 2 h, but the levels returned to normal 1 h later (Supplementary Fig. 3, Fig. 1F). In addition to the increased ROS levels, the viability of the neutrophils was reduced in the presence of SLE serum (Fig. 1G). Notably, both the induction of intracellular ROS production and the reduction in the viability of neutrophils cultured with SLE serum were completely abolished by the addition of the antioxidant N-acetyl cysteine (NAC) (Fig. 1H, I). Interestingly, pharmacological suppression of either NETosis (GSK484, a peptidylarginine deiminase 4 inhibitor) or apoptosis (VX765, a caspase 1 inhibitor; z-VAD, a pancaspase inhibitor) but not necroptosis partially rescued SLE serum-induced cell death (Supplementary Fig. 4). Taken together, these data demonstrate that SLE serum induces neutrophil death by enhancing intracellular ROS production and that the presence of an antioxidant effectively prevents this process.
The inhibitory receptor VSTM1 contributes to increased ROS production and decreased viability of SLE neutrophils
ITIM-containing inhibitory receptors have been proposed to control ROS production and cell death in neutrophils [24,25,26,27]. To test this hypothesis, we performed a functional siRNA screening of the inhibitory receptors in neutrophil-like dHL60 cells and found that knockdown of eight of thirteen inhibitory receptors (CEACAM1, CD300A, CD300LF, CIEC12 A, LILRB3, SIGLEC9, SIRPA, and VSTM1) increased the intracellular ROS levels and that among these receptors, Vstm1 deficiency resulted in decreased cell viability (Supplementary Fig. 5).
It has been reported that VSTM1 ligation inhibits FcR-induced ROS production in HC neutrophils [28]. We validated the function of VSTM1 using an anti-VSTM1 agonist mAb (clone 1A5) [11]. As expected, ligation of VSTM1 significantly reduced ROS levels in both HC and SLE neutrophils (Fig. 2A), and this outcome was found in the presence of HC or SLE serum (Fig. 2B). Consistently, ligation of VSTM1 helped preserve neutrophil viability by inhibiting cell death induced by the addition of SLE serum (Fig. 2C, D). Taken together, these results suggest that activation of VSTM1 preserves neutrophil viability by suppressing ROS production.
VSTM1 contributes to ROS regulation and cell viability in SLE neutrophils. A The ROS production of neutrophils from HCs (n = 12) or SLE patients (n = 10) stimulated with plate-bound VSTM1 antibody (5 μg/mL) or isotype control was measured with a fluorescence microplate reader. B The ROS production of neutrophils from HCs (n = 18) cultured in 10% serum pooled HC or SLE serum with plate-bound VSTM1 antibody (5 μg/mL) or isotype control was measured using a fluorescence microplate reader. C, D Flow cytometric quantification of the viability of neutrophils from HCs (n = 6 or 7) cultured in the (C) absence or (D) presence of 10% serum pooled HC or SLE serum samples stimulated with plate-bound VSTM1 antibody (5 μg/mL) or isotype control. E qPCR analysis of VSTM1-v1 expression in neutrophils from HCs (n = 19) and SLE patients (n = 30). F, G Western blot analysis of VSTM1 expression on the neutrophil membrane (HC: n = 5, SLE: n = 6). H Flow cytometry quantification of VSTM1 expression on the membranes of neutrophils from HCs (n = 19) and SLE patients (n = 17). I The expression of VSTM1 on the surface membrane of neutrophils from SLE patients did not correlate with disease activity as measured by the SLEDAI (n = 17). The data are shown as the mean ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; two-tailed unpaired or paired Student’s t test. MFI mean fluorescence intensity
VSTM1 is expressed predominantly on neutrophils and at low levels on monocytes but not on T and B cells (Supplementary Fig. 6A, B). In contrast to VSTM1 activity in neutrophils, VSTM1 activity in monocytes did not significantly affect ROS production levels (Supplementary Fig. 6C). Neutrophil subsets with distinct phenotypes and functions have been recognized. [29,30,31] We measured the expression of VSTM1 in different neutrophil subsets, including immunosuppressive (CD16hiCD62Ldim), aging (CXCR4 + CXCR2-), senescent (CXCR4hiCD62Ldim), mature (CD16hiCD10 + ), and reverse transendothelial migration-related (rTEM) (CD54 + CXCR1 + ) neutrophil subsets. We found that VSTM1 expression did not differ between various neutrophil subsets in either HC or SLE patients (Supplementary Fig. 6D–F). VSTM1 is expressed in either membrane form (VSTM1-v1, an ITIM-bearing immune receptor) or soluble form (VSTM1-v2) [32]. The expression of total VSTM1 (both membrane and soluble forms) in neutrophils did not differ significantly between SLE patients and HCs (Supplementary Fig. 6G). Notably, the expression of VSTM1 on the cell surface was generally lower in neutrophils from SLE patients than in those from HCs (Fig. 2E–H), although this downregulation did not correlate with SLE disease activity (Fig. 2I). It remains to be determined whether reduced expression of VSTM1 on the surface of neutrophils, low binding affinity for its natural ligands or both are involved in the pathogenesis of SLE.
Gal1 was identified as a ligand for VSTM1
Next, we performed a screening assay to identify potential VSTM1 ligands. First, performing an in vitro pull-down assay with either GST-labeled extracellular segment of the VSTM1 protein incubated with lysate from HC serum or anti-VSTM1 antibody with the VSTM1 complex on the membrane of neutrophils, we identified potential VSTM1 ligands via mass spectrometry (MS). Among the enriched targets, Gal1 was identified because it was the most abundant (Supplementary Table 4). We confirmed the interaction between VSTM1 and Gal1 via coimmunoprecipitation experiments using neutrophils and HEK 293 T cells transfected with VSTM1 (Fig. 3A, Supplementary Fig. 7A, B). The binding assay showed that fluorescence-conjugated Gal1 efficiently bound to dHL60 cells, but silencing of VSTM1 abolished this interaction (Fig. 3B, Supplementary Fig. 7C). We examined the function of Gal1 in neutrophils and confirmed that, similar to effect of the VSTM1 agonistic antibody, Gal1 decreased ROS production in a dose-dependent manner (Fig. 3C, D). Moreover, the addition of Gal1 prevented SLE serum-induced cell death (Fig. 3E, F). Considering that Gal1 is a β-galactoside-binding lectin [33], we pretreated fixed dHL60 cells with or without β-galactosidase or PNGase F and performed a Gal1 binding assay. Deglycosylation exerted no effect on the binding ability of Gal1 to dHL60 cells (Supplementary Fig. 7D). Lactose was also added to the cultures, but it exerted no effect on ROS production in the presence of Gal1 (Supplementary Fig. 7E).
Gal1 acts as a ligand for VSTM1. A Neutrophil lysates were immunoprecipitated using anti-VSTM1 or anti-Gal1 antibody, followed by immunoblot analysis with VSTM1 and Gal1 antibodies. B Flow cytometric analysis of AF488-labeled Gal1 binding to dHL60 cells with or without VSTM1 knocked down. C The ROS production in neutrophils from HCs (n = 10 or 20) cultured with increasing doses of Gal1 protein (1/5/25/50 μg/mL) was measured with a fluorescence microplate reader. D The ROS production of neutrophils from HCs (n = 8) cultured in 10% mixed HC serum or SLE serum with or without Gal1 protein (25 μg/mL) was measured by a fluorescence microplate reader. E, F Flow cytometry quantification of the viability of neutrophils from HCs (n = 5 or 6) cultured in the (E) absence or (F) presence of 10% serum from mixed HC serum or SLE serum stimulated with or without Gal1 protein (25 μg/mL). G, H Effect of Gal1 treatment on ROS production in (G) dHL60 cells after knockdown of VSTM1 and (H) THP-1 cells after overexpression of VSTM1. The data are shown as the mean ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; two-tailed paired Student’s t test
To confirm that Gal1 inhibits ROS production by binding to VSTM1, we knocked down VSTM1 in dHL60 cells or overexpressed VSTM1 in THP-1 cells. As expected, Gal1 efficiently inhibited ROS production in a time-dependent manner in wild-type dHL60 cells but not in dHL60 cells transfected with VSTM1-silencing siRNA (Supplementary Fig. 7C, Fig. 3G). Moreover, Gal1 failed to inhibit the production of ROS in THP-1 cells that did not express VSTM1, and overexpression of VSTM1 rescued the inhibitory effect of Gal1 (Supplementary Fig. 7F, G and Fig. 3H). Taken together, these results demonstrate that Gal1 acts as an agonistic ligand of VSTM1.
Attenuated binding of Gal1 to VSTM1 in the neutrophils of SLE patients
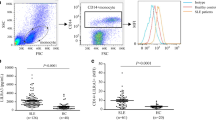
We sought to determine whether the levels of Gal1 in SLE serum correlate with SLE disease activity. Surprisingly, Gal1 levels were slightly higher in SLE serum than in HC serum (Fig. 4A), which was not consistent with the proposal that Gal1 binds to the inhibitory VSTM1 receptor. Using immunofluorescence staining, we confirmed the colocalization of VSTM1 and Gal1 on the membrane of neutrophils, but this colocalization was reduced in SLE neutrophils even though SLE serum contained more Gal1 (Fig. 4B). This observation suggests that Gal1 binding to VSTM1 is attenuated in SLE neutrophils. Moreover, lower levels of Gal1 were immunoprecipitated by an anti-VSTM1 antibody obtained from neutrophil lysates exposed to serum from SLE patients than from those exposed to serum from HCs (Fig. 4C). In a competitive binding assay, fluorescence-conjugated Gal1 bound to neutrophils, and the addition of HC but not SLE serum effectively blocked this binding. Notably, the depletion of Gal1 from HC serum completely abolished its competitive binding capacity. (Fig. 4D). These results suggest that Gal1 acts as a key ligand for binding VSTM1 and that Gal1 is much less effective binding VSTM1 in SLE serum than VSTM1 in HC serum.
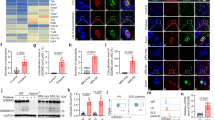
Decreased binding of Gal1 on the surface membrane of SLE neutrophils. A Measurement of Gal1 levels in serum from HCs (n = 22) and SLE patients (n = 38) as determined by ELISA. B Immunofluorescence staining of neutrophils immediately isolated from HC and SLE patients. Colocalization of VSTM1 (green) and Gal1 (red) can be observed. DAPI (blue) was used as a DNA marker. Scale bar, 5 μm. C Membrane protein extracted from HC (n = 3) and SLE (n = 3) neutrophils mixed with serum depleted of highly abundant serum proteins was immunoprecipitated using an anti-VSTM1 antibody, followed by immunoblot analysis with anti-VSTM1 and anti-Gal1 antibodies. D Flow cytometry analysis of AF488-labeled Gal1 competitively binding to neutrophils in HC and SLE serum with or without Gal1 depleted. The data are shown as the mean ± SEM from three independent experiments. **p < 0.01; Mann‒Whitney test
Gal1 is excessively oxidized in SLE serum
The reactive sulfhydryl of reduced cysteine residues (-SH) in proteins can be oxidized and converted into disulfide bonds (-S-S-) and is therefore considered a reliable indicator of the redox state of a protein. Interestingly, we observed that less Gal1 was present in the reduced form (with free sulfhydryl groups) in SLE serum than in HC serum (Fig. 5A) and in contrast was present mainly in the oxidized, which was characterized by excessive disulfide bonds (Cys3-Cys43 is a representative disulfide bond) (Fig. 5B). By labeling the sulfhydryl groups with biotin-conjugated iodoacetamide (BIAM), we confirmed that in HC serum, Gal1 was present mainly in the reduced form (with reduced cysteine residues), whereas in SLE serum, it was present in the oxidative state (with more in dimeric form) (Fig. 5C–E). As oxidized Gal1 has shown functions opposite those of reduced Gal1 [34], we hypothesized that the oxidation of Gal1 may interfere with its interactions with VSTM1.
Gal1 is excessively oxidized in SLE serum. A MS peak area of Gal1 peptides containing free sulfhydryl groups. B Secondary-ion mass spectrometry of Gal1 peptides containing disulfide bonds (Cys3-Cys43). C–E The reduced cysteine residues in Gal1 were detected by biotin-conjugated iodoacetamide (BIAM) labeling and streptavidin immunoprecipitation. Western blot analysis of reduced Gal1 and total Gal1 contents in serum from HCs (n = 13) and SLE patients (n = 16) under reduced conditions and the dimerization of oxidized Gal1 under nonreduced conditions. The data are shown as the mean ± SEM from three independent experiments. ***p < 0.001; two-tailed unpaired Student’s t test
Oxidized Gal1 in SLE serum is unable to bind VSTM1
We then evaluated whether oxidized Gal1 showed lower binding affinity for VSTM1 than reduced Gal1. Oxidized Gal1 protein was obtained from reduced recombinant human Gal1 protein. The average molecular masses of the reduced and oxidized Gal1 were determined by Q-TOF MS. A monomer of oxidized Gal1 (14578 Da) was slightly smaller than its reduced form (14584 Da) due to the former carrying three pairs of disulfide bonds. Oxidized Gal1 also exists as an oxidized dimer with a molecular weight of 29156 Da (Supplementary Fig. 8).
Using biolayer interferometry (BLI), we found that the reduced form of Gal1 bound to VSTM1 with a dissociation constant (KD) of 1.288E-07 M, whereas oxidized Gal1 failed to bind (Fig. 6A). Similarly, reduced Gal1, but not oxidized Gal1, bound to dHL60 cells (Fig. 6B). Consistent with its poor binding ability, oxidized Gal1 did not decrease ROS production in neutrophils (Fig. 6C, D) and did not increase neutrophil viability (Fig. 6E, F). The MAPK/ERK axis has been shown to be essential for ROS production [28], and as expected, both an anti-VSTM1 mAb and Gal1 reduced the phosphorylation of p42 and p38 (Fig. 6G). However, oxidized Gal1 did not reduce the phosphorylation of p42 or p38. Taken together, the findings suggest that oxidized Gal1 present in SLE serum lost the ability to bind VSTM1 and failed to activate the VSTM1 downstream signaling pathway.
Oxidized Gal1 fails to bind to VSTM1. A Affinity measurement Gal1 and oxGal1 binding immobilized VSTM1 using biolayer interferometry (ligand concentrations from top to bottom: 2, 1, 0.5, 0.25, 0.125 μM). B Flow cytometry analysis of AF488-labeled Gal1 or oxGal1 binding to dHL60 cells. C–F The ROS production of neutrophils from HCs (n = 8) cultured in the (C) absence or (D) presence of 10% mixed HC serum or SLE serum stimulated with or without Gal1 or oxGal1 protein (25 μg/mL) was measured by a fluorescence microplate reader. Flow cytometry quantification of the viability of neutrophils from HCs (n = 4 or 5) cultured in the (E) absence or (F) presence of 10% pooled HC or SLE serum stimulated with or without Gal1 and oxGal1 protein (25 μg/mL). G HC neutrophils were treated with anti-VSTM1 antibody (5 μg/mL) or isotype, Gal1/oxGal1 protein (25 μg/mL) or BSA control. The phosphorylation of ERK (p42) and MAPK (p38) was assessed over time by western blotting. The data are shown as the mean ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; two-tailed paired Student’s t test. oxGal1, oxidized Galectin-1
Redox imbalance in SLE patients decreases the rate of Gal1 binding to VSTM1
We next asked, what leads to Gal1 overoxidation in patients with SLE? Since dimerized Gal1 was not detectable in the cell lysates of HC and SLE neutrophils, the oxidation of Gal1 was determined to take place in the extracellular space after it is secreted (Fig. 7A). Reduced glutathione (GSH) is the most abundant nonprotein thiol present in all mammalian tissues and can defend cells against oxidative stress [35]. Interestingly, the circulating GSH levels (as determined by the ratio of GSH/GSH + GSSG) were significantly reduced in SLE patients but not in patients with other autoimmune diseases, such as rheumatoid arthritis (RA), Behcet’s disease (BD) and primary Sjögren’s syndrome (PSS) (Fig. 7B). Furthermore, the circulating GSH levels were inversely correlated with disease activity (Fig. 7C). These data suggest that the redox imbalance in SLE patients may contribute to disease pathogenesis by enhancing the oxidation of Gal1 in serum.
The redox imbalance in SLE patients decreases the binding of Gal1 to VSTM1. A Western blot analysis of Gal1 contents in neutrophil lysates from HCs (n = 2) and SLE patients (n = 2) under nonreduced conditions. B The GSH/(GSH + GSSG) ratio in serum from HCs or patients with autoimmune diseases, including SLE, rheumatoid arthritis (RA), Behcet’s disease (BD) and primary Sjögren’s syndrome (PSS) (HC: n = 22, SLE: n = 20, RA: n = 10, BD: n = 10, PSS: n = 10). C The GSH/(GSH + GSSG) ratio in serum from SLE patients was negatively correlated with disease activity as measured by the SLEDAI (n = 20). D Gal1 protein was treated with H2O2 (300 nM) and simultaneously added with increasing doses of NAC (0/1/10/50 μM) or GSH (0/1/10 μM). E Gal1 protein was first treated with H2O2 (300 nM) and then added along with increasing doses of NAC (0/1/10/50 μM) or GSH (0/1/10 μM). F Serum protein was treated with increasing doses of GSH (0/0.1/1/10 μM). G Serum from five SLE patients was treated with GSH (10 μM). The formation and dissociation of the oxidized Gal1 dimer and multimer were analyzed by western blotting after nonreduced SDS‒PAGE. H–J The reduced Gal1/total Gal1 ratio in serum from SLE patients positively correlated with the GSH/(GSH + GSSG) ratio and the numbers of peripheral neutrophils and negatively correlated with disease activity as measured by the SLEDAI (n = 16). The data are shown as the mean ± SEM from three independent experiments. **p < 0.01; two-tailed paired Student’s t test. GSH glutathione, NAC N-acetyl cysteine
We then asked whether antioxidants might remove/repair the oxidative modification of Gal1 in SLE serum. Although both GSH and NAC remarkably inhibited Gal1 dimerization and multimerization after H2O2 treatment (Fig. 7D), only GSH eliminated oxidized Gal1 in vitro, and its effect was dose dependent (Fig. 7E). GSH efficiently reduced the amount of oxidized Gal1 in SLE serum by converting it to the reduced form (Fig. 7F, G). Furthermore, our data revealed that reduced Gal1 was positively correlated with GSH levels and neutrophil count and negatively correlated with SLEDAI score (Fig. 7H–J). Therefore, our results demonstrated that the decreased GSH levels in SLE serum play a pivotal role in triggering the excessive oxidation of Gal1 in SLE patients. These results were authenticated by the effect of antioxidants on the excessive generation of reactive oxygen species (ROS) induced by SLE serum, which led to the rescue of cell viability (Fig. 1H and I).
Discussion
The importance of the innate immune system to SLE pathogenesis has recently been revealed. As the most abundant innate immune cells, neutrophils have a short life cycle and are predominant sources of dead cell debris-derived autoantigens. Increased rates of neutrophil death, including death via NETosis, apoptosis and ferroptosis, have been observed in SLE patients, explaining the neutropenia associated with the disease [20, 36,37,38]. ROS are major inducers of neutrophil death [39,40,41]. Neutrophil behavior, including both ROS production and cell death, is tightly regulated by ITIM-bearing inhibitory receptors [42, 43]. VSTM1, also known as signal inhibitory receptor on leukocytes-1 (SIRL-1), is a member of the transmembrane receptor Ig superfamily [11]. VSTM1 binding leads to the phosphorylation of ITIMs, which then recruit the Src homology 2 domain-containing tyrosine phosphatases SHP-1 and SHP-2, which attenuate signaling through the MEK-ERK pathway [27, 28, 44]. With the transient accumulation of ROS induced by SLE serum, several programmed forms of neutrophil death are induced, which can be suppressed by the ligation of VSTM1.
Here, we report for the first time that serum Gal1 functions as a ligand of VSTM1. Galectins constitute a family of β-galactoside-binding lectins with at least one carbohydrate recognition domain (CRD) [33, 45]. Gal1, which is expressed as a monomer or as a noncovalent homodimer containing a CRD, plays important roles in a wide variety of biological processes, including cell proliferation, cell communications and survival [33, 46, 47]. As a secretory protein produced by virtually all hematopoietic lineages, serum Gal1 is considered a hallmark biomarker for a variety of cancers and immunological diseases [48, 49]. Notably, Gal1 has been shown to attenuate several inflammatory diseases in mice, including arthritis [50], hepatitis [51] and colitis [52], and Gal1-deficient mice develop spontaneous lupus-like symptoms [53, 54]. Gal1 can modulate ROS production and phosphatidylserine exposure on fMet-Leu-Phe-activated neutrophils in a carbohydrate-dependent manner [55,56,57]. Here, we present a different mechanism by which Gal1 regulates ROS production in neutrophils in a carbohydrate-independent manner. The possible downregulation of VSTM1 on neutrophils during fMet-Leu-Phe stimulation may explain the dominance of different ROS regulatory mechanisms under different conditions.
Gal1 is oxidized at a cysteine residue and oligomerized in SLE serum, resulting in the loss of its binding affinity for VSTM1. The presence and number of disulfide bonds in Gal1 contribute to the redox state of the protein. Previous research has shown that Gal1 exists in different states, including both oxidized and reduced forms, with each showing different lectin-related activities [58, 59]. Three intramolecular disulfide bridges (Cys3, Cys17 and Cys89) can be formed during Gal1 oxidation [60, 61]. Under pathophysiological conditions, Cys3, Cys43 and Cys61 in Gal1 have been shown to be oxidized, and Gal1 may be fully or partially oxidized in serum. In the absence of a reducing agent, Gal1 is unstable and inactive [60, 62]. Oxidation of Gal1 resulted in loss of lectin activity, ligand recognition and immune system viability [59,60,61]. However, Gal1 functionality was restored when the oxidative environment was abrogated or mitigated through reduction [61]. In the present study, we confirmed that some of the cysteine residues were oxidized and that the levels of reduced Gal1 in SLE serum were markedly reduced. The low levels of functional ligands lead to defective function of inhibitory receptors on neutrophils in SLE patients and may further aggravate the disease. The increased oxidative stress in SLE patients, particularly that caused by low GSH levels in SLE serum, may explain the increased oxidation of Gal1. GSH is an important endogenous antioxidant that protects cells from oxidative damage. It can scavenge reactive oxygen species directly or act as a cofactor for redox-related enzymes [63]. Notably, the antioxidant NAC protected Gal1 from oxidation, and GSH even reverse oxidized Gal1 into the nonoxidized form. Compared to that of other redox-active compounds, the concentration of GSH is extremely high, reaching between 1 and 11 mM in the cell [64] and with 8 nmol/mg hemoglobin in the blood [65]. Thus, the GSH-GSSG pair serves as the most abundant ROS scavenging system and is an important indicator of the redox environment. These findings confirm the value of using antioxidants in the treatment of SLE (ClinicalTrials.gov Identifier: NCT00775476) [15, 66, 67].
Immune inhibitory receptors are thought to dampen immune responses and maintain immune homeostasis. Defects in immune inhibitory receptor signaling contribute to the development of autoimmune diseases [2,3,4,5, 68]. However, a better understanding of the specialized functions of different inhibitory receptors in different diseases is warranted for the rational application of targeted therapies. Our study first revealed the importance of the Gal1-VSTM1 inhibitory axis in the pathogenesis of SLE. The interaction between circulating Gal1 and the inhibitory receptor VSTM1 is essential for neutrophil homeostasis, and the oxidation of Gal1 in patients with SLE disrupts key interactions and leads to a series of neutrophil abnormalities, including increased intracellular ROS production and cell death rate, which are frequently observed in SLE patients.
In conclusion, we identify for the first time Gal1 as a natural ligand of VSTM1 and provide novel information showing the importance of the Gal1/VSTM1 axis in the pathogenesis of SLE. Our findings provide a theoretical framework that elucidates the potential consequences of impaired ligand self-recognition by an immune inhibitory receptor, resulting in diminished immunosuppression. Furthermore, our findings broaden the understanding of immune inhibitory receptors in immune homeostasis and have important clinical implications for targeting oxidative stress as an SLE treatment.
Data availability
The data are available upon reasonable request. All data relevant to the study are included in the paper or uploaded as supplementary information. The data are available from the corresponding author (email:zxpumch2003@sina.com ORCID: https://orcid.org/0000-0001-8775-1699) upon reasonable request.
References
Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. https://doi.org/10.1126/science.290.5489.84
Grebinoski S, Vignali DA. Inhibitory receptor agonists: the future of autoimmune disease therapeutics? Curr Opin Immunol. 2020;67:1–9. https://doi.org/10.1016/j.coi.2020.06.001
Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016;44:1034–51. https://doi.org/10.1016/j.immuni.2016.04.017
Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–85. https://doi.org/10.1016/s1074-7613(00)00027-3
Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–93. https://doi.org/10.1182/blood-2002-01-0027
Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–88. https://doi.org/10.1016/S0140-6736(14)60128-8
Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. https://doi.org/10.1056/NEJMra1100359
Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. https://doi.org/10.1126/scitranslmed.3001201
Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7:691–9. https://doi.org/10.1038/nrrheum.2011.132
Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–53. https://doi.org/10.1038/nm.4027
Steevels TA, Lebbink RJ, Westerlaken GH, Coffer PJ, Meyaard L. Signal inhibitory receptor on leukocytes-1 is a novel functional inhibitory immune receptor expressed on human phagocytes. J Immunol. 2010;184:4741–8. https://doi.org/10.4049/jimmunol.0902039
Wang XF, En Z, Li DJ, Mao CY, He Q, Zhang JF, et al. VSTM1 regulates monocyte/macrophage function via the NF-kappaB signaling pathway. Open Med (Wars). 2021;16:1513–24. https://doi.org/10.1515/med-2021-0353
Otaki N, Chikazawa M, Nagae R, Shimozu Y, Shibata T, Ito S, et al. Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies. J Biol Chem. 2010;285:33834–42. https://doi.org/10.1074/jbc.M110.165175
Passam FH, Giannakopoulos B, Mirarabshahi P, Krilis SA. Molecular pathophysiology of the antiphospholipid syndrome: the role of oxidative post-translational modification of beta 2 glycoprotein I. J Thromb Haemost. 2011;9:275–82. https://doi.org/10.1111/j.1538-7836.2011.04301.x
Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:674–86. https://doi.org/10.1038/nrrheum.2013.147
Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697–713. https://doi.org/10.1084/jem.20151876
Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol. 2017;13:280–90. https://doi.org/10.1038/nrrheum.2017.43
Miao N, Wang Z, Wang Q, Xie H, Yang N, Wang Y, et al. Oxidized mitochondrial DNA induces gasdermin D oligomerization in systemic lupus erythematosus. Nat Commun. 2023;14:872. https://doi.org/10.1038/s41467-023-36522-z
Sergiev PV, Dontsova OA, Berezkin GV. Theories of aging: an ever-evolving field. Acta Nat. 2015;7:9–18.
Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol. 2021;22:1107–17. https://doi.org/10.1038/s41590-021-00993-3
Fukaya K, Hasegawa M, Mashitani T, Kadoya T, Horie H, Hayashi Y, et al. Oxidized galectin-1 stimulates the migration of Schwann cells from both proximal and distal stumps of transected nerves and promotes axonal regeneration after peripheral nerve injury. J Neuropathol Exp Neurol. 2003;62:162–72. https://doi.org/10.1093/jnen/62.2.162
Pei JF, Li XK, Li WQ, Gao Q, Zhang Y, Wang XM, et al. Diurnal oscillations of endogenous H2O2 sustained by p66(Shc) regulate circadian clocks. Nat Cell Biol. 2019;21:1553–64. https://doi.org/10.1038/s41556-019-0420-4
Armstrong DJ, Crockard AD, Wisdom BG, Whitehead EM, Bell AL. Accelerated apoptosis in SLE neutrophils cultured with anti-dsDNA antibody isolated from SLE patient serum: a pilot study. Rheumatol Int. 2006;27:153–6. https://doi.org/10.1007/s00296-006-0219-z
Favier B. Regulation of neutrophil functions through inhibitory receptors: an emerging paradigm in health and disease. Immunol Rev. 2016;273:140–55. https://doi.org/10.1111/imr.12457
Baudhuin J, Migraine J, Faivre V, Loumagne L, Lukaszewicz AC, Payen D, et al. Exocytosis acts as a modulator of the ILT4-mediated inhibition of neutrophil functions. Proc Natl Acad Sci USA. 2013;110:17957–62. https://doi.org/10.1073/pnas.1221535110
Schwarz F, Pearce OM, Wang X, Samraj AN, Laubli H, Garcia JO, et al. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 2015;4. https://doi.org/10.7554/eLife.06184
Van Avondt K, van der Linden M, Naccache PH, Egan DA, Meyaard L. Signal Inhibitory Receptor on Leukocytes-1 Limits the Formation of Neutrophil Extracellular Traps, but Preserves Intracellular Bacterial Killing. J Immunol. 2016;196:3686–94. https://doi.org/10.4049/jimmunol.1501650
Steevels TA, Lebbink RJ, Westerlaken GH, Coffer PJ, Meyaard L. Signal inhibitory receptor on leukocytes-1 (SIRL-1) negatively regulates the oxidative burst in human phagocytes. Eur J Immunol. 2013;43:1297–308. https://doi.org/10.1002/eji.201242916
Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. https://doi.org/10.1098/rsob.120134
Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. https://doi.org/10.1371/journal.ppat.1004651
Loyer C, Lapostolle A, Urbina T, Elabbadi A, Lavillegrand JR, Chaigneau T, et al. Impairment of neutrophil functions and homeostasis in COVID-19 patients: association with disease severity. Crit Care. 2022;26:155. https://doi.org/10.1186/s13054-022-04002-3
Guo X, Zhang Y, Wang P, Li T, Fu W, Mo X, et al. VSTM1-v2, a novel soluble glycoprotein, promotes the differentiation and activation of Th17 cells. Cell Immunol. 2012;278:136–42. https://doi.org/10.1016/j.cellimm.2012.07.009
Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–8. https://doi.org/10.1016/0092-8674(94)90498-7
Inagaki Y, Sohma Y, Horie H, Nozawa R, Kadoya T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur J Biochem. 2000;267:2955–64. https://doi.org/10.1046/j.1432-1033.2000.01311.x
Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. https://doi.org/10.1515/BC.2009.033
Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2888–97. https://doi.org/10.1002/art.11237
Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun. 2010;2:216–27. https://doi.org/10.1159/000284367
Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015;27:448–53. https://doi.org/10.1097/BOR.0000000000000197
Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18:1457–69. https://doi.org/10.1038/cdd.2011.75
Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS). Apoptosis Induct Apoptosis. 2000;5:415–8. https://doi.org/10.1023/a:1009616228304
Xu Y, Loison F, Luo HR. Neutrophil spontaneous death is mediated by down-regulation of autocrine signaling through GPCR, PI3Kgamma, ROS, and actin. Proc Natl Acad Sci USA. 2010;107:2950–5. https://doi.org/10.1073/pnas.0912717107
Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. https://doi.org/10.1111/j.1600-065X.2008.00666.x
van Rees DJ, Szilagyi K, Kuijpers TW, Matlung HL, van den Berg TK. Immunoreceptors on neutrophils. Semin Immunol. 2016;28:94–108. https://doi.org/10.1016/j.smim.2016.02.004
Van Avondt K, Fritsch-Stork R, Derksen RH, Meyaard L. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS ONE. 2013;8:e78459. https://doi.org/10.1371/journal.pone.0078459
Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. https://doi.org/10.1038/nrc1527
Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–57R. https://doi.org/10.1093/glycob/cwl025
Sundblad V, Morosi LG, Geffner JR, Rabinovich GA. Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation. J Immunol. 2017;199:3721–30. https://doi.org/10.4049/jimmunol.1701172
Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007;120:2331–8. https://doi.org/10.1002/ijc.22434
Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, et al. Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2010;20:507–20. https://doi.org/10.1093/glycob/cwp203
Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–98. https://doi.org/10.1084/jem.190.3.385
Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399–406. https://doi.org/10.1002/hep.510310220
Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, et al. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–94. https://doi.org/10.1016/s0016-5085(03)00267-1
Liu SD, Lee S, La Cava A, Motran CC, Hahn BH, Miceli MC. Galectin-1-induced down-regulation of T lymphocyte activation protects (NZB x NZW) F1 mice from lupus-like disease. Lupus. 2011;20:473–84. https://doi.org/10.1177/0961203310388444
Martinez Allo VC, Hauk V, Sarbia N, Pinto NA, Croci DO, Dalotto-Moreno T, et al. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc Natl Acad Sci USA. 2020;117:6630–9. https://doi.org/10.1073/pnas.1922778117
Rodrigues LC, Kabeya LM, Azzolini A, Cerri DG, Stowell SR, Cummings RD, et al. Galectin-1 modulation of neutrophil reactive oxygen species production depends on the cell activation state. Mol Immunol. 2019;116:80–9. https://doi.org/10.1016/j.molimm.2019.10.001
Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–27. https://doi.org/10.1182/blood-2006-03-007153
Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–93. https://doi.org/10.1074/jbc.M306624200
Lopez-Lucendo MF, Solis D, Andre S, Hirabayashi J, Kasai K, Kaltner H, et al. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343:957–70. https://doi.org/10.1016/j.jmb.2004.08.078
Yu X, Scott SA, Pritchard R, Houston TA, Ralph SJ, Blanchard H. Redox state influence on human galectin-1 function. Biochimie. 2015;116:8–16. https://doi.org/10.1016/j.biochi.2015.06.013
Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, et al. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284:4989–99. https://doi.org/10.1074/jbc.M808925200
Guardia CM, Caramelo JJ, Trujillo M, Mendez-Huergo SP, Radi R, Estrin DA, et al. Structural basis of redox-dependent modulation of galectin-1 dynamics and function. Glycobiology. 2014;24:428–41. https://doi.org/10.1093/glycob/cwu008
Hirabayashi J, Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991;266:23648–53.
Mailloux RJ. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020;32:101472 https://doi.org/10.1016/j.redox.2020.101472
Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharm. 1996;140:1–12. https://doi.org/10.1006/taap.1996.0191
Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, et al. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin Chem. 2001;47:1467–9.
Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–46. https://doi.org/10.1002/art.34502
Nasr S, Perl A. Principles behind SLE treatment with N-acetylcysteine. Immunometabolism (Cobham). 2022;4:e00010. https://doi.org/10.1097/IN9.0000000000000010
Olde Nordkamp MJ, Koeleman BP, Meyaard L. Do inhibitory immune receptors play a role in the etiology of autoimmune disease. Clin Immunol. 2014;150:31–42. https://doi.org/10.1016/j.clim.2013.11.007
Acknowledgements
The authors are grateful to all of the patients who participated in the study. We thank Prof Dalong Ma Lab and Prof Wenling Han Lab at Peking University Health Science Center for providing plasmids and helpful discussions. We also thank the Center for Biomarker Discovery and Validation, National Infrastructures for Translational Medicine (PUMCH), Institute of Clinical Medicine, Peking Union Medical College Hospital for instrument support and assistance. This study was supported by grants from the National Natural Science Foundation of China (81788101, 82171799, 82100942, 82171726, 82171798, 82230060, 32141004), National Key R&D Program of China (2022YFC3602000), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2021-I2M-1-017, 2022-I2M-JB-003, 2021-I2M-1-047, 2021-I2M-1-040, 2021-I2M-1-016, and 2021-I2M-1-026), Capital’s Funds for Health Improvement and Research (2020-2-4019), and National High Level Hospital Clinical Research Funding (BJ-2022-116, BJ-2023-084).
Author information
Authors and Affiliations
Contributions
XZ, GCT, and MJ designed and supervised the study; XJ, XX, MW, HY, LZ, SM, YX, YL, YJ, and YW collected samples and performed clinical analysis; XJ, XX, and YG designed and performed experiments; XJ, YG, CZ, JL, YC, SF, and HD performed and analyzed the MS data; and XJ, XX, HL, YG, MJ, GCT and XZ drafted and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Ethics Committee of PUMC Hospital Review Board.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, X., Xiao, X., Li, H. et al. Oxidized galectin-1 in SLE fails to bind the inhibitory receptor VSTM1 and increases reactive oxygen species levels in neutrophils. Cell Mol Immunol 20, 1339–1351 (2023). https://doi.org/10.1038/s41423-023-01084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01084-z
- Springer Nature Limited
Keywords
This article is cited by
-
New insights into the role of the immune inhibitory receptor VSTM1 in autoimmune disease
Cellular & Molecular Immunology (2023)