Abstract
Background
The potential of using magnetic resonance image tumour-regression grading (MRI-TRG) system to predict pathological TRG is debatable for locally advanced rectal cancer treated by neoadjuvant radiochemotherapy.
Methods
Referring to the American Joint Committee on Cancer/College of American Pathologists (AJCC/CAP) TRG classification scheme, a new four-category MRI-TRG system based on the volumetric analysis of the residual tumour and radiochemotherapy induced anorectal fibrosis was established. The agreement between them was evaluated by Kendall’s tau-b test, while Kaplan–Meier analysis was used to calculate survival outcomes.
Results
In total, 1033 patients were included. Good agreement between MRI-TRG and AJCC/CAP TRG classifications was observed (k = 0.671). Particularly, as compared with other pairs, MRI-TRG 0 displayed the highest sensitivity [90.1% (95% CI: 84.3–93.9)] and specificity [92.8% (95% CI: 90.4–94.7)] in identifying AJCC/CAP TRG 0 category patients. Except for the survival ratios that were comparable between the MRI-TRG 0 and MRI-TRG 1 categories, any two of the four categories had distinguished 3-year prognosis (all P < 0.05). Cox regression analysis further proved that the MRI-TRG system was an independent prognostic factor (all P < 0.05).
Conclusion
The new MRI-TRG system might be a surrogate for AJCC/CAP TRG classification scheme. Importantly, the system is a reliable and non-invasive way to identify patients with complete pathological responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Nowadays, various imaging examinations have been widely used in tumour diagnosis, treatment and curative effect prediction [1,2,3,4]. In particular, high-resolution magnetic resonance imaging (MRI) is the gold standard for rectal cancer TNM staging due to its high consistency with pathological findings, particularly in the evaluation of tumour involvement of the mesorectal fascia (MRF) and extramural venous invasion (EMVI) [5,6,7,8]. Importantly, MRI-based TNM staging guides the stratified treatment selection for rectal cancer among options such as surgery alone for early-stage cases, neoadjuvant treatment to achieve downstaging and downsizing, adjuvant chemotherapy for locally advanced individuals and an intensified chemotherapy regimen with or without target therapy for distant metastatic patients [9,10,11,12].
Moreover, MRI is accurate and sensitive enough to assess the tumour response to neoadjuvant chemoradiotherapy (nCRT) or neoadjuvant chemotherapy (nCT) for locally advanced rectal cancer (LARC) [13]. In those patients who respond to neoadjuvant treatment, the tumour mass undergoes volume regression, and responsive tumours are replaced by dense fibrosis and appear with low signal intensity on T2-weighed images. In contrast, for those patients with no response to neoadjuvant treatment, the tumour mass triggers a persistent intermediate-level or higher signal intensity on T2-weighed images [14, 15]. By distinguishing signal differences, the MERCURY study proposed an MRI tumour-regression grading (MRI-TRG) system that closely resembled the five-category pathological TRG (pTRG) developed by Dworak et al. and Mandard et al. [5, 16, 17]. In previous reports, the MRI-TRG system identified a favourable-outcomes subgroup with prolonged overall survival (OS) and disease-free survival (DFS) rates [5, 18]. Though similar to pTRG principles, whether the MRI-TRG system could be used as a surrogate of the pTRG classification scheme remains controversial [13, 15, 19].
Recently, an organ- and function-preserving strategy has been suggested for patients with neoadjuvant treatment-induced complete clinical response (CCR), which accounts for 11 to 38% of all LARC patients [20,21,22]. The “watch and wait” strategy could be alternatively employed for CCR patients given their good long-term OS, DFS and distant metastasis-free survival (DMFS) rates [20,21,22]. Given its powerful capacity of discriminating between the tumour and fibrosis, MRI has been an alternative means by which to identify CCR patients for organ-preserving management [23]. However, the consistency in stratification between CCR and pathological complete response (pCR) by the MRI-TRG system has not yet been clearly addressed for LARC.
Here, we recruited 1033 consecutive LARC patients from two institutions, with one institution’s patients used as the training set and the other institution’s patients used as the validation set for this investigation, respectively. Further, referring to the American Joint Committee on Cancer/College of American Pathologists (AJCC/CAP) four-category TRG classification scheme [24, 25], we proposed a four-category MRI-TRG system based on the volumetric analysis and investigated its prognostic value for LARC and consistency with AJCC/CAP TRG system, particularly for patients in the pCR subgroup.
Methods
Patient characteristics
LARC patients were retrospectively recruited from the Guangdong Institute of Gastroenterology, Sixth Affiliated Hospital of Sun Yat-sen University and Sir Run Run Shaw Hospital, College of Medicine Zhejiang University from July 2011 to December 2018. The study inclusion criteria were as follows: pathologically confirmed rectal adenocarcinoma; tumour located within 15 cm from the anal verge; clinical stage of T3–4 or/and N-positive; receipt of neoadjuvant treatment followed by total mesorectal excision (TME) surgery; and availability of both primary and restaged MRI scans, with the primary MRI scan performed within two weeks before neoadjuvant treatment and the restaged MRI performed within one week before TME surgery, respectively. Exclusion criteria were as follows: patients with distant metastasis occurring before or during treatment; primary or restaged MRI scans missing or with poor quality (such as artefact). This study was approved by the Clinical Ethics Review Committee at the Sixth Affiliated Hospital of Sun Yat-sen University (2021ZSLYEC-121), with the requirement for informed consent waived.
Treatment
The treatment strategy was determined by the multidisciplinary team at each institution. Briefly, the neoadjuvant radiotherapy was delivered at 45 Gy in 25 fractions to the whole pelvis with a boost of 5.4 Gy aimed directly at the gross tumour, and patients were concurrently given 5-fluorouracil–based chemotherapy regimens orally or intravenously. The choice of 5-fluorouracil, FOLFOX (leucovorin 400 mg/m2 intravenously followed by fluorouracil 400 mg/m2 intravenously and fluorouracil 2.4 g/m2 by 48-h continuous intravenous infusion and oxaliplatin 85 mg/m2 intravenously on day 1), or CAPOX (Capecitabine was given 1000 mg/m2 twice daily on days 1–14 and Oxaliplatin was given 130 mg/m2 on day 1) chemotherapy would be given to each patient was at the discretion of the multidisciplinary team [26]. Meanwhile, a subgroup of patients were enrolled from a prospective clinical trial, so their preoperative radiotherapy was omitted from this investigation [27]. Surgery with curative intent was performed according to TME principles at 6–8 weeks following the completion of neoadjuvant radiotherapy or 2 weeks after completion of neoadjuvant chemotherapy. Standard FOLFOX or CAPOX adjuvant chemotherapy was administered to most cases [26].
MRI-TRG system
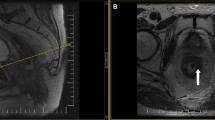
As summarised in Supplementary Table 1, MRI scans were performed by a 1.5-Tesla or 3.0-Tesla MRI unit. The bowel preparation consisting of a Fleet’s™ enema was performed before scanning. The high-spatial-resolution axial plane of the T2-weighted sequence was perpendicular to the long axis of the tumour in restaged pelvic MRI scans. Oblique axial T2-weighted images were used to outline the region of interest; meanwhile, the other sequences in the restaged pelvic MRI and primary MRI scans were used as the reference images. Two physicians with 7 (Dr. Xiaolin Pang) and 9 years (Dr. Peiyi Xie) of experience in rectal cancer imagining were recruited, whom were blinded to the patient’s clinical characteristics and follow-up information. The outline criteria were as follows: radiochemotherapy induced anorectal fibrosis and residual tumour were delineated on each axial plane of T2-weighted images by the ITK-snap version 3.4.0 software (http://itk-snap.org), respectively. In detail, areas presenting with intermediate or high signal intensity on T2 and DWI images (b = 800/1200 s/mm2) were judged as a residual tumour. In contrast, zones displaying a low signal on T2 and DWI images were judged as exhibiting fibrosis. For the images with artefacts that obscured interpretation, T2 images, DWI and apparent diffusion coefficient (ADC) should be combined for response assessment. The signal of ADC referred the background of the whole image. The areas displaying a low signal on T2 and unrestricted diffusion images (low signal on DWI and low signal on ADC) were judged as exhibiting fibrosis, and a high signal on T2 and restricted diffusion images (high signal on DWI and low signal on ADC) were recognised as tumour [28]. After contouring at each axial plane, the ITK-snap software reconstructed the outlined tumour and fibrosis at all planes into the three-dimensional tissue mass. The percentage of residual tumour was calculated using the following formula: tumour volume/ (tumour volume + fibrosis volume) × 100%. To test the interobserver variability, another 50 LARC patients were enrolled and their T2 images were delineated simultaneously. Discrepancies in assessment between the two physicians were arbitrated by the third physician (Dr. Jian Zheng) with 20 years of imaging experience. We developed a four-category MRI-TRG system as a non-invasive surrogate for the tissue-based AJCC/CAP TRG scheme [24, 25]. We hypothesised that the fractional residual tumour volume on MRI is correlated with pathological residual tumour volume. Based on this hypothesis, we divided the observed MRI fractional residual tumour volumes in our training set into four ordered subgroups (MRI-TRG 0-3) and set the thresholds between groups such that each MRI-TRG subgroup included the same fraction of the population as the corresponding AJCC/CAP TRG stage group [29]. The final MRI-TRG subgroups were defined as follows: MRI-TRG 0 (complete response), where there was no remaining tumour tissue; MRI-TRG 1 (moderate response), where no more than 30% of the tumour remained; MRI-TRG 2 (minimal response), where 30 to 80% of the tumour remained and MRI-TRG 3 (poor response), where more than 80% of the tumour remained (Fig. 1 and Supplementary Table 2).
The four columns shown from left to right display the axial plane of T2-weighted for MRI-TRG 0 to MRI-TRG three category patients, respectively. Row a present the representative axial plane of a T2-weighted image before neoadjuvant treatment. Row b denotes the representative axial plane of a T2-weighted image following the completion of neoadjuvant treatment but before the TME surgery. Row c displays the ITK-snap software–delineated residual tumour and the replaced fibrosis in the row b T2 image. Based on the delineation in each plane (row c), the ITK-snap software reconstructed a three-dimensional residual tumour (red block) and radiochemotherapy induced anorectal fibrosis (yellow block) mass (row d). The red arrows indicate the tumour zone, while the yellow arrows point to the radiochemotherapy induced anorectal fibrosis area.
Pathological TRG assessment
The AJCC/CAP TRG category was scored by two pathologists (Dr. Xinjuan Fan and Dr. Yan Huang), who had more than 12 years of experience in gastrointestinal pathological diagnosis. Two pathologists were blinded to patient characteristics and follow-up information. The four-category AJCC/CAP TRG system was classified as follows [24, 25]: grade 0 (complete response), where there were no remaining viable cancer cells; grade 1 (moderate response), where only a small cluster or single cancer cells remained; grade 2 (minimal response), where there was some cancer remaining, but with predominant fibrosis; grade 3 (poor response), where there was minimal or no tumour death with extensive cancer remaining (Supplementary Table 2).
Follow-up
After the TME operation, patients were followed up with a 3-month interval during the first 3 years and then at six-month intervals thereafter. Clinical follow-up included physical examinations, blood tests and chest and abdomen computed tomography and pelvic MRI scans [26]. OS was defined as the data of diagnosis to death or when censored at the latest date if patients were still alive. DFS was defined as the time from surgery to the date of relapse or death or when censored at the latest date. LRFS (local recurrence-free survival) and DMFS were defined as the time of diagnosis to the date of local recurrence or distant metastases, respectively, or death or when censored at the latest date.
Statistical analysis
Intraclass correlation coefficient analysis was employed to evaluate the interobserver agreement regarding the percentage of residual tumour between the two physicians (poor agreement, k < 0.2; fair agreement, k = 0.21–0.40; moderate agreement, k = 0.41–0.60; good agreement, k = 0.61–0.80; very good agreement, k = 0.81–1.00). The correlation between the grade of the MRI-TRG system and pathological TRG classification was determined by Kendall’s tau-b analysis (0–0.20: poor agreement; 0.21–0.40: fair agreement; 0.41–0.60: moderate agreement; 0.61–0.80: good agreement; 0.81–1.00: very good agreement) for each MRI-TRG category. Further, the sensitivity (SE), specificity (SP), positive predictive value (PPV) and negative predictive value (NPV) in predicting the corresponding AJCC/CAP TRG category were also reported. The Kaplan–Meier method and log-rank test were used to assess the survival probability difference between MRI-TRG categories and patient outcomes (OS, LRFS, DFS and DMFS). The area under the receiver operating characteristic (ROC) curve (AUC) was generated by the ROC curve analysis to evaluate the predictive value of MRI-TRG in predicting outcome status. Cox proportional hazards modelling was employed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for patient outcomes. Variables with P < 0.05 in the univariate analysis were subjected to further multivariate Cox regression analysis. P values of less than 0.05 with two sides were considered as statistically significant differences. Statistical analysis was performed with the Statistical Package for the Social Sciences version 26 software programme (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
A total of 1033 LARC patients treated with nCRT or nCT followed by TME surgery were enrolled, consisting of 252 cases in the training set and 781 cases in the validation set (Supplementary Fig. 1). As shown in Table 1, we found 3, 43, 793 and 194 patients have stage cT1, cT2, cT3 and cT4 disease, respectively. Stages cN0, cN1 and cN2 were detected in 207, 411 and 415 cases, respectively. The median time from the date of restaged MRI scan to surgery was 4 days (range: 1–7 days).
In the training set, the MRI-TRG category was positively correlated with perineural invasion (P = 0.002), ypT (P < 0.001) and ypN (P = 0.001) stages. In contrast, MRI-TRG category was not correlated with tumour cell-differentiation status (P = 0.050), age (P = 0.414), gender (P = 0.736), cT stage (P = 0.155), cN stage (P = 0.271), tumour distance to the anal verge (P = 0.720), lymph–vascular invasion (P = 0.064), or neoadjuvant radiotherapy (P = 0.311). In the validation set, however, MRI-TRG category was positively correlated with perineural invasion (P < 0.001), neoadjuvant radiotherapy (P < 0.001), ypT (P < 0.001) and ypN (P < 0.001) stages (Table 1).
Association between four-category MRI-TRG system and AJCC/CAP TRG classification scheme
Among 1033 LARC patients, 23 patients with relatively poor quality restaged DWI sequence but still available as the reference for the response assessment. After delineation and calculation, 25.0% (258/1033) of MRI-TRG 0 patients (training set: 60/252, 23.8%; validation set: 198/781, 25.4%), 20.7% (214/1033) of MRI-TRG 1 patients (training set: 36/252, 14.3%; validation set: 178/781, 22.8%), 31.2% (322/1033) of MRI-TRG 2 patients (training set: 88/252, 34.9%; validation set: 234/781, 30.0%), and 23.1% (239/1033) of MRI-TRG 3 patients (training set: 68/252, 27.0%; validation set: 171/781, 21.9%) were identified, respectively (Table 2).
The mean time for one physician to draw the volumetric segmentation was 9 min for a patient. The intraclass correlation coefficient between the two physicians was 0.72. Good agreement was confirmed between the four-category MRI-TRG system and AJCC/CAP TRG classification scheme in both the training and validation subsets (Kendall’s tau-b = 0.645 and 0.671, respectively). Specifically, compared with other pairs (MRI-TRG 1 and AJCC/CAP TRG 1, MRI-TRG 2 and AJCC/CAP TRG 2, or MRI-TRG 3 and AJCC/CAP TRG 3), the MRI-TRG 0 category displayed the greatest sensitivity [training set: 91.7% (95% CI: 79.1–97.3); validation set: 90.1% (95% CI: 84.3–93.9)], specificity [training set: 92.2% (95% CI: 87.4–95.3); validation set: 92.8% (95% CI: 90.4–94.7)], PPV [training set: 73.3% (95% CI: 60.1–83.5); validation set: 77.8% (95% CI: 71.2–83.2)] and NPV [training set: 97.9% (95% CI: 94.4–99.3); validation set: 97.1% (95% CI: 95.3–98.2)] in identifying AJCC/CAP TRG 0 patients (Table 3).
Association of four-category MRI-TRG system with survival outcome
The median follow-up time for the overall study population was 40 months (range: 4–101 months). In the training set, a significant 3-year survival (OS, DFS, LRFS, and DMFS) difference was observed among the four MRI-TRG categories (all P < 0.001) (Supplementary Fig. 2). A similar association between the four MRI-TRG categories and OS, DFS, LRFS, and DMFS was also detected in the validation set (all P < 0.001) (Fig. 2). The pairwise comparison showed that, except for the comparable survival (OS, DFS, LRFS and DMFS) ratios between MRI-TRG 0 and MRI-TRG 1 patients, any two of the four MRI-TRG categories had distinguished survival (OS, DFS, LRFS and DMFS) ratios (all P < 0.05) (Supplementary Table 3).
ROC and multivariate regression analysis
In the training set, ROC plot analysis confirmed that, the MRI-TRG system was closely correlated with individual outcomes (OS AUC = 0.829, 95% CI: 0.759–0.899, Supplementary Fig. 3A; LRFS AUC = 0.842, 95% CI: 0.775–0. 909, Supplementary Fig. 3B; DFS AUC = 0.762, 95% CI: 0.697–0.826, Supplementary Fig. 3C; DMFS AUC = 0.748, 95% CI: 0.681–0.815, Supplementary Fig. 3D). A similar finding was further confirmed in the validation set (OS AUC = 0.740, 95% CI: 0.674–0.805, Fig. 3a; LRFS AUC = 0.783, 95% CI: 0.732–0.835, Fig. 3b; DFS AUC = 0.786, 95% CI: 0.736–0.837, Fig. 3c; DMFS AUC = 0.722, 95% CI: 0.677–0.767, Fig. 3d).
Moreover, the variables found to be statistically significant in the univariate analysis were subjected to multivariate analysis (Supplementary Table 4, Supplementary Table 5). In the validation set, lymph–vascular invasion (P = 0.011), ypT stage (P = 0.010), cN stage (P = 0.049) and MRI-TRG system (P < 0.001) were independent prognosticators of OS. For LRFS, ypN stage (P < 0.001) and the MRI-TRG system (P = 0.002) were identified as independent prognostic factors. Moreover, ypN stage (P < 0.001) and the MRI-TRG system (P < 0.001) were associated with disease progression. Also, ypT stage (P = 0.007), ypN stage (P < 0.001), lymph–vascular invasion (P = 0.032) and the MRI-TRG system (P < 0.001) were independent predictive variables of DMFS.
Discussion
Here, a four-category MRI-TRG system was proposed similar to the four-tier AJCC/CAP TRG classification for LARC patients. This four-category MRI-TRG system quantitatively assessed images through three-dimensionally delineating the degree of tumour replacement by fibrosis. Following testing in the training set, an independent validation set further confirmed that our four-category MRI-TRG system had good agreement with the AJCC/CAP TRG classification scheme. Particularly, the MRI-TRG 0 category had the greatest power to predict AJCC/CAP TRG 0 category. Moreover, pairwise comparison showed that, except for the MRI-TRG 0 and MRI-TRG 1 categories, patients in any two of the four MRI-TRG categories exhibited a distinguished prognosis. Therefore, this four-category MRI-TRG system might be a surrogate for the AJCC/CAP TRG system in estimating prognosis and may be a promising non-invasive way to identify pCR patients for organ-preserving management strategies.
The advantage of MRI lies in its dynamic, sensitive and non-invasive capacity to assess the degree of tumour regression following neoadjuvant treatment before TME surgery for LARC [5, 30]. Nevertheless, the central question that remains controversial is whether and to what extent MRI-TRG can reflect the degree of pathological tumour regression [13, 15, 19]. In previous studies, the agreement between MRI-TRG and pathological TRG system ranged from poor to perfect [13, 15, 18, 19]. Considering the findings of Patel UB et al. for example, the MRI-TRG system was strongly associated with histopathological parameters [15]. Conversely, the pooled PAN-EX (EXPERT and EXPERT-C) study suggested that the agreement between MRI-TRG and pathological TRG category was poor (k = 0.25) [18]. Similarly, in another small cohort studies, MRI was found to have low accuracy in predicting pathological TRG [13, 19]. The underlying reason for these different findings might be the structure of the MRI-TRG system. In the MERCURY study, based on principles similar to those of the Mandard pathological TRG criteria [17], Patel UB et al. proposed a five-category MRI-TRG system for the first time [5]. The MERCURY five-category MRI-TRG system categorised individual patients qualitatively rather than quantitatively when determining the degree of tumour replacement by fibrotic stroma [5]. Therefore, the classification process was prone to being subjective and showed great potential for increasing the interobserver variability, as the fibrosis degree of two parts adjacent within the intermediate three parts (MRI-TRG 2 vs. MRI-TRG 3; MRI-TRG 3 vs. MRI-TRG 4) was often too subtle.
Actually, a similar issue was also found in the MERCURY study using the Mandard and Dworak pathological TRG system [16, 17]. Excluding patients with pathological complete response and non-responders, the microscopic difference between the intermediate response categories (e.g., TRG 2 vs. TRG 3 and TRG 3 vs. TRG 4 in the Mandard system, TRG 3 vs. TRG 2 and TRG 2 vs. TRG 1 in the Dworak system) was less clear using the five-category pathological TRG system [16, 17, 31]. In contrast, the AJCC/CAP TRG system adopted a four-category approach with two intermediate groups: good responders (AJCC/CAP TRG 1 category) and a transitional subset of patients who evolve from good to poor responders (AJCC/CAP TRG 2 category), which is more easily applied [25]. Indeed, as compared with the Mandard/Dowrak/MSKCC pathological TRG systems [16, 17, 32], the four-category AJCC/CAP TRG system was more accurate in predicting recurrence and had the highest concordance index value (0.694) [31]. Currently, the AJCC/CAP TRG system is recommended as the main pathological tool to evaluate nCRT treatment response by the NCCN Rectal Cancer Guideline Panel [26].
To achieve perfect predictive performance, we herein proposed a four-category MRI-TRG system based on the principle of AJCC/CAP TRG classification by quantitatively assessing the volume of both the residual tumour and fibrosis replacement (Fig. 1). Because the individual degree of tumour replacement by fibrosis was delineated at each image layer and further reconstructed into a three-dimensional volume, our approach is objective and more accurate when assigning the MRI-TRG category. As expected, our study found in the training set and further confirmed in the validation set that the agreement between our four-category MRI-TRG system and the AJCC/CAP TRG classification scheme was good (k = 0.671), and we proposed that our four-category MRI-TRG system could be used as a surrogate for AJCC/CAP TRG classification. Significantly, the Kaplan–Meier analysis also proved that, except for the MRI-TRG 0 and 1 categories, any two categories in our four-tier MRI-TRG system had distinguished survival ratios (Fig. 2 and Supplementary Table 3). In contrast, the significant survival difference between any two categories of the five-category MRI-TRG and five-category pathological TRG system has not yet been confirmed [5, 13].
Importantly, our study proved that the MRI-TRG system is a reliable method to identify possible complete responders for organ-preserving management. Given the perfect outcome among patients in the pCR subgroup, the possibility of the organ-preserving strategies (e.g., local excision, “watch and wait”) has been raised for the pCR patients in the last decade to reduce treatment-related toxicities and deterioration of the quality of life (e.g., anal leakage, and sexual and urinary dysfunction) without compromising the satisfactory outcome [21, 33]. However, due to the limited pCR group size (9–48 patients) included for analysis [13, 18, 34], whether MRI is sensitive and reliable for predicting pCR is still debatable thus far. Here, to improve the PPV of MRI-TRG 0, we utilised this simple binary system instead of the volumetric analysis. Once the signal of the residual signal was captured in the T2, the response assessment would not be MRI-TRG 0. Furthermore, using a consecutive large group of pCR patients (n = 219), we demonstrated that, even with good agreement between the four-category MRI-TRG and AJCC/CAP TRG systems, the MRI-TRG 0 category still had the highest power to predict pCR (Table 3). In contrast with the finding that the MRI-TRG 1 through three categories were sensitive to detect pCR (sensitivity: 94.0%) [34], we found that radiochemotherapy induced anorectal injury and changes, such as oedema, inflammation, necrosis, and particularly fibrosis, were hard to be distinguished from the residual tumour on the restaged MRI (Supplementary Fig. 4) [35, 36]. Thus, the MRI-TRG 0 showed the highest specificity and sensitivity in both the validation and training sets, while the MRI-TRG 2&3 stage had relatively lower accuracy in predicting pTRG2&3. Therefore, the patients who were assessed as the MRI-TRG 0 could employ the Watch & Wait policy in the prospective clinical studies, but should also be warranted by the other necessary examinations, such as the digital rectal examination, enteroscopy and serological tumour biomarkers, while the close inspection is essential in the follow-up period [37, 38].
In addition, as shown in Fig. 1 and Supplementary Fig. 4, the MRI images from MRI-TRG 2&3 stage individuals included not only residual tumour tissue, but also treatment-induced injury and changes, such as oedema, necrosis and fibrosis. The signals of tumour tissue and non-tumour tissue were similar and mixed. This made it was difficult to distinguish them by the naked eye [35, 36, 39]. It was notable that artificial intelligence has been widely used in tumour diagnosis, treatment and curative effect prediction [40,41,42,43]. Also, we and others groups ever reported that artificial intelligence based on pre-treatment or post-treatment multi-parametric MRI performed well in predicting the response after neoadjuvant chemoradiotherapy for LARC patients [39, 44, 45]. Therefore, a radiomics-based deep-learning approach might be a promising selection to differentiate residual tumour and treatment-induced injury in the future. In the following study, the combination of experienced radiologists with artificial intelligence might be an accurate way to identify the radiochemotherapy induced anorectal injury from the residual tumour.
It should be noted that our study had some limitations. Firstly, this retrospective study may have introduced potential bias and confounding variables, especially in the neoadjuvant treatment, and in enrolling a cohort of patients from a prospective study who were not treated with radiotherapy. We addressed this issue by selecting a large consecutively recruited patient group. In the survival analysis, radiotherapy was confirmed not to be an independent prognostic factor. Thus, the confounding effect of the treatment regimen is controlled to a minimal. In addition, radiologists are prone to misinterpret fibrosis as a residual tumour [46], and greater experience is important to narrow the gap of interobserver variability. To achieve perfect interobserver agreement, two experienced physicians specialising in rectal cancer for more than seven years joined our project. The agreement analysis showed that the interobserver variability was small (k = 0.72). Moreover, we outlined the tumour and fibrosis at T2 sequence, and simultaneously employed the DWI sequence as supportive information to determine the zone of tumour as well as fibrosis in our study to improve the accuracy of the assessment of tumour response. Theoretically, the integration of T2 and DWI would achieve an improved prediction efficacy [47,48,49]. Previously reported studies have confirmed that the combination DWI with T2 sequences intensified the response assessment for LARC after neoadjuvant treatment [49,50,51]. Moreover, artefacts have an evident influence in accurate assessment of treatment response based on DWI [28, 51]. In order to reduce the artefact, bowel preparation consisting of a Fleet’s™ enema was performed routinely before the MRI scan in most of the enroled cases [50]. In further clinical practice, bowel preparation should be advised to reduce the artefact. In addition, the innovation techniques such as reduced FoV EPI DWI might be novel selection [51].
In conclusion, we proposed a four-category MRI-TRG system as a surrogate of the AJCC/CAP TRG classification scheme to predict the neoadjuvant treatment response. Importantly, this tool might be a powerful and non-invasive means to identify complete responders for organ-preserving management strategies.
Data availability
The datasets used during this study are available from the corresponding author on reasonable request.
References
Bilal M, Raza SEA, Azam A, Graham S, Ilyas M, Cree IA, et al. Development and validation of a weakly supervised deep learning framework to predict the status of molecular pathways and key mutations in colorectal cancer from routine histology images: a retrospective study. Lancet Digit Health. 2021;3:e763–72.
Lee C, Light A, Alaa A, Thurtle D, Van der Schaar M, Gnanapragasam VJ. Application of a novel machine learning framework for predicting non-metastatic prostate cancer-specific mortality in men using the Surveillance, Epidemiology, and End Results (SEER) database. Lancet Digit Health. 2021;3:e158–65.
Vaidya P, Bera K, Gupta A, Wang X, Corredor G, Fu P, et al. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multicohort study for outcome prediction. Lancet Digit Health. 2020;2:e116–28.
Jayachandran Preetha C, Meredig H, Brugnara G, Mahmutoglu MA, Foltyn M, Isensee F, et al. Deep-learning-based synthesis of post-contrast T1-weighted MRI for tumour response assessment in neuro-oncology: a multicentre, retrospective cohort study. Lancet Digit Health. 2021;3:e784–94.
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–60.
Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, et al. Magnetic resonance imaging-detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance. Eur Radiol. 2018;28:496–505.
Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43.
Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110:19–25.
Deng Y. The “Chinese Expert Consensus on the Clinical Application of the Chinese Modified Triplet Combination with Irinotecan (CPT-11), Oxaliplatin (LOHP), Continuous Infusion 5-Fluorouracil, and Leucovorin for Colorectal Cancer”. Gastroenterol Rep. 2021;9:279–89.
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40.
Maas M, Nelemans PJ, Valentini V, Crane CH, Capirci C, Rodel C, et al. Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer. 2015;137:212–20.
Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–9.
Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117:1478–85.
Battersby NJ, Moran B, Yu S, Tekkis P, Brown G. MR imaging for rectal cancer: the role in staging the primary and response to neoadjuvant therapy. Expert Rev Gastroenterol Hepatol. 2014;8:703–19.
Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19:2842–52.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic Correlations Cancer 1994;73:2680–6.
Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27:1557–65.
van den Broek JJ, van der Wolf FS, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, et al. Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum. 2017;60:274–83.
Habr-Gama A, Sao JGP, Fernandez LM, Vailati BB, Andrade A, Araujo SEA, et al. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think! Dis Colon Rectum. 2019;62:802–8.
Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–83.
Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40.
Battersby NJ, Dattani M, Rao S, Cunningham D, Tait D, Adams R, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials. 2017;18:394.
Mace AG, Pai RK, Stocchi L, Kalady MF. American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum. 2015;58:32–44.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874–901.
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–7.
Lambregts DM, Boellaard TN, Beets-Tan RG. Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: a pictorial review. Insights Imaging. 2019;10:15.
Chen H, Feng L, Li M, Ju H, Ding Y, Lan M, et al. College of American pathologists tumor regression grading system for long-term outcome in patients with locally advanced rectal cancer. Oncologist. 2021;26:e780–93.
Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522–31.
Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106:dju248.
Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113:57–64.
van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein KE, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45.
Bhoday J, Smith F, Siddiqui MR, Balyasnikova S, Swift RI, Perez R, et al. Magnetic resonance tumor regression grade and residual mucosal abnormality as predictors for pathological complete response in rectal cancer postneoadjuvant chemoradiotherapy. Dis Colon Rectum. 2016;59:925–33.
van der Sluis FJ, Couwenberg AM, de Bock GH, Intven MPW, Reerink O, van Leeuwen BL, et al. Population-based study of morbidity risk associated with pathological complete response after chemoradiotherapy for rectal cancer. Br J Surg. 2020;107:131–9.
Petresc B, Lebovici A, Caraiani C, Feier DS, Graur F, Buruian MM. Pre-treatment T2-WI based radiomics features for prediction of locally advanced rectal cancer non-response to neoadjuvant chemoradiotherapy: a preliminary study. Cancers. 2020;12:1894.
Habr-Gama A, Sabbaga J, Gama-Rodrigues J, Sao JGP, Proscurshim I, Bailao Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109–17.
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767.
Liu Z, Zhang X, Shi Y, Wang L, Zhu H, Tang Z, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. 2017;23:7253–62.
Peng S, Liu Y, Lv W, Liu L, Zhou Q, Yang H, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study. Lancet Digit Health. 2021;3:e250–9.
Xiao W, Huang X, Wang JH, Lin DR, Zhu Y, Chen C, et al. Screening and identifying hepatobiliary diseases through deep learning using ocular images: a prospective, multicentre study. Lancet Digit Health. 2021;3:e88–e97.
Lu C, Bera K, Wang X, Prasanna P, Xu J, Janowczyk A, et al. A prognostic model for overall survival of patients with early-stage non-small cell lung cancer: a multicentre, retrospective study. Lancet Digit Health. 2020;2:e594–e606.
Liu KL, Wu T, Chen PT, Tsai YM, Roth H, Wu MS, et al. Deep learning to distinguish pancreatic cancer tissue from non-cancerous pancreatic tissue: a retrospective study with cross-racial external validation. Lancet Digit Health. 2020;2:e303–13.
Feng L, Liu Z, Li C, Li Z, Lou X, Shao L, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. 2022;4:e8–e17.
Shao L, Liu Z, Feng L, Lou X, Li Z, Zhang XY, et al. Multiparametric MRI and whole slide image-based pretreatment prediction of pathological response to neoadjuvant chemoradiotherapy in rectal cancer: a multicenter radiopathomic study. Ann Surg Oncol. 2020;27:4296–306.
Siddiqui MR, Gormly KL, Bhoday J, Balyansikova S, Battersby NJ, Chand M, et al. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG). Clin Radiol. 2016;71:854–62.
Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res. 2016;22:5256–64.
Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy-conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011;260:734–43.
Cao W, Li B, Gong J, Hu M, Li W, Pan X, et al. Diffusion-weighted magnetic resonance imaging of mucin pools in locally advanced rectal mucinous adenocarcinoma predicts tumor response to neoadjuvant therapy. Eur J Radiol. 2020;125:108890.
Coskun M, Mehralivand S, Shih JH, Merino MJ, Wood BJ, Pinto PA, et al. Impact of bowel preparation with Fleet’s enema on prostate MRI quality. Abdom Radiol. 2020;45:4252–9.
Attenberger UI, Tavakoli A, Stocker D, Stieb S, Riesterer O, Turina M, et al. Reduced and standard field-of-view diffusion weighted imaging in patients with rectal cancer at 3 T-Comparison of image quality and apparent diffusion coefficient measurements. Eur J Radiol. 2020;131:109257.
Acknowledgements
We thank Dr. XinJuan Fan and Dr. Yan Huang for their professional assistance in the pathological analysis, and Dr. Lishuo Shi (the Clinical Research Center, the Sixth Affiliated Hospital of Sun Yat-Sen University) for providing valuable advice on statistical analyses.
Funding
This research was funded by the Sixth Affiliated Hospital of Sun Yat-Sen University Clinical Research 1010 Program, grant number 1010PY (2020)-09; Guangdong Science and Technology Project (2017B090901065); Natural Science Foundation of China (No. 81872188), and Beijing Bethune Charitable Foundation (flzh202102).
Author information
Authors and Affiliations
Contributions
XLP and XBW contributed to the study design. XBW, HYC, LY and JZ recruited study patients. XLP, PYX, LY, XCM and XBW collected, audited, and assembled these data. XLP and XBW wrote the initial draft. PYX and LY revised the draft. XBW supervised this study. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The protocol was approved by the central ethics committee of The Sixth Affiliated Hospital, Sun Yat-sen University. (2021ZSLYEC-121).
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pang, X., Xie, P., Yu, L. et al. A new magnetic resonance imaging tumour response grading scheme for locally advanced rectal cancer. Br J Cancer 127, 268–277 (2022). https://doi.org/10.1038/s41416-022-01801-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01801-x
- Springer Nature Limited
This article is cited by
-
Fundamentals of Bowel Cancer for Biomedical Engineers
Annals of Biomedical Engineering (2023)
-
Can patients with good tumor regression grading after neoadjuvant chemoradiotherapy be exempted from lateral lymph node dissection?
Discover Oncology (2022)