Abstract
Background
No targeted drugs are currently available against small cell lung cancer (SCLC). BCL-2 family members are involved in apoptosis regulation and represent therapeutic targets in many malignancies.
Methods
Expression of BCL-2 family members in 27 SCLC cell lines representing all known four SCLC molecular subtypes was assessed by qPCR, Western blot and mass spectrometry-based proteomics. BCL-2 and MCL-1 inhibition (venetoclax and S63845, respectively) was assessed by MTT assay and flow cytometry and in mice bearing human SCLC tumours. Drug interactions were calculated using the Combenefit software. Ectopic BAX overexpression was achieved by expression plasmids.
Results
The highest BCL-2 expression levels were detected in ASCL1- and POU2F3-driven SCLC cells. Although sensitivity to venetoclax was reflected by BCL-2 levels, not all cell lines responded consistently despite their high BCL-2 expression. MCL-1 overexpression and low BAX levels were both characteristic for venetoclax resistance in SCLC, whereas the expression of other BCL-2 family members did not affect therapeutic efficacy. Combination of venetoclax and S63845 resulted in significant, synergistic in vitro and in vivo anti-tumour activity and apoptosis induction in double-resistant cells; however, this was seen only in a subset with detectable BAX. In non-responding cells, ectopic BAX overexpression sensitised to venetoclax and S63845 and, furthermore, induced synergistic drug interaction.
Conclusions
The current study reveals the subtype specificity of BCL-2 expression and sheds light on the mechanism of venetoclax resistance in SCLC. Additionally, we provide preclinical evidence that combined BCL-2 and MCL-1 targeting is an effective approach to overcome venetoclax resistance in high BCL-2-expressing SCLCs with intact BAX.
Similar content being viewed by others
Background
Small cell lung cancer (SCLC) is characterised by rapid doubling time and a high propensity to metastasise [1,2,3]. SCLC makes up about 13–15% of lung cancer cases, and with a 5-year survival rate of <7%, it remains one of the most lethal forms of malignant diseases [4, 5]. Due to its aggressive course, two-thirds of patients already present with a metastatic spread outside the chest at initial diagnosis [3]. Accordingly, patients are being treated usually with systemic cytotoxic chemotherapy with or without concurrent radiation [1, 6]. While SCLC is initially exceptionally responsive to platinum-based therapies with response rates consistently over 60%, recurrence arises rapidly in the majority of cases [3, 7]. Of note, the addition of immunotherapy to the armamentarium of anti-SCLC therapies failed so far to mimic the efficacy seen in non-SCLC (NSCLC) [8,9,10]. Additionally, in contrast to NSCLC, where genotype-based targeted therapies paved the way for personalised therapeutic approaches, SCLC is still regarded and treated both in the clinic and in the laboratory as a single disease.
The worldwide resurgence of SCLC profiling studies and the development of new preclinical models converged on a new model of SCLC subtypes defined by differential expression of four key transcription regulators: ASCL1 (SCLC-A), NEUROD1 (SCLC-N), POU2F3 (SCLC-P) and YAP1 (SCLC-Y) [11, 12]. These biologically distinct molecular subsets have major differences in morphology, growth properties, genetic alterations and prognosis [3, 13,14,15]. SCLC-A tumours show high expression of neuroendocrine (NE) markers and have a classic morphology compared to SCLC-N which is associated with less NE features and variant morphology [1, 2, 11]. Meanwhile SCLC-P tumours have mostly non-NE features and they represent a specific tuft-cell variant of SCLC [11, 16]. YAP1 is also preferentially expressed in non-NE SCLCs, immunohistochemical analyses, however, failed so far to confirm a unique SCLC-Y subtype in human tissue specimens [11, 14, 15]. These phenotypic differences are also mirrored in prognosis since high ASCL1 expression is linked with worse prognosis, whereas high POU2F3 associates with improved survival in surgically treated SCLC patients [15].
As transcription factors are difficult drug targets, the development of targeted therapies for SCLC has been lacking [17]. In 1988, the BCL-2 protein was shown to promote carcinogenesis and cancer progression through cell death resistance [18]. In the following years, several proteins have been added to this family, each being involved in regulating cellular apoptosis and containing one or more BCL-2 homology (BH) domain [19]. Proteomic profiling of SCLCs revealed elevated levels of BCL-2, which was later associated with increased cisplatin resistance [20]. In addition, in-depth whole-genome sequencing of primary SCLC tumours and cell lines suggests that BCL-2 expression might vary among the newly defined molecular subtypes, thus defining a potential subtype-specific therapy [11].
MCL-1 is another BCL-2 family member that promotes cell survival by preventing the activation of structurally similar but pro-apoptotic BAX and BAK on the mitochondrial outer membrane [21]. However, in contrast to BCL-2 expression which leads to greater sensitivity to BCL-2 inhibitors, bench-to-bedside studies identified MCL-1 as a critical factor in acquired resistance to these antagonists [22].
In recent years, the emergence of BCL-2 family interacting disruptors has provided a new arsenal of drugs for treating various malignancies, especially blood cancers [23]. Venetoclax, the first Food and Drug Administration-approved pure BCL-2 BH3 mimetic dramatically improved patient care in chronic lymphocytic leukaemia (CLL) and acute myeloid leukaemia (AML), and is currently undergoing preclinical and clinical testing in solid tumours as well [24, 25]. Given the elevated expression of BCL-2 in certain SCLC cases [26], venetoclax along with other BCL-2 antagonists might represent an attractive option for treating these patients [23]. Nevertheless, despite the initially encouraging results [17], the sensitivity to these inhibitors is inconsistent both in preclinical models and in early-phase clinical trials even in high BCL-2-expressing cases [23]. One of the key mechanisms of resistance to these inhibitors is suspected to be the overexpression of MCL-1, through sequestering BH3-only proteins released from BCL-2, and preventing activation of BAX/BAK [27, 28]. In our study, in order to facilitate the development of personalised therapeutic approaches, we assessed the differences in BCL-2 expression among different SCLC subtypes and investigated the mechanisms underlying resistance to BCL-2 inhibition. Additionally, the efficacy of combined BCL-2/MCL-1 inhibition was assessed in vitro and in vivo.
Materials and methods
Cell lines
Cells lines were maintained in RPMI-1640 with 10% fetal calf serum (FCS) (Sigma, St. Louis, MO, USA), 100 U/ml penicillin and 10 mg/ml streptomycin (Sigma) at 37 °C in a humidified incubator with 5% CO2 and regularly screened for Mycoplasma contamination. All cell lines used, their sources, molecular subtype as well as their in vitro growth pattern (adherent, semi-adherent, suspension) are listed in Supplementary Table S1.
Proteomic analysis
The proteomics dataset analysed in this study was recently published by our group [29]. Briefly, proteins were extracted from cell pellets of 27 SCLC cell lines and enzymatically digested. Mass spectrometry analysis of the peptides was performed on an Ultimate 3000 RSLC nano pump coupled to a Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) as previously described [30]. Raw files were searched in Proteome Discoverer 2.4 (Thermo Fisher Scientific) using Spectral Library (Proteome tools human spectrum library) and SEQUEST HT searches against UniProtKB human database (v.15/01/2019) [15, 31]. Raw protein intensities were then log2-transformed and the samples were median-normalised. Triplicate measurements were averaged, followed by a filter for minimum 80% valid values and left-censored missing data imputation.
Drug treatment
Venetoclax and S63845 were purchased from Medchemexpress (Monmouth Junction, NJ, USA) and dissolved in DMSO and used in vitro at the indicated concentrations. For the in vivo part, venetoclax was dissolved in 60% phosal 50 propylene glycol (PG) (Medchemexpress), 30% polyethylene glycol 400 (PEG400) (Medchemexpress) and 10% ethanol. S63845 was prepared in a solution of 20% hydroxypropyl β‐cyclodextrin (Sigma) in 20 mM HCl.
Transfection with expression plasmids
In all, 1 × 106 cells were seeded into T25 flask and, on the following day, transfected with 2.5 µg pCMV6-BAX (OriGene Technologies, Rockville, MD, USA) or pEGFP-n3 (Takara Bio, Kusatsu, Japan) using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
RNA isolation and qPCR
Total RNA was isolated with TRIzol and reverse transcribed with MMLV reverse transcriptase (Thermo Fisher Scientific) as previously described [32]. The cDNAs were analysed using TaqMan gene expression assays (Thermo Fisher Scientific, BCL-2: Hs04986394; GAPDH: Hs02786624; BAX: Hs00180269) on a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). GAPDH was used as reference gene.
Protein isolation and Western blot analysis
Cells were collected in RIPA buffer containing protease inhibitor cocktail (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE, blotted on nitrocellulose membranes and Western blots were performed according to the manufacturer’s protocol using the following primary antibodies (all from Cell Signaling Technology, Danvers, MA, USA): BCL-2 (1:3000, #15071), MCL-1 (1:1000, #39224), BAX (1:1000, #5023), Caspase 3 (1:3000 #9662), Cleaved Caspase 3 (1:1000 #9661) PARP (1:3000, #9542) and GAPDH (1:5000, #5174).
Cell viability assay
A previously defined optimal number of cells in 100 µl medium was seeded in triplicate in 96 well plates. Accurate cell counting was ensured by generating single cell suspension using trypsin, also in the cell lines which grow in 3D clusters. On the next day, cells were treated with another 100 µl medium containing drugs as indicated. In case of previous transfection with plasmids, cells were seeded 24 h after transfection. After 72 h, cell survival was measured using an MTT assay according to the manufacturer’s instruction (EZ4U, Biomedica, Vienna, Austria).
Colony formation assay
Clonogenic assays were implemented to test the long term effects of venetoclax and S63845 as single drugs and in combination. Therefore, cells were seeded in low density into 6- or 12-well culture plates in triplicate and on the next day treated as indicated. After 10–21 days, depending on the cell line, colonies were fixed with 70% ethanol and stained with 0.1% crystal violet. For quantification, plates were destained with 2% SDS and absorbance was measured at 562 nm.
Apoptosis assay
In all, 3 × 105 cells were seeded in a 6-well tissue culture plate and, on the next day, treated with DMSO, 2.5 μM venetoclax, 2.5 μM S63845 or a combination as indicated. Following 24 or 48 h incubation, cells were collected and stained with Annexin V-FITC (BD Pharmingen, Franklin Lakes, NJ, USA) and propidium iodide. After 15 min incubation at room temperature, cells were analysed by flow cytometry on a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA).
In vivo xenograft model
In all, 1 × 107 SHP77 cells were injected subcutaneously into male SCID mice using a 1:1 ratio of serum-free medium and matrigel. After 7 days when tumours were palpable, mice were randomly assigned into 4 treatment groups (N = 7 per group), vehicle control, venetoclax, S63846 and venetoclax+S63845. Venetoclax-treated mice were treated with 100 mg/kg of venetoclax by oral gavage 5 days per week. S63845 was intraperitoneally administered at 25 mg/kg twice a week. All mentioned animal experiments were performed according to the ARRIVE guidelines [33] and to the animal welfare regulations of the host institutes (permission number: PEI/001/2574–6/2015). Tumours were measured thrice a week with a caliper and expressed in mm3 by the formula for the volume of a prolate ellipsoid (length × width2 × π/6), as described previously [34]. Mice were sacrificed after 17 days of treatment.
Histological analysis of tumours
Tumours were fresh-frozen and cut into 4 µm sections. To assess cell proliferation, slides were incubated with a Ki-67 antibody (1:100, clone MIB-1, Dako, Glostrup, Denmark) for 30 min after endogenous peroxidase blocking. The ImmPRESS Goat Anti-Mouse detection system (Vector Laboratories, Burlingame, CA, USA) was used to detect antibody binding according to the manufacturer’s recommendations. 3-3-Diaminobenzidine (Dako) was implemented for colour development. Each slide was counterstained with Mayer’s haematoxylin (Sigma) and mounted with Entellan (Merck, Darmstadt, Germany). For the analysis of apoptosis, terminal deoxynucleotidyl transferase– mediated dUTP nick-end labelling (TUNEL) was carried out according to the manufacturer’s instructions (Roche Diagnostics, Basel, Switzerland). Counterstaining of nuclei was accomplished using DAPI.
Slides were scanned by a TissueFAXS System (TissueGnostics GmbH, Vienna, Austria). At least 3000 tumour cells were counted manually with the ImageJ software to evaluate the percentage of Ki-67 and TUNEL-positive tumour cells per sample.
Statistical analysis
Unless stated otherwise, data were analysed using GraphPad Prism 8 and are shown as means or means ± SD or boxplots (min to max) of at least three independent experiments, each performed in triplicate. IC50 values were calculated from dose–response curves with GraphPad Prism 8. For correlation analysis, Anderson–Darling test for normal distribution was used, and accordingly, either Pearson or Spearman r was calculated with GraphPad Prism 8. Drug interactions were assessed using the Combenefit software. Differences were evaluated by Student’s t test or analysis of variance for comparisons of two or multiple groups, respectively, and considered statistically significant at p < 0.05.
Results
BCL-2 is highly expressed in SCLC subtypes A and P
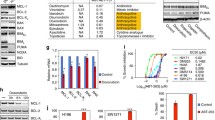
We recently performed a comprehensive proteomic analysis of 27 SCLC cell lines representing all 4 main molecular subtypes (9× ASCL1, 7× NEUROD1, 4× POU2F3, 7× YAP1) [29]. We further analysed this dataset with respect to differences between the subtypes and found that BCL-2 is significantly higher expressed in cell lines established from tumours representing the SCLC-A and SCLC-P subtypes (Fig. 1a). We confirmed this expression pattern in an extended panel of cell lines (N = 28) by Western blot and qPCR (Fig. 1b, c). Of note, we observed a significant positive correlation between BCL-2 mRNA and protein levels (Supplementary Fig. S1).
BCL-2 protein levels in SCLC cell lines, grouped according to SCLC molecular subtypes, from a proteomics analysis (N = 27). b BCL-2 levels of SCLC cell lines (N = 28) deriving from densitometric analysis of Western blots relative to GAPDH and representative Western blot pictures. Bars are shown as mean ± SD of 4–5 replicates. c BCL-2 mRNA levels (N = 28) relative to GAPDH measured by qPCR. d IC50 values of venetoclax, calculated from dose–response curves after 72 h, assessed by MTT assay. The black line (4 µM) indicates the threshold between sensitive and resistant cells. e Spearman correlation between BCL-2 expression levels deriving from Western blot analysis and venetoclax IC50 values (blue: SCLC-A, red: SCLC-N, yellow: SCLC-P, green: SCLC-Y). Black arrows show “outliers”. f BCL-2 expression deriving from Western blot analyses in SCLC cell lines categorised into venetoclax sensitive and resistant groups, according to the 4 µM threshold. Each dot represents the mean of one cell line. *p < 0.05, **p < 0.01, ***p < 0.001.
Sensitivity to BCL-2 inhibition does not depend solely on BCL-2 levels
We next treated our SCLC cell lines with different concentrations of the BCL-2 inhibitor venetoclax, which showed a wide range of activity among the cell lines resulting in calculated IC50 values ranging from 75 nM to >25 µM (Supplementary Fig. S2 and Supplementary Table S2). Generally, sensitivity to BCL-2 inhibition was partially reflected by SCLC subtypes expressing different levels of BCL-2, with SCLC-A, SCLC-N and SCLC-P being more sensitive than SCLC-Y (Fig. 1d). When we compared venetoclax sensitivity with BCL-2 expression levels in the individual cell lines, we found a significant but poor correlation (Fig. 1e). Using a cut-off IC50 value of 4 µM, we stratified our panel into venetoclax sensitive (venS) and resistant (venR) groups, consisting of 8 (DMS53, H146, DMS153, COR-L311, H187, H211, H524, N417) and 18 (H526, H1882, H1048, H446, SHP77, H1694, H1688, H372, H69, H82, H2171, GLC4, H196, H841, H1341, CRL-2066, CRL-2177, HLHE) cell lines, respectively (Supplementary Table S2). No significant difference in BCL-2 levels between the venS and venR groups was observed, since there were several cell lines resistant to venetoclax despite their very high levels of BCL-2, as indicated by black arrows (Fig. 1e, f).
MCL-1 overexpression and low BAX levels are characteristic for venetoclax resistance
In search for potential candidates responsible for the observed resistance to BCL-2 inhibition, focusing only on high BCL-2 expressing cell lines (venSBCL2high, venRBCL2high), we assessed the expression of other BCL-2 family members in the venetoclax sensitive and resistant cell lines using our proteomic data. Therefore, cell lines with a normalised log2 intensity >19 in the proteomics dataset (Fig. 1a) and with detectable BCL-2 by Western blot (Fig. 1b) were assigned into the BCL-2 high group, resulting in 6 venSBCL2high (DMS53, H146, DMS153, COR-L311, H187, H211) and 8 venRBCL2high (H526, H1882, H1048, H446, SHP77, H1694, H1688, H372) cell lines (Supplementary Table S2). While no difference in NOXA, BAD, BOK, BID, BAK1, Bcl-XL and BCL-2L12 expression levels was observed (Supplementary Fig. S3), we found significantly higher levels of the anti-apoptotic MCL-1 and lower expression of the pro-apoptotic BAX in the venetoclax resistant group in our proteomic data (Fig. 2a, b). This observation was confirmed by Western blot data, showing a positive correlation between venetoclax IC50 values and MCL-1 expression (Fig. 2c) and, moreover, a significant negative correlation between venetoclax sensitivity and BAX levels (Fig. 2d). Generally, in contrast to BCL-2, MCL-1 and BAX were not differently expressed between the 4 SCLC subtypes and BCL-2 expression neither correlated with MCL-1 nor with BAX (Supplementary Figs. S4 and S5).
Normalised log2 intensity of a MCL-1 and b BAX deriving from proteomic analysis of cell lines categorised into BCL-2 high-venetoclax sensitive (venSBCL2high) and BCL-2 high-venetoclax resistant (venRBCL2high) groups. c Pearson correlation between MCL-1 expression levels (deriving from Western blot analysis) and venetoclax IC50 values. d Spearman correlation between BAX expression levels (deriving from Western blot analysis) and venetoclax IC50 values (in c and d: blue: SCLC-A, red: SCLC-N, yellow: SCLC-P, green: SCLC-Y). e Dose–response curves of H146 and H146venR cells after 72 h, treated with venetoclax at the indicated doses. f Expression levels in H146venR cells relative to H146 deriving from Western blot analysis. Bars are shown as mean ± SD. g IC50 values of S63845, calculated from dose-response curves after 72 h, assessed by MTT assay. The black line (2 µM) indicates the threshold between sensitive and resistant cells. h IC50 values of venetoclax and S63845 (blue: SCLC-A, red: SCLC-N, yellow: SCLC-P, green: SCLC-Y). i BAX expression deriving from Western blot analysis in double sensitive (S/S), venetoclax sensitive (vS/R), S63845 sensitive (vR/S) and double resistant (R/R) cells. Each dot represents the mean of one cell line. ANOVA and Tukey’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001.
Next, we established a venetoclax-resistant cell line (H146venR) by treating H146 cells with increasing doses of venetoclax over several months (Fig. 2e). When we compared the levels of BCL-2, MCL-1 and BAX to the venetoclax-sensitive parental cell line, we found a trend for lower BCL-2, higher MCL-1 and significantly lower BAX expression levels (Fig. 2f). We, therefore, hypothesised that MCL-1 mediates venetoclax resistance in high BCL-2 expressing cells. Thus, we treated our cell line panel with the MCL-1 inhibitor S63845, and found IC50 values ranging from 50 nM to >10 µM (Fig. 2g and Supplementary Fig. S6). A value of 2 µM was used as the cut-off for stratification into S63845 sensitive and resistant cells, as indicated by the black line. There was no correlation between S63845 IC50 values and venetoclax IC50 values or MCL-1 levels (Supplementary Fig. S7). Using the cut-offs defined previously (venetoclax: 4 µM; S63845: 2 µM), we next categorised our panel of cell lines with respect to sensitivity to BCL-2 and MCL-1 inhibition into 4 groups: Sensitive to both inhibitors (S/S, N = 2), sensitive to venetoclax but not to S63845 (vS/R, N = 6), resistant to venetoclax but sensitive to S63845 (vR/S, N = 8) and resistant to both drugs (R/R, N = 10) (Fig. 2h and Supplementary Table S3).
Looking at the BCL-2, MCL-1 and BAX expression patterns in these groups, we found a non-significant trend of higher BCL-2 and MCL-1 levels in vS/R and vR/S cells, respectively (Supplementary Fig. S8). Interestingly, low BAX expression remained clearly associated with venetoclax resistance (Fig. 2i), suggesting that SCLC cells, despite expressing high levels of BCL-2 or MCL-1, can overcome sensitivity to venetoclax by downregulating BAX.
Inhibition of BCL-2 and MCL-1 synergise in a subset of double-resistant SCLC cell lines
We next treated the 10 double-resistant cell lines (R/R) in a combined setting with different doses of venetoclax and S63845 to assess potential effects of drug interaction. Interestingly, some cell lines showed strong growth inhibition in response to the combination treatment while in others, neither treatment had any effect (Fig. 3a and Supplementary Fig. S9). Using the HSA model in the Combenefit software to calculate synergism or antagonism, we found that five cell lines showed moderate to strong synergism. In the other five cell lines, combination treatment resulted in mainly additive or weak antagonistic effects and no response in terms of growth inhibition (Fig. 3b and Supplementary Fig. S9). The strong synergism in the adherently growing SHP77 cells was also clearly visible in a colony formation assay (Fig. 3c).
a Dose–response curves of SCLC cell lines after 72 h treatment with venetoclax and S63845 at the indicated doses, deriving from MTT assays and b HSA synergy/antagonism plots generated by the Combenefit software. Data are shown as mean ± SD. c Quantification of colony formation assays and representative pictures of SHP77 cells treated with DMSO (Co), 2.5 µM venetoclax (Ven), 2.5 µM S63845 (S6) or a combination of both (Ven+S6). Data are shown as mean ± SD of three biological replicates (ANOVA and Tukey’s multiple comparison test). d Tumour volume over time and tumour weight at day 24. SCID mice (n = 4–7 per group) were subcutaneously injected with SHP77 cells and treated with vehicle (Co), 100 mg/kg venetoclax 5× per week (Ven), 25 mg/kg S63845 2× per week (S6) or a combination of both (Ven+S6). Percentage of e TUNEL or f Ki67-positive cells in xenografts. *p < 0.05, **p < 0.01, ***p < 0.001.
Next, to in vivo validate the highly synergistic interaction between venetoclax and S63845, we subcutaneously injected double-resistant SHP77 cells into SCID mice. When tumours became measurable, mice were treated with venetoclax, S63845 or a combination of both. Of note, we found a significant decrease of tumour burden in the combination group, while neither drug alone exhibited an effect on tumour growth, thereby validating our in vitro results (Fig. 3d). No signs of severe toxicity or weight loss were observed (Supplementary Fig. S10). Subsequent analysis of the SCLC xenografts showed significantly increased apoptosis and decreased proliferation in the combination group (vs. controls), as indicated by TUNEL-positive double-strand breaks (Fig. 3e) and reduced KI-67 expression (Fig. 3f), respectively.
Venetoclax and S63845 clearly induce apoptosis in sensitive cells
To evaluate the effect of BCL-2 and MCL-1 inhibition on apoptosis induction, we performed Western blots for cleaved PARP and caspase 3 in one cell line representative for each group (S/S, vS/R, vR/S, R/R) treated with venetoclax, S63845 and in case of the double-resistant SHP77 cell line, a combination of both. We found induction of cleaved PARP and cleaved caspase 3 in the cell lines according to their sensitivity profiles (Fig. 4a). This was also observed in flow cytometry analyses. Of note, in case of the double-resistant SHP77 cell line, a significantly higher number of apoptotic cells was found in the combination group, while neither drug alone had an effect (Fig. 4b, c and Supplementary Fig. S11).
a Western blot of cleaved PARP and Caspase 3 in SCLC cells 1 or 2 days after treatment with 2.5 µM venetoclax (Ven), S63845 (S6) or a combination of both. Bars show densitometry relative to GAPDH of 2 biological replicates as mean ± SD. b Cells were treated with 2.5 µM venetoclax or a S63845, or a combination of both. Apoptosis was measured after 48 h by propidium iodide (PI) or AnnexinV staining via flow cytometry. Data are shown as mean + SD of 3 biological replicates. The high number of dead cells in the control groups is due to the non-adherent properties of the cell lines. c Representative flow cytometry plots after 48 h.
BAX is required for synergistic interactions between venetoclax and S63845
In order to find the mechanism responsible for the different response to the combination of venetoclax and S63845, we compared the expression profiles between the additive/antagonistic (Add) and synergistic (Syn) cell lines. To that aim, we grouped the cells by their max. synergism score deriving from the HSA/Combenefit analysis using a cut-off value of 15, as indicated by the black line in Fig. 5a, resulting in 5 cell lines per group. In line with our previous findings, BAX expression was significantly lower in the non-responding, additive cell lines, while BCL-2 and MCL-1 expression were not significantly altered (Fig. 5b). We, therefore, overexpressed BAX using expression plasmids in H1048, a cell line with low BAX but detectable BCL-2, to test if restored BAX could sensitise the cells to venetoclax, S63845 or the combination of both (Supplementary Fig. S12). Indeed, H1048 cells which were previously not responding to the combination treatment and also did not respond when transfected with the control plasmid (eGFP), showed synergistic effects when BAX was overexpressed (Fig. 5c). When we compared the scores deriving from the HSA synergy model, we obtained a max. synergy score of 25.86 in the BAX-transfected H1048BAX versus 11.89 in the control transfected H1048eGFP cells, which is well above the previously defined threshold of 15 (Fig. 5d). Also, the sum of the synergy and antagonistic scores was higher in the H1048BAX cells (Fig. 5e). Finally, colony formation assays confirmed the enhanced activity of venetoclax and S63845 alone and, especially pronounced, in the combination setting in the BAX overexpressing cell line (Fig. 5f).
a Max. synergy score deriving from HSA/Combenefit analysis. The red line indicates the threshold between additive (<15, Add) and synergistic (>15, Syn), as indicated by the black line, resulting in 5 cell lines per group. b Expression profiles deriving from Western blot analysis between the synergistic (Syn) and additive/antagonistic (Add) cell line groups. Each dot represents the mean of one cell line. c Dose–response curves of H1048 cells transfected with eGFP (H1048eGFP) or BAX (H1048BAX) after 72 h treatment with venetoclax and S63845 at the indicated doses, deriving from MTT assays (left) and corresponding HSA synergy/antagonism plots generated by the Combenefit software (right). Data are shown as mean ± SD. d Max. synergy score and e synergism sum score, deriving from HSA analysis. f Colony formation assays of H1048eGFP and H1048BAX after 10 days of incubation with DMSO (Co), 1 µM venetoclax (Ven), 1 µM S63845 (S6) or a combination of both (Ven+S6) (ANOVA and Dunnett’s multiple comparison test), *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Despite the encouraging results with various targeted agents in preclinical models and early-phase clinical trials, no significant breakthroughs have been achieved recently in the treatment of SCLC patients. The endless loop of negative phase II clinical trials is mainly due to the high plasticity of SCLC and to the non-selected patient groups. Accordingly, stratifying the patients by their dominant molecular subtypes and specific protein-level alterations may contribute to the development of novel targeted strategies in this recalcitrant disease. In the current study, we evaluated the preclinical efficacy of the BCL-2 inhibitor venetoclax and the MCL-1 inhibitor S63845 according to the expression of other BCL-2 family proteins, and moreover, we assessed the mechanisms that might contribute to their therapeutic failures.
By analysing the molecular subtype-specificity of BCL-2, we found widely elevated expression levels in ASCL1- and POU2F3-dominant subtypes. This is in line with the findings of others suggesting that the SCLC-A subtype is highly dependent on BCL-2 and INSM1 levels [11, 13, 35, 36]. In light of these outcomes, BCL-2 might indeed represent a potential subtype-specific therapeutic target for this subset of SCLC. Previously, BCL-2 gene expression levels were also found to correlate with POU2F3 expression, yet these results did not reach statistical significance in earlier studies [11]. Notably, in our study, BCL-2 levels were significantly increased in SCLC-P cells concerning both their RNA and protein expression. Recent preclinical work argues that POU2F3 expression may as well be valuable in identifying tumours susceptible to PARP inhibition [13, 37]. Since BCL-2 frequently interacts with PARP-1 and therefore suppresses PARP-1-dependent DNA repair [38], targeted disruption of the BCL-2–PARP-1 interaction might represent an attractive subtype-specific approach in these cases.
Next, we investigated whether BCL-2 expression correlates with in vitro venetoclax efficacy and found that, although the majority of venetoclax-sensitive cell lines indeed belonged to SCLC-A or SCLC-P subtypes, not all cell lines expressing high levels of BCL-2 responded properly to BCL-2 inhibition. In line with this, Lochmann et al. also found that a substantial subset of SCLCs are sensitive to venetoclax administration [17]. These findings might provide some optimism that there may be a new therapeutic strategy on the horizon, yet the therapy refractory cases and the dose-limiting toxicities raise serious concerns. Indeed, the multicentre phase 1/2 trial (NCT04543916) designed to establish the recommended phase 2 dose of oral venetoclax in patients with relapsed or refractory SCLC was terminated because of occurring toxicities. To overcome these issues, determining the key mechanisms of resistance along with appropriate patient selection will be crucial. Importantly, we found that both MCL-1 overexpression and low BAX levels were characteristic for venetoclax resistance in SCLC, whereas NOXA, BAD, BOK, BID, BAK1, BCL-XL and BCL-2L12 levels did not affect the therapeutic efficacy. Similar conclusions were reached by Punnoose et al. in multiple myeloma where MCL-1 overexpression conferred resistance to the BCL-2 inhibitors venetoclax and navitoclax [39]. Interestingly, their preliminary analysis also suggested that increased expression of BCL-XL relative to BCL-2 may be associated with decreased sensitivity to venetoclax [39]. Of note, these findings could not be validated in our study. Instead, in our study, another protein of interest concerning therapeutic resistance was BAX, a pro-apoptotic protein, which under the influence of the death signal undergoes oligomerization and forms holes in the outer mitochondrial membrane [40, 41]. This facilitates the entry of cytochrome c and the other pro-apoptotic molecules into the cytoplasm, and activates caspases, thus leading to cellular apoptosis [40, 41]. Previous studies in acute lymphoid leukaemia (ALL) also suggests that BAX-deficiency and missense BAX mutations induce acquired venetoclax resistance [27, 42].
Since elevated MCL-1 levels associate with venetoclax resistance in SCLC cell lines with high BCL-2 expression, targeting MCL-1 with S63845 might as well constitute a feasible approach in these refractory cases. However, not all venetoclax resistant BCL-2-expressing cell lines with upregulated MCL-1 responded appropriately to MCL-1 inhibition either and, surprisingly, MCL-1 expression did not correlate with S63845 efficacy. In order to overcome therapeutic resistance in these double-resistant cell lines, we administered the two targeted agents simultaneously. There are several studies on double inhibition of MCL-1 and BCL-2 [43]. The combination of S63845 with venetoclax exhibited potent activity against nasopharyngeal carcinoma [44], AML [45] and mantle cell lymphoma [46]. Consistent with this, a recent in vitro study on SCLC cell lines also suggested potential synergism between S63845 and navitoclax, a dual BCL-XL and BCL-2 inhibitor [43]. Yet, the therapeutic range of navitoclax with S63845 was narrow in additional in vivo studies [43]. Altogether, the dual administration of venetoclax and S63845 remains controversial in SCLC. The current work shows that simultaneous inhibition of BCL-2 and MCL-1 with the aforementioned two therapeutic agents is synergistic in a subset of double-resistant SCLC cell lines. In addition, we also found that venetoclax/S63845 combination exhibited marked anti-tumour activity in the SHP77 xenograft model resistant to both inhibitors alone, in agreement with studies reporting efficacy of venetoclax combined with MCL1 inhibitors in myeloma models [47,48,49]. Importantly, no signs of toxicity were observed in our in vivo model. Notably, a proportion of our SCLC samples remained resistant even to BCL-2/MCL-1 dual targeting.
Although both BCL-2 and MCL-1 inhibitors are clinically available and tolerable in diverse cancer models [47, 50, 51], simultaneous administration of venetoclax and S63845 has not yet been translated into clinical trials. In the current and prior studies no dose-dependent toxicities were detected [47], yet the concomitant use of BCL-2 and MCL-1 inhibitors might still increase the toxicity rates in some cases. In addition, the efficacy of dual inhibition is not consistent in every cell line and not all tumours respond ubiquitously to BCL-2/MCL-1 inhibition [47]. Therefore, besides appropriate patient selection, the assessment of the biological features concerning therapeutic resistance will be fundamental prior to future clinical trials. In the current study, we demonstrate that mechanistically the combination of both inhibitors favours apoptosis in a BAX-dependent manner. Specifically, in accordance with previous findings in AML [45] and multiple myeloma [47], we show that intact and activated BAX is obligatory for effective inhibition by the venetoclax/S63845 combination also in SCLC. Importantly, restoration of BAX levels sensitised H1048 cell to venetoclax/S63845. The in-depth biological characteristics of BCL-2/MCL-1/BAX interaction remains to be determined, however, a possible cause of this BAX-dependent efficacy might lie in the pro-apoptotic features of BAX itself. Namely, dual inhibition induces cell death by permitting BAX to form heterocomplexes with BAK which is a crucial step for apoptosis [47]. Additionally, a cooperative binding between BAX and BAK for the formation of mitochondrial membrane pores was also observed under dual inhibition, further supporting the hypothesis that appropriate BAX level is a necessity for cell death [52, 53]. Although these biological concepts partly explain the poor response rates of BAXlow cell lines to dual BCL-2/MCL-1 inhibition, further examination of these possibilities should be the subject of future research.
Conclusions
Although the specific genetic and proteomic landscape of SCLC molecular subtypes might provide insights into individualised therapy, the expression and distribution of subtype-specific therapeutic vulnerabilities are still controversial in SCLC. In our study, we show significantly elevated BCL-2 levels in SCLC-A and SCLC-P subtypes, suggesting that venetoclax might be a promising subtype-specific therapeutic agent in these subsets. In addition, we also demonstrate that venetoclax resistance of BCL2high SCLC cell lines is mainly due to the high expression of the anti-apoptotic protein MCL-1 and to the low expression of the pro-apoptotic protein BAX. Prior to this study, the feasibility of simultaneous pharmacological BCL-2 and MCL-1 targeting was uncertain in SCLC. The current study presents preclinical evidence that dual inhibition of BCL-2/MCL-1 with venetoclax and S63845 is an effective approach to overcome venetoclax resistance in high BCL-2-expressing SCLCs. Nevertheless, in order to maximise the impact of MCL-1 and BCL-2 inhibition, intact BAX is required. Altogether, besides revealing the subtype-specificity of BCL-2 and shedding light on the mechanism of venetoclax resistance in human SCLC, our study provides the proof-of-concept demonstration that simultaneous BCL-2 and MCL-1 targeting is a potent strategy to inhibit SCLC growth in vitro and in vivo. Nevertheless, the concept of dual BCL-2/MCL-1 inhibition in SCLC needs further preclinical validation before being considered for clinical testing.
Data availability
Data were generated by the authors and are available on request.
References
Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–37.
Schwendenwein A, Megyesfalvi Z, Barany N, Valko Z, Bugyik E, Lang C, et al. Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics. 2021;20:470–83.
Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Prim. 2021;7:3.
Kahnert K, Kauffmann-Guerrero D, Huber RM. SCLC-state of the art and what does the future have in store? Clin Lung Cancer. 2016;17:325–33.
Karachaliou N, Pilotto S, Lazzari C, Bria E, de Marinis F, Rosell R. Cellular and molecular biology of small cell lung cancer: an overview. Transl Lung Cancer Res. 2016;5:2–15.
Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D’Amico TA, et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:1441–64.
Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract. 2018;14:359–66.
Esposito G, Palumbo G, Carillio G, Manzo A, Montanino A, Sforza V, et al. Immunotherapy in small cell lung cancer. Cancers. 2020;12:2522.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9.
Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14:237–44.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–97.
Drapkin BJ, Rudin CM. Advances in small-cell lung cancer (SCLC) translational research. Cold Spring Harb Perspect Med. 2021;11:a038240.
Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39:346–60.e7.
Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15:1823–35.
Megyesfalvi Z, Barany N, Lantos A, Valko Z, Pipek O, Lang C, et al. Expression patterns and prognostic relevance of subtype-specific transcription factors in surgically resected small-cell lung cancer: an international multicenter study. J Pathol. 2022;257:674–86.
Huang YH, Klingbeil O, He XY, Wu XS, Arun G, Lu B, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32:915–28.
Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, et al. Venetoclax Is effective in small-cell lung cancers with high BCL-2 expression. Clin Cancer Res. 2018;24:360.
Schenk RL, Strasser A, Dewson G. BCL-2: long and winding path from discovery to therapeutic target. Biochem Biophys Res Commun. 2017;482:459–69.
Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002.
Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811.
Anstee NS, Bilardi RA, Ng AP, Xu Z, Robati M, Vandenberg CJ, et al. Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice. Cell Death Differ. 2019;26:1316–31.
Pan R, Ruvolo VR, Wei J, Konopleva M, Reed JC, Pellecchia M, et al. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015;126:363–72.
Lochmann TL, Bouck YM, Faber AC. BCL-2 inhibition is a promising therapeutic strategy for small cell lung cancer. Oncoscience. 2018;5:218–9.
Roberts AW, Stilgenbauer S, Seymour JF, Huang DCS. Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2017;23:4527–33.
Juárez-Salcedo LM, Desai V, Dalia S. Venetoclax: evidence to date and clinical potential. Drugs Context. 2019;8:212574.
Ben-Ezra JM, Kornstein MJ, Grimes MM, Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994;145:1036–40.
Tahir SK, Smith ML, Hessler P, Rapp LR, Idler KB, Park CH, et al. Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer. 2017;17:399.
Zhang Q, Riley-Gillis B, Han L, Jia Y, Lodi A, Zhang H, et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct Target Ther. 2022;7:51.
Szeitz B, Megyesfalvi Z, Woldmar N, Valko Z, Schwendenwein A, Barany N, et al. In-depth proteomic analysis reveals unique subtype-specific signatures in human small cell lung cancer. Clin Transl Med. 2022;12:e1060.
Kuras M, Woldmar N, Kim Y, Hefner M, Malm J, Moldvay J, et al. Proteomic workflows for high-quality quantitative proteome and post-translational modification analysis of clinically relevant samples from formalin-fixed paraffin-embedded archives. J Proteome Res. 2021;20:1027–39.
Szeitz B, Woldmar N, Valko Z, Megyesfalvi Z, Barany N, Paku S, et al. Proteomic characterization of small cell lung cancer subtypes. 16th Annual US HUPO Conference; Seattle Washington, USA, 2020.
Laszlo V, Valko Z, Ozsvar J, Kovacs I, Garay T, Hoda MA, et al. The FAK inhibitor BI 853520 inhibits spheroid formation and orthotopic tumor growth in malignant pleural mesothelioma. J Mol Med. 2019;97:231–42.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
Torok S, Rezeli M, Kelemen O, Vegvari A, Watanabe K, Sugihara Y, et al. Limited tumor tissue drug penetration contributes to primary resistance against angiogenesis inhibitors. Theranostics. 2017;7:400–12.
Costanzo F, Martínez Diez M, Santamaría Nuñez G, Díaz-Hernandéz JI, Genes Robles CM, Díez, et al. Promoters of ASCL1- and NEUROD1-dependent genes are specific targets of lurbinectedin in SCLC cells. EMBO Mol Med. 2022;14:e14841.
Augustyn A, Borromeo M, Wang T, Fujimoto J, Shao C, Dospoy PD, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci USA. 2014;111:14788–93.
Knelson EH, Patel SA, Sands JM. PARP inhibitors in small-cell lung cancer: rational combinations to improve responses. Cancers. 2021;13:727.
Dutta C, Day T, Kopp N, van Bodegom D, Davids MS, Ryan J, et al. BCL2 suppresses PARP1 function and nonapoptotic cell death. Cancer Res. 2012;72:4193–203.
Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15:1132–44.
Yue X, Chen Q, He J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int. 2020;20:524.
Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58:1–17.
Fresquet V, Rieger M, Carolis C, García-Barchino MJ, Martinez-Climent JA. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood. 2014;123:4111–9.
Yasuda Y, Ozasa H, Kim YH, Yamazoe M, Ajimizu H, Yamamoto Funazo T, et al. MCL1 inhibition is effective against a subset of small-cell lung cancer with high MCL1 and low BCL-X(L) expression. Cell Death Dis. 2020;11:177.
Wang Y, Wang Y, Fan X, Song J, Wu H, Han J, et al. ABT-199-mediated inhibition of Bcl-2 as a potential therapeutic strategy for nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2018;503:1214–20.
Moujalled DM, Pomilio G, Ghiurau C, Ivey A, Salmon J, Rijal S, et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia. 2019;33:905–17.
Prukova D, Andera L, Nahacka Z, Karolova J, Svaton M, Klanova M, et al. Cotargeting of BCL2 with venetoclax and MCL1 with S63845 is synthetically lethal in vivo in relapsed mantle cell lymphoma. Clin Cancer Res. 2019;25:4455–65.
Seiller C, Maiga S, Touzeau C, Bellanger C, Kervoëlen C, Descamps G, et al. Dual targeting of BCL2 and MCL1 rescues myeloma cells resistant to BCL2 and MCL1 inhibitors associated with the formation of BAX/BAK hetero-complexes. Cell Death Dis. 2020;11:316.
Algarín EM, Díaz-Tejedor A, Mogollón P, Hernández-García S, Corchete LA, San-Segundo L, et al. Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica. 2020;105:e116–20.
Siu KT, Huang C, Panaroni C, Mukaihara K, Fulzele K, Soucy R, et al. BCL2 blockade overcomes MCL1 resistance in multiple myeloma. Leukemia. 2019;33:2098–102.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–82.
Sundararajan R, Cuconati A, Nelson D, White E. Tumor Necrosis Factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem. 2001;276:45120–7.
Debernardi J, Hollville E, Lipinski M, Wiels J, Robert A. Differential role of FL-BID and t-BID during verotoxin-1-induced apoptosis in Burkitt’s lymphoma cells. Oncogene. 2018;37:2410–21.
Acknowledgements
We thank Barbara Dekan, Barbara Peter-Vörösmarty and Erzsebet Nagy for technical assistance and Nicole Woldmar and Beáta Szeitz for their help with the proteomic analyses.
Funding
KS was supported the Austrian Science Fund (FWF No. T 1062-B33) and the City of Vienna Fund for Innovative Interdisciplinary Cancer Research. BD, ZM and AR acknowledge funding from the Hungarian National Research, Development and Innovation Office (KH130356 to BD; 2020-1.1.6-JÖVŐ and TKP2021-EGA-33 to BD and ZM; FK-143751 to BD, ZM and AH-R). BD was also supported by the Austrian Science Fund (FWF I3522, FWF I3977 and I4677). ZM was supported by the UNKP-20-3 and UNKP-21-3 New National Excellence Program of the Ministry for Innovation and Technology of Hungary and by the Hungarian Respiratory Society (MPA #2020). ZM is recipient of the IASLC/ILCF Young Investigator Grant 2023. VL is a recipient of the Bolyai Research Scholarship of the Hungarian Academy of Sciences and the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. MG was supported by the City of Vienna Fund for Innovative Interdisciplinary Cancer Research and the Berndorf Private Foundation. MR acknowledges funding from the Mrs. Berta Kamprad´s Cancer Foundation (FBKS-2020-22-(291)).
Author information
Authors and Affiliations
Contributions
ZV was responsible for conceptualisation, data curation, formal analysis, investigation, methodology, software, visualisation, writing the original draft, review and editing. ZM was responsible for conceptualisation, data curation, formal analysis, investigation, methodology, software, resources, writing the original draft, review and editing. AS, NB, BF, AH-R, IK, and ES were responsible for data curation, writing—review and editing. CL and VP were responsible for formal analysis, writing—review and editing. SP was responsible for investigation, resources, writing—review and editing. KB was responsible for investigation, writing—review and editing. MR was responsible for data curation, software, visualisation, resources, writing—review and editing. GM-V was responsible for resources, writing—review and editing. FR-V and KH were responsible for project administration, resources, writing—review and editing. MAH, TK, and MG were responsible for project administration, writing—review and editing. VL was responsible for conceptualisation, methodology, supervision, writing—review and editing. BD was responsible for conceptualisation, investigation, methodology, project administration, resources, supervision, writing—original draft and writing—review and editing. KS was responsible for conceptualisation, data curation, investigation, methodology, project administration, software, visualisation, validation, resources, supervision, writing—original draft and writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All mentioned animal experiments were performed according to the ARRIVE guidelines and to the animal welfare regulations of the host institutes (permission number: PEI/001/2574-6/2015).
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valko, Z., Megyesfalvi, Z., Schwendenwein, A. et al. Dual targeting of BCL-2 and MCL-1 in the presence of BAX breaks venetoclax resistance in human small cell lung cancer. Br J Cancer 128, 1850–1861 (2023). https://doi.org/10.1038/s41416-023-02219-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02219-9
- Springer Nature Limited
This article is cited by
-
Small cells – big issues: biological implications and preclinical advancements in small cell lung cancer
Molecular Cancer (2024)
-
Emerging advances in defining the molecular and therapeutic landscape of small-cell lung cancer
Nature Reviews Clinical Oncology (2024)