Abstract
The combination of immune checkpoint inhibitors and anti-angiogenic agents is a promising new approach in cancer treatment. Immune checkpoint inhibitors block the signals that help cancer cells evade the immune system, while anti-angiogenic agents target the blood vessels that supply the tumour with nutrients and oxygen, limiting its growth. Importantly, this combination triggers synergistic effects based on molecular and cellular mechanisms, leading to better response rates and longer progression-free survival than treatment alone. However, these combinations can also lead to increased side effects and require close monitoring.
Similar content being viewed by others
Introduction
There is a sustained interest in the development of therapeutic combinations with immune checkpoint inhibitors (ICIs) [1]. A main reason for this undeniable interest lies in the efficacy of ICIs in cancer treatment, which, however, remains limited in terms of the number of patients who benefit from this category of drugs in the long term. This phenomenon is mainly due to intrinsic and induced resistance mechanisms pointing to the necessity to combine ICIs with other treatments [2]. An emerging strategy for combinations with ICIs is the association with anti-angiogenic agents [3] based on a strong rationale [4]. This rationale lies in mechanistic interactions in which vascular endothelial growth factor (VEGF) occupies the central place with the subsequent VEGF receptor (VEGFR) downstream cellular signalling and pathophysiological consequences. Preclinical knowledge underlines the role of VEGF in the development of an abnormal vascular network that can disrupt both immune effectors trafficking and delivery of ICIs to the tumour [5]. VEGF can also directly affect immune cell function and impair optimal anti-tumour immunity [6]. Therefore, there are both cellular and molecular signalling pathways that can be restored by anti-angiogenic agents in favour of improved efficacy of ICIs. Successful therapeutic strategies in renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) have echoed this view clinically [7].
The aim of this review is to highlight the rationale for combining of ICIs with anti-angiogenic agent, to describe their clinical success, and to point out the limitations of this association in terms of induced toxicity, lack of knowledge of predictive markers, and the need to identify resistance mechanisms and corrective strategies.
Impact of the combination on vessel structuration
Long before the concrete clinical application of mechanistic and molecular considerations of ICIs and their combination with anti-angiogenic agents, Motz and Coukos [8] disclosed key molecular players on endothelial cells that later emerged as the cornerstones of this drug association. The authors emphasised the role of the endothelium in acting as a physical barrier to the passage of lymphocytes to sites of inflammation or tumour growth. Indeed, during the first physiological step of inflammation, there are molecular interactions such as tumour necrosis factor (TNF), which upregulates the expression of chemotactic proteins and adhesion molecules in endothelial cells including intercellular adhesion molecule 1 (ICAM1), and vascular adhesion molecule 1 (VCAM1). These molecules, by direct interaction with circulating T lymphocytes, promote their migration (diapedesis) to the inflamed tissues. The presence of tumour cells is responsible for the local diffusion of several growth factors, including VEGFA which downregulates the expression of ICAM1 and VCAM1 through its specific endothelial cell membrane receptor signalling. This phenomenon reduces T lymphocyte diapedesis. Moreover, this VEGFA-receptor interaction upregulates the expression of VEGFR1, which favours the diapedesis of T Reg cells, thereby downregulating the potential anti-tumour immune response of T lymphocytes.
This visionary conception of the role that endothelial cells may play in cellular modulation of the anti-tumour immune response is echoed by recent arguments suggesting that tumour neo-angiogenesis favours immunosuppression [9]. Converging experimental data focused on a beneficial modification of the immune cellular trafficking by anti-angiogenic agents. For instance, Hodi and co-workers reported on the combination of CTLA4 blockade with ipilimumab and VEGF inhibition by bevacizumab in cancer patients [10]. The presence of bevacizumab significantly affected the changes in tumour vasculature and immune response.
Tumours exhibit abnormal vasculature, not only in terms of the molecular properties of the constitutive endothelial cells but also in terms of the global architectural loss of organisation of tumour vessels [4]. Globally, this abnormal tumour vasculature leads to hypoxia, acidosis and ultimately immune dysfunction. A more pronounced impact of anti-angiogenic treatment on vascular normalisation rather than vessel pruning has been reported [4]. This status of normalised tumour vasculature by anti-angiogenic agents is temporary and results in the intra-tumoral diffusion of immunological cells especially T lymphocytes and macrophages [11]. This so-called phenomenon of tumour vasculature normalisation is complemented by a change in the endothelial cell membrane composition resulting from the direct impact on VEGFR and its intracellular signalling. This relationship introduces some complexity as excessive dosage and duration of anti-VEGFR therapy could exacerbate tumour immunosuppression by reducing perfusion rate due to direct alteration of intratumor vessels [4]. So, it is clear that more experimental basis is needed in order to improve clinically translatable combinations of anti-angiogenic agents and ICIs. In particular, more preclinical data are expected regarding the applied doses of the combination partners as well as the effect of exposure time on final effects of the drug combination compared to the drugs alone.
Impact of the combination on tumour microenvironment
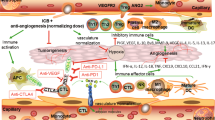
The tumour microenvironment (TME) refers to the cellular and molecular components that surround and interact with cancer cells in a tumour. TME includes various cell types such as cancer-associated fibroblasts, immune cells, and endothelial cells [12, 13]. An important aspect of the TME is the tumour immune microenvironment (TIME), which refers to the immune cells and molecules present in the tumour. TIME includes tumour-infiltrating immune cells, such as T cells and macrophages, as well as immune cells in the surrounding stroma. The presence of these immune cells in the TME can have critical effects on cancer progression [14]. For instance, tumour-infiltrating T cells can help limit the growth and spread of cancer by recognising and attacking cancer cells (Fig. 1).
Anti-angiogenic therapy targets the formation of new blood vessels needed for the tumours to grow and spread, while ICIs reinvigorate the immune system’s ability to attack cancer cells. This combination may win by improving the infiltration of immune cells and the delivery of ICIs to the tumour bed, potentially improving their effectiveness. Several clinical trials are underway to evaluate which patients will benefit most from this approach and how to optimise the promise of this combination. DCs Dendritic cells, TAM tumour-associated macrophages, MDSC myeloid-derived suppressor cells.
However, the TIME is a complex and dynamic system that is composed of various cells and molecules that modulate the immune response to cancer. This microenvironment can be influenced by several factors, including the genetic status of the cancer cells, the presence of other cells and molecules in the microenvironment, and the host immune response to the cancer [12]. The TIME may also be influenced by the stage of the cancer and the patient’s overall health and immune status. For example, in early-stage cancer, the immune system may be able to effectively fight and eliminate cancer cells, whereas in advanced-stage cancer, it may be less effective at recognising and fighting cancer cells. In addition, since the efficacy of anti-PD-1 therapy in cancer patients depends in a great part on PD-1+CD8+T-cell proliferation and the activation and expression of CD28 in PD-1+CD8+T cells, the accumulation of senescent T cells with downregulation or loss of CD28 may compromise the response to immunotherapy [15,16,17,18].
Apart from immune cells itself, the TME can also promote the development of an immunosuppressive microenvironment that can inhibit the immune system’s ability to effectively fight cancer cells. Since VEGFR signalling in immune cells is also associated with pro-tumoral TIME development, VEGF/VEGFR inhibitors also appear to act as factors to restore immune cell differentiation and function. However, previous experiments have shown that anti-angiogenic therapy upregulates PD-L1 in tumour tissues and RCC cell lines [19]. These results suggest that anti-angiogenic agents contribute to the restoration of anti-tumour TIME development, but this positive response may be suppressed by immune checkpoint expression, which is more likely to lead to tumour progression. In this context, then, ICIs should help to release extensive immune activity.
Dendritic cells are a type of immune cell that plays a critical role in the immune response by recognising and presenting foreign antigens to other immune cells [20]. They act as sentinels of the immune system, constantly surveying the body for signs of infection or abnormal cells. When dendritic cells encounter antigens, they engulf them and present them on their surface along with molecules of the major histocompatibility complex (MHC) [21]. This allows other immune cells, such as T cells, to recognise and respond to the antigens. VEGF, through the inhibition of NF-κB; decreases the production of certain inflammatory cytokines that play critical roles in both activation/maturation and migration and numbers of dendritic cells [22, 23]. Specifically, VEGF suppresses the migration of dendritic cells to lymph nodes, through the RhoA‐cofilin1 pathway mediated by the VEGFR2, and to decrease the expression of MHC class II molecules on their surface [24]. Decreased expression of MHC class II molecules may also hinder the activation of T cells, which is essential for the destruction of cancer cells. In addition, VEGF decreases the number of dendritic cells in tumours, turning them in a pro-inflammatory immature population. This may be a double-edged sword, as reducing the number of dendritic cells may limit the immune system’s ability to fight cancer, but it may also reduce the risk of immunologic side effects [20, 21]. In summary, VEGF has a significant impact on dendritic cells, affecting their activation, maturation, migration, and number. Therefore, VEGF inhibitors, by releasing the NFκB axis, may contribute to the recruitment and the maturation of DCs and their anti-tumour effects [22, 25].
The relationship between VEGF and T cells depends on the lymphocyte subpopulation. Treg cells can promote the development and progression of cancer by suppressing the immune response against cancer cells by favouring overexpression of checkpoints on T cells including PD-1, CTLA4, TIM3 and LAG3 [26]. Under hypoxia, Tregs can increase the expression of VEGF in tumours, which can promote the formation of new blood vessels and support the growth of cancer cells [27]. The use of anti-angiogenic agents (anti-VEGF and anti-ANG2) reduces Tregs influence and activates both CD4 and CD8 T cells and increases IFN-γ [28]. While cancer cells can use the loss of MHC-I expression to avoid CD8 recognition, the combination of anti-angiogenic and ICIs promotes antigen-specific T-cell migration and elevated MHC-I, Th1 and T effector cell markers and soluble biomarkers. In the clinic, bevacizumab administration in patients resulted in massive tumour infiltration by CD3+CD8+T cells [29, 30].
MDSCs (myeloid-derived suppressor cells) are a type of immune cell that can suppress the immune response against tumours. They accumulate in the tumour microenvironment and contribute to the growth and progression of tumours [12]. MDSCs promote the formation of new blood vessels in tumours through the production of VEGF and directly repress DC, NK cells, and T cells promoting immune tolerance. Hypoxic conditions fine tunes suppressive M2 tumour-associated macrophages (TAMs) [31]. Vessel normalisation will help to reverse the TAMs differentiation to M1 (anti-tumoral) phenotype promoting immune cell recruitment through soluble factors secretion [32].
Altogether, the combination of anti-VEGF therapy with other immunotherapies may be more effective at treating cancer than anti-VEGF therapy or ICIs alone, as it may target both the tumour’s blood supply and the suppressive immune cells that promote its growth. In this context, studies on tumour-bearing mouse models have demonstrated that multi-targeted anti-angiogenic tyrosine kinase inhibitors (TKIs) increased tumour infiltration of CD8+ and CD4+ T cells by downregulating PD-1 expression, and decreased the number and activity of Tregs and MDSCs [26, 33, 34]. Similarly, sunitinib, a multi-tyrosine kinase receptor inhibitor (TKI), inhibited the expansion of Tregs and MDSCs in patients with RCC [35]. The VEGFR2-targeting TKI cabozantinib was also associated with a reduction in the number of Tregs and MDSCs, and simultaneously promoted tumour infiltration of CD4+ and CD8 + T lymphocytes, both alone and in combination with the anti-cancer vaccine MVA/rF-CEA/TRICOM [35].
Clinical applications and their limits
More than 100 clinical trials are currently investigating the associations between immunotherapy and anti-angiogenic agents. The synergistic efficacy of combining immune checkpoint inhibitors (ICIs) with anti-angiogenic drugs, which results in considerable therapeutic advantages in a variety of solid tumours, is highly supported by the preclinical and experimental evidence now available. Notably, controlled studies show outstanding success in treating RCC [36,37,38,39,40,41,42] and HCC [43] (as highlighted in Table 1).
There is also rising interest in this combination used in other tumour types such as endometrial carcinoma. Extended efficacy and tolerability of the combination of lenvatinib plus pembrolizumab were demonstrated in patients with previously treated advanced endometrial carcinoma (EC) who were neither MSI-high or dMMr and who experienced progression on initial drug treatment [44, 45]. Advanced ovarian cancer represents an urgent medical need. In this latter tumour site, promising response rates have been recorded following treatment with a combination of bevacizumab and nivolumab [46]. More generally there are several indications of positive preliminary results that strengthen the notion of therapeutic benefit from the association of anti-angiogenic agents and ICIs across a broader spectrum of tumour sites [47]. Promising clinical data are also emerging from lung cancers [48]. For this latter localisation, a sustained interest in the combination is undeniable as stressed by real-world studies recently reported [49,50,51].
It is worth noting that the expression of vascular endothelial growth factor (VEGF) and programmed death-ligand 1 (PD-L1) is mainly regulated by hypoxia-inducible factor 2 alpha (HIF2A), which makes it a critical player at the intersection of anti-angiogenic and anti-immune checkpoint therapies. Promisingly, belzutifan, a HIF2A inhibitor, has demonstrated great potential alone or in combination with cabozantinib in treating von Hippel-Lindau disease and RCC [52, 53].
It is important to keep in mind the mechanism underlying the potential beneficial effect of the association between anti-angiogenic agents and ICIs. More specifically, when considering bevacizumab, the depletion of VEGF is related to the quenching of VEGFR2 signalling. In contrast, such a pure effect against VEGFR2 signalling does not occur with multi-target TKIs that have a more or less extensive effect on other membrane receptors with their own signalling [47]. This situation may complicate the final interpretation of the pharmacodynamic effects that follow the application of the combination especially regarding the potential supplement of toxicity introduced by multiple cellular pathways affected by TKIs. Other important aspects to consider as potentially influencing the final pharmacodynamic effects of the combination are the sequence order of drugs, their dosages and timing [4, 11]. For example, a study by Hamuro and co-workers showed a comparable benefit-risk ratio in patients with RCC treated with a less frequent schedule of nivolumab 480 mg every 4 weeks plus cabozantinib 40 mg daily compared with to nivolumab 240 mg every 2 weeks plus cabozantinib 40 mg daily [54]. The lessons from the use of ultra-low dose of immunotherapy are of value in the present context. This relates to a recent article by Patil et al. Patil et al., ($year$) [55], which showed that in the treatment of advanced head and neck cancer, a flat dose of nivolumab at 20 mg once every 3 weeks provided a significant overall survival benefit over treatment with methotrexate and erlotinib.
Cardiovascular toxicity is reported to occur more frequently during and after treatments with ICIs than previously thought [56]. Since cardiac function is affected not only by the use of anti-angiogenic agents but also by ICIs, it is obvious that their combination schedule should be particularly well-weighted with respect to cardiovascular risk. It must be considered as central in the context of the drug combination. Therefore, the use of indicators of toxicity risk as well as predictive markers of treatment efficacy should be particularly welcome in the context of combination usage [57].
In this context of efficacy vs. toxicity risk ratio, there is a critical need to develop prognostic and predictive biomarkers of response to VEGFR-TKIs and ICIs., Measurement of plasma interleukin 8 (IL-8) is emerging as a potential candidate in this respect since elevated levels have been associated with enhanced intra-tumour neutrophils and reduced clinical benefit of ICIs [58, 59]. Other biomarkers such as ctDNA concentration or MIKI67 mutations have also been recently proposed to analyse the benefit of the combination [60]. Taken together, well-designed clinical trials in which the selection of optimal combination regimens and the setting of appropriate doses and sequences should be conducted in order to improve the therapeutic index of immunotherapy.
Perspectives
There is compelling preclinical and experimental data to support the clinical evidence for a beneficial combination of anti-angiogenic agents and ICIs. The evidence is particularly strong in patients with kidney, lung and liver cancers.
However, in the context of association, more data would be desirable. On one hand, one of the most neglected issues for combination with ICIs is a well-defined sequencing that could be explored on an experimental basis with relevant models like optimised tumour organoids [61, 62] or using implantable microdevices [63].
On the other hand, the preclinical setting has put the focus mainly on the effect of anti-angiogenic agents on the activity CPI, the reverse sequence (effects of CPIs on anti-angiogenic agents) could reveal complementary aspects and should not be neglected. For example, tumour-associated B lymphocytes could accelerate tumour progression by enhancing tumour angiogenesis by promoting the secretion of proangiogenic factors such as VEGF, FGF-2 and MMP-9 [64]. This implies that a larger understanding of the interaction between anti-angiogenic agents and ICIs could lead to optimal use of these agents in the clinical setting.
Better clinical use of the combination would also result from the identification of predictive clinical biomarkers. They would allow optimal application of this treatment option in potentially responsive patients as well as for potentially toxic effects. In addition, almost nothing is known about the mechanisms of acquired resistance resulting from the use of the combination. One possibility is that the drugs destroying blood vessels stimulate the development of tumour lymphatic vessels contributing to treatment failure [65]. Tumours from sunitinib-treated RCC patients in a neoadjuvant setting exhibit increased lymphatic vessels and increased lymph node invasion. This detrimental effect is explained at least by the stimulation of VEGFC expression following sunitinib administration [66]. This kind of information should pave the way for complementary treatments following clinical use of the combination. In final, peculiar attention could also be paid to the possibility that normalisation of tumour vasculature under the effect of anti-angiogenic agents could favour a better diffusion of therapeutic monoclonal antibodies.
So, while it is very satisfying to have a strong rationale for the use of the combination, more remains to be done to optimise this promising therapeutic option at the interface between immunotherapy and targeted therapy.
References
Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, et al. Beyond conventional immune-checkpoint inhibition—novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18:199–214. https://doi.org/10.1038/s41571-020-00455-z
Vukadin S, Khaznadar F, Kizivat T, Vcev A, Smolic M. Molecular mechanisms of resistance to immune checkpoint inhibitors in melanoma treatment: an update. Biomedicines. 2021;9:835. https://doi.org/10.3390/biomedicines9070835
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. https://doi.org/10.1186/s12943-019-0974-6
Duda DG, Jain RK. Revisiting antiangiogenic multikinase inhibitors in the era of immune checkpoint blockade: the case of sorafenib. Cancer Res. 2022;82:3665–7. https://doi.org/10.1158/0008-5472.CAN-22-2639
Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res. 2023;29:30–9. https://doi.org/10.1158/1078-0432.CCR-22-1366
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–86. https://doi.org/10.1182/blood-2002-07-1956
Zheng W, Qian C, Tang Y, Yang C, Zhou Y, Shen P, et al. Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade. Front Immunol. 2022;13:1–24. https://doi.org/10.3389/fimmu.2022.1035323
Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–11. https://doi.org/10.1038/nri3064
Orr BA, Eberhart CG. Molecular pathways: not a simple tube-the many functions of blood vessels. Clin Cancer Res. 2015;21:18–23. https://doi.org/10.1158/1078-0432.CCR-13-1641
Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–42. https://doi.org/10.1158/2326-6066.CIR-14-0053
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. https://doi.org/10.1038/nrclinonc.2018.29
Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell. 2020;37:471–84. https://doi.org/10.1016/j.ccell.2020.03.007
Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. https://doi.org/10.1016/j.tig.2008.10.012
Li Z, Seehawer M, Polyak K. Untangling the web of intratumour heterogeneity. Nat Cell Biol. 2022;24:1192–201. https://doi.org/10.1038/s41556-022-00969-x
Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1–targeted therapies is CD28-dependent. Science (80-). 2017;355:1423–7. https://doi.org/10.1126/science.aaf0683
Liu X, Hoft DF, Peng G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J Clin Invest. 2020;130:1073–83. https://doi.org/10.1172/JCI133679
Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–31. https://doi.org/10.1182/blood-2012-03-416040
Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, et al. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190:2403–14. https://doi.org/10.4049/jimmunol.1202369
Liu X-D, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3:1017–29. https://doi.org/10.1158/2326-6066.CIR-14-0244
Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol. 2019;9:1–18. https://doi.org/10.3389/fimmu.2018.03176
Wang Y, Xiang Y, Xin VW, Wang X-W, Peng X-C, Liu X-Q, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13:107 https://doi.org/10.1186/s13045-020-00939-6.
Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–24. https://doi.org/10.1007/s00262-007-0441-x
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. https://doi.org/10.4049/jimmunol.160.3.1224
Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, et al. Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2‐RhoA‐cofilin1 pathway. Cancer Sci. 2019;110:2357–67. https://doi.org/10.1111/cas.14091
Malo CS, Khadka RH, Ayasoufi K, Jin F, AbouChehade JE, Hansen MJ, et al. Immunomodulation mediated by anti-angiogenic therapy improves CD8 T cell immunity against experimental glioma. Front Oncol. 2018;8:1–6. https://doi.org/10.3389/fonc.2018.00320
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8++ T cells in tumors. J Exp Med. 2015;212:139–48. https://doi.org/10.1084/jem.20140559
Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–71. https://doi.org/10.1158/0008-5472.CAN-11-3687
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–85. https://doi.org/10.1038/s12276-020-00500-y
Tamura R, Tanaka T, Ohara K, Miyake K, Morimoto Y, Yamamoto Y, et al. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 2019;110:499–508. https://doi.org/10.1111/cas.13889
Wang Y, Dong J, Quan Q, Liu S, Chen X, Cai X, et al. Immune cell infiltration of the primary tumor microenvironment predicted the treatment outcome of chemotherapy with or without bevacizumab in metastatic colorectal cancer patients. Front Oncol. 2021; 10. https://doi.org/10.3389/fonc.2020.581051.
Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. https://doi.org/10.1158/0008-5472.CAN-13-1196
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. https://doi.org/10.1016/j.ccr.2010.11.009
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. https://doi.org/10.1158/1078-0432.CCR-08-1332
Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–49. https://doi.org/10.1158/0008-5472.CAN-12-2325
Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12:294. https://doi.org/10.1186/s12967-014-0294-y
Lee C-H, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946–58. https://doi.org/10.1016/S1470-2045(21)00241-2
Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300. https://doi.org/10.1056/nejmoa2035716
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. https://doi.org/10.1056/NEJMoa1816047
Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023a;24:228–38. https://doi.org/10.1016/S1470-2045(23)00049-9
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019a;380:1116–27. https://doi.org/10.1056/NEJMoa1816714
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019b;393:2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl J Med. 2021;384:829–41. https://doi.org/10.1056/NEJMoa2026982
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. https://doi.org/10.1056/NEJMoa1915745
Makker V, Aghajanian C, Cohn AL, Romeo M, Bratos R, Brose MS, et al. A phase Ib/II study of lenvatinib and pembrolizumab in advanced endometrial carcinoma (Study 111/KEYNOTE-146): long-term efficacy and safety update. J Clin Oncol. 2023;41:974–9. https://doi.org/10.1200/JCO.22.01021
Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386:437–48. https://doi.org/10.1056/NEJMoa2108330
Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer. JAMA Oncol. 2019;5:1731. https://doi.org/10.1001/jamaoncol.2019.3343
Li S, Chen J, Sun Z. Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021;41:830–50. https://doi.org/10.1002/cac2.12183
Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. 2021;12:1–12. https://doi.org/10.3389/fimmu.2021.689132
Ikeuchi N, Igata F, Kinoshita E, Kawabata T, Tan I, Osaki Y, et al. Real-world efficacy and safety of atezolizumab plus bevacizumab, paclitaxel and carboplatin for first-line treatment of Japanese patients with metastatic non-squamous non-small cell lung cancer. Anticancer Res. 2023;43:713–24. https://doi.org/10.21873/anticanres.16210
Chen S, Wei H, Zhao W, Jiang W, Ning R, Zhou S, et al. PD-1/PD-L1 inhibitors plus anti-angiogenic agents with or without chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy as second or later-line treatment for patients with advanced non-small cell lung cancer: A real-world retrospective cohort study. Front Immunol. 2022;13:1–20. https://doi.org/10.3389/fimmu.2022.1059995
Provencio M, Ortega AL, Coves-Sarto J, Calvo V, Marsé-Fabregat R, Dómine M, et al. (2022) Atezolizumab Plus Bevacizumab as First-line Treatment for Patients With Metastatic Nonsquamous Non–Small Cell Lung Cancer With High Tumor Mutation Burden. JAMA Oncol. 1–9, https://doi.org/10.1001/jamaoncol.2022.5959.
Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau Disease. N Engl J Med. 2021;385:2036–46. https://doi.org/10.1056/nejmoa2103425
Choueiri TK, McDermott DF, Merchan J, Bauer TM, Figlin R, Heath EI, et al. (2023b) Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: an open-label, single-arm, phase 2 study. Lancet Oncol. 2045: 1–10, https://doi.org/10.1016/S1470-2045(23)00097-9.
Hamuro L, Hu Z, Passarell J, Barcomb H, Zhang J, Goldstein S, et al. Exposure-response analysis to support nivolumab once every 4 weeks dosing in combination with cabozantinib in renal cell carcinoma. Clin Cancer Res. 2022;28:1603–13. https://doi.org/10.1158/1078-0432.CCR-21-3149
Patil VM, Noronha V, Menon N, Rai R, Bhattacharjee A, Singh A, et al. Low-dose immunotherapy in head and neck cancer: a randomized Study. J Clin Oncol. 2023;41:222–32. https://doi.org/10.1200/JCO.22.01015
Laenens D, Yu Y, Santens B, Jacobs J, Beuselinck B, Bechter O, et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;40:3430–8. https://doi.org/10.1200/JCO.21.01808
Hertz DL, McShane LM, Hayes DF. Defining clinical utility of germline indicators of toxicity risk: a perspective. J Clin Oncol. 2022;40:1721–31. https://doi.org/10.1200/JCO.21.02209
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92. https://doi.org/10.1038/s41591-020-0856-x
Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26:693–8. https://doi.org/10.1038/s41591-020-0860-1
Chen Y, Li X, Liu G, Chen S, Xu M, Song L, et al. ctDNA concentration, MIKI67 mutations and hyper-progressive disease related gene mutations are prognostic markers for camrelizumab and apatinib combined multiline treatment in advanced NSCLC. Front Oncol. 2020;10:1–11. https://doi.org/10.3389/fonc.2020.01706
Palasantzas VEJM, Tamargo-Rubio I, Le K, Slager J, Wijmenga C, Jonkers IH, et al. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. 2023;39:268–84. https://doi.org/10.1016/j.tig.2023.01.002
Schuster B, Junkin M, Kashaf SS, Romero-Calvo I, Kirby K, Matthews J, et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat Commun. 2020;11:1–12. https://doi.org/10.1038/s41467-020-19058-4
Tatarova Z, Blumberg DC, Korkola JE, Heiser LM, Muschler JL, Schedin PJ, et al. A multiplex implantable microdevice assay identifies synergistic combinations of cancer immunotherapies and conventional drugs. Nat Biotechnol. 2022;40:1823–33. https://doi.org/10.1038/s41587-022-01379-y
Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W, et al. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS ONE. 2013;8:e64159. https://doi.org/10.1371/journal.pone.0064159
Montemagno C, Pagès G. Resistance to anti-angiogenic therapies: a mechanism depending on the time of exposure to the drugs. Front Cell Dev Biol. 2020;8:1–21. https://doi.org/10.3389/fcell.2020.00584
Dufies M, Giuliano S, Ambrosetti D, Claren A, Ndiaye PD, Mastri M, et al. Sunitinib stimulates expression of VEGFC by tumor cells and promotes lymphangiogenesis in clear cell renal cell carcinomas. Cancer Res. 2017;77:1212–26. https://doi.org/10.1158/0008-5472.CAN-16-3088
Funding
Funding is acknowledged from the French Government (Agence Nationale de Recherche, ANR) through the ‘Investments for the Future’ LABEX SIGNALIFE (ANR-11-LABX-0028-01 and IDEX UCAJedi ANR-15-IDEX-01) and [AD-ME project R19162DD]; CANC’AIR Genexposomic project, Canceropole PACA; DREAL PACA, ARS PACA, Région Sud, INSERM cancer; INCA Plan Cancer; ITMO Cancer, Children Medical Safety Research Institute (CMSRI, Vaccinophagy project R17033DJA), The Fondation Max et Yvonne de Foras, La Ligue contre le Cancer-Equipe labellisée 2019, Fondation ARC de la Recherche contre le Cancer Programme labellisé 2022, Fondation Amgen, Fondation Flavien.
Author information
Authors and Affiliations
Contributions
GM, PB: conceptualisation; investigation; writing—original draft; GP, BM, PH: visualisation; writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brest, P., Mograbi, B., Pagès, G. et al. Checkpoint inhibitors and anti-angiogenic agents: a winning combination. Br J Cancer 129, 1367–1372 (2023). https://doi.org/10.1038/s41416-023-02437-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02437-1
- Springer Nature Limited
This article is cited by
-
Clinical efficacy and immune response of neoadjuvant camrelizumab plus chemotherapy in resectable locally advanced oesophageal squamous cell carcinoma: a phase 2 trial
British Journal of Cancer (2024)
-
Immune check points in cancer treatment: current challenges and perspectives
British Journal of Cancer (2023)