Abstract
Background
A high Mandard score implies a non-response to chemotherapy in oesophageal adenocarcinoma. However, some patients exhibit tumour volume reduction and a nodal response despite a high score. This study examines survival and recurrence patterns in these patients.
Methods
Clinicopathological factors were analysed using multivariable Cox regression assessing time to death and recurrence. Computed tomography-estimated tumour volume change was examined in a subgroup of consecutive patients.
Results
Five hundred and fifty-five patients were included. Median survival was 55 months (Mandard 1–3) and 21 months (Mandard 4 and 5). In the Mandard 4 and 5 group (332 patients), comparison between complete nodal responders and persistent nodal disease showed improved survival (90 vs 18 months), recurrence rates (locoregional 14.75 vs 28.74%, systemic 24.59 vs 48.42%) and circumferential resection margin positivity (22.95 vs 68.11%). Complete nodal response independently predicted improved survival (hazard ratio 0.34 (0.16–0.74). Post-chemotherapy tumour volume reduction was greater in patients with a complete nodal response (−16.3 vs −7.7 cm3, p = 0.033) with no significant difference between Mandard groups.
Conclusion
Patients with a complete nodal response to chemotherapy have significantly improved outcomes despite a poor Mandard score. High Mandard score does not correspond with a non-response to chemotherapy in all cases and patients with nodal downstaging may still benefit from adjuvant chemotherapy.
Similar content being viewed by others
Background
Neoadjuvant oncological therapy has become the standard of care in oesophageal cancer.1,2,3,4 A good response to neoadjuvant treatment is associated with improved survival.5,6 The current standard measure of chemotherapy response is the Mandard tumour regression grade. This describes the proportion of the primary tumour, which has been replaced with fibrosis on histopathological analysis. Mandard 1 describes complete fibrosis (complete pathological response) and Mandard 5 corresponds with no tumour fibrosis (no chemotherapy response).6 Many studies have used the Mandard tumour regression grade as the gold standard measure of chemotherapy response when investigating the validity of preoperative imaging modalities in estimating treatment response.7
However, there are patients who seem to have an obvious radiological response to chemotherapy, with a reduction in the size of the tumour and lymph nodes, who have little or no evidence of response in their primary tumour on pathological analysis.8,9 Computed tomography (CT)-estimated tumour volume reduction is commonly seen following neoadjuvant treatment and has been shown to correlate with improved survival in some studies8 but not others.9 Studies have also demonstrated that histopathological evidence of lymph node fibrosis following chemotherapy correlates with improved survival and that the amount of nodal fibrosis does not always correspond to the amount of fibrosis in the original tumour.10 These studies suggest that a poor response using the Mandard scoring system (Mandard 4 and 5) is not indicative of chemoresistance in all cases. It is conceivable that either tumour shrinkage occurs without replacement of tumour cells with fibrosis or that clonal populations of oesophageal cancer cells have varying degrees of response to chemotherapy resulting in a differential response within the primary tumour and nodal metastases.10
Recognising varying locoregional and systemic chemotherapy responses is an important concept in designing future neoadjuvant and adjuvant treatment strategies. This study aimed to assess survival and recurrence in the context of the Mandard tumour regression grade. Of specific interest are patients with a poor histological response to chemotherapy (Mandard 4 and 5), and the extent to which subgroups of patients with a complete nodal response or tumour volume reduction exhibit improved outcomes. This may in turn affect how patients are categorised into responders or non-responders and in turn whether the rationale for adjuvant treatment is strengthened or weakened.
Methods
Study design
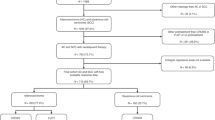
This was a cohort study based on a prospectively collected database of consecutive resections performed at Guy’s & St Thomas’ Oesophago-Gastric Centre (London, UK). Included in the study cohort were patients who underwent neoadjuvant chemotherapy and oesophagectomy between 2000 and 2017 for adenocarcinoma of the oesophagus. Follow-up ended in December 2019.
Outcomes
The primary outcome measure of the study was time to death. The secondary outcome measure was time to recurrence further categorised into systemic or locoregional recurrence. Locoregional recurrence was defined as any recurrence within the original surgical resection field. Locoregional and systemic recurrence were analysed as independent outcome measures even though many presented with a mixed pattern. A patient was deemed to have suffered from synchronous locoregional and systemic recurrence if both were found within 6 weeks of their first presentation recurrent disease. Circumferential resection margin positivity (CRM) was also analysed. A patient was classified as CRM positive if tumour was found within 1 mm of the cut margin as per the Royal College of Pathologist definition.11
Clinical management
All patients staging and management were decided by a multidisciplinary team (MDT). Patients underwent a standard protocol of investigations, including oesophago-gastro-duodenoscopy, CT and endoscopic ultrasound (EUS), and since 2007, fluorodeoxyglucose positron emission tomography. Neoadjuvant chemotherapy practice evolved during the study period and followed standard indications and regimens as supported by randomised controlled trial evidence. Histological staging was standardised to meet the 7th edition TNM (tumour, node, metastasis) criteria. Patients underwent either a transthoracic oesophagectomy (THO) or transhiatal oesophagectomy. Adjuvant therapy was determined by the MDT and was based on the positivity of resection margins, pathological nodal status and the post-operative performance status of the patient.
Study exposure
All tumours were given a categorical score (1, 2, 3, 4, 5) based on the proportion of fibrosis to residual viable tumour cells on pathological analysis of the primary tumour as originally described by Mandard.6 Mandard 1 was defined as complete fibrosis and a complete pathological response. Mandard 5 was defined as no fibrosis seen. Mandard 4 and 5 tumours were considered to be poor or non-responders to chemotherapy. To confirm the validity of this subgrouping, Kaplan–Meier analysis was performed on the five groups (see Supplementary Fig. 1).
Clinical nodal staging was determined by the MDT based on CT, EUS and positron emission tomography (PET) CT. Patients were categorised as naturally node negative (cN0 to ypN0), complete nodal response (cN+ to ypN0) or persistent nodal disease (cN+ to ypN+).
CT estimated tumour volumes were available for a sequential cohort of patients undergoing resection between 2009 and 2016. CT tumour volumes were estimated using open-source, Mac-based DICOM Viewer (OsiriX 3.9) software. Volumes were calculated using CT volume software and a validated formula, using longest axial and craniocaudal tumour dimensions.12 Tumour volumes were assessed retrospectively by a consultant radiologist before and after chemotherapy and tumour volume change was recorded in cm3. This consultant radiologist was blinded to clinical and pathological tumour stage and CRM status.
Statistical analysis
To evaluate which patient and tumour characteristics influenced survival and each recurrence type, univariable and multivariable Cox regression was performed to give hazard ratios (HRs) with 95% confidence intervals (CIs). The first model examined survival in the entire cohort and then was stratified by Mandard group (Mandard 1–3 vs Mandard 4 and 5). This adjusted for gender (male or female), age (continuous), surgical approach (transthoracic oesophagectomy TTO vs transhiatal oesophagectomy THO), CRM positive (positive vs negative), lymphovascular invasion (LVI) (yes or no), pathological T stage (ypT0–ypT4), pathological nodal status (ypN0–ypN3), nodal response (cN0 to ypN0, complete nodal response vs persistent nodal disease), pathological grade (poorly differentiated, moderately differentiated and well differentiated), Mandard tumour regression score in the whole cohort (Mandard 1–3 vs Mandard 4 and 5) and adjuvant treatment (yes/no). Only variables significant on univariable analysis were brought forward into the multivariable analysis. The second model examined systemic and locoregional recurrence in patients stratified by pathological response (Mandard 1–3 vs Mandard 4 and 5). The second model used nodal response (cN0-ypN0, complete nodal response or persistent nodal disease) as the sole nodal variable to compensate for the colinearity of nodal variables in an analysis with a less frequent outcome measure. Mean tumour volume change was compared between Mandard 1–5 and nodal response groups using the Kruskal–Wallis test. All statistics were calculated using SPSS v26.
Results
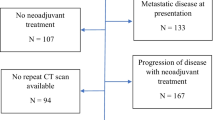
Five hundred and fifty-five patients were included in the analysis. Baseline patient characteristics are shown in Table 1. All had received neoadjuvant chemotherapy, followed by oesophagogastrectomy between 2000 and 2017. Five hundred and three (90.63%) received epirubicin, cisplatin and 5-FU or capecitabine, with the majority of the remaining patients receiving a combination involving platinum-based chemotherapy. Forty-five of 555 (8.18%) were naturally node negative (cN0-ypN0), 154/555 (27.75%) had a complete nodal response and 356/555 (64.14%) had persistent nodal disease. Three hundred and thirty-two of 555 (59.82%) patients were non-responders to chemotherapy (Mandard 4 and 5). The Mandard 4 and 5 group contained less patients with a complete nodal response compared to the Mandard 1–3 group (18.37 vs 41.70%). Median follow-up was 18 months.
cN0-ypN0
Forty-five patients were naturally node negative. Twenty-seven (60%) of these patients were clinically staged with EUS and 31 (68.9%) were staged with PET CT. Twenty-one of these patients have CT volumetrics available for analysis. Pre-chemotherapy volumes were smaller on average than the rest of the cohort. (29.1 cm3 naturally node negative vs 36.7 cm3 in complete nodal responders and 34.9 cm3 in those with persistent nodal disease).
Complete nodal responders
One hundred and fifty-four patients had a complete nodal response. Sixty-one patients had a Mandard 4/5 tumours and 93 had Mandard 1/2/3 tumours. One hundred and seventeen were staged with PET CT and 121 were staged with EUS. One hundred and twenty-nine had cN1 disease, 24 had cN2 disease and 3 had cN3 disease.
Survival
Median survival (Table 2) was 21 months in patients with a pathological poor response to chemotherapy (Mandard 4 and 5) and 55 months in the pathological responders (Mandard 1–3). In the Mandard 1–3 group, median survival was 125 months in the cN0-ypN0 group, 124 months in the complete nodal response group and 30 months in patients with persistent nodal disease (p < 0.001). In the Mandard 4 and 5 group, the median survival was 19 months in the cN0-ypN0 group, 90 months in the complete nodal response group and 18 months in patients with persistent nodal disease (p < 0.001). Kaplan–Meier curves showing overall survival by nodal response groups in non-responders and responders are shown in Fig. 1.
On multivariable survival analysis of the entire cohort (Table 3), independent predictors of poor outcome were ypT4 tumours (HR 3.12, 95% CI 1.02–9.57), LVI (HR 1.51, 95% CI 1.14–2.01), CRM positivity (HR 1.41, 95% CI 1.07–1.85) and poor response to chemotherapy in the primary tumour (HR 1.20, 95% CI 1.06–1.86). Multivariable analysis in the non-responder group (Mandard 4 and 5) found a complete nodal response to chemotherapy to be the only independent predictor of survival significance (HR 0.34, 95% CI 0.16–0.74). Complete multivariable analysis of survival can be seen in Supplementary Table 1.
Recurrence
One hundred and ninety-five of 555 (35.14%) suffered systemic recurrence and 126/555 (22.70%) suffered locoregional recurrence within the follow-up period. Systemic and locoregional recurrence rates are shown in Table 2. The systemic recurrence rate was significantly lower in the Mandard 4 and 5 group with a complete nodal response compared to patients with persistent nodal disease (24.59 vs 48.42.11%, p = 0.003). In Mandard 4 and 5 patients, locoregional recurrence rates were significantly lower in the complete nodal responders (14.75%) than in patients with persistent nodal disease (28.74) (p = 0.005). Locoregional recurrence rates were similar between Mandard 1–3 and Mandard 4 and 5 patients when there was a complete nodal response (12.90 and 14.75%). Kaplan–Meier analysis of systemic recurrence-free survival by nodal response in each Mandard group is shown in Fig. 2.
(a) Locoregional disease free survival in Mandard 4 and 5 patients by nodal group. (b) Locoregional disease free survival in Mandard 1, 2, 3 patients by nodal group. (c) Systemic disease free survival in Mandard 4 and 5 patients by nodal group. (d) Systemic disease free survival in Mandard 1, 2, 3 patients by nodal group.
On multivariable analysis, the only independent predictor of both locoregional and systemic recurrence in the Mandard 4 and 5 group was a complete nodal response to chemotherapy (locoregional HR 0.43, 95% CI 0.21–0.89 and systemic HR 0.42, 95% CI 0.24–0.75). In the Mandard 1–3 group, LVI and cN0-ypN0 independently influenced locoregional recurrence (HR 1.99, 95% CI 1.01–3.99 and HR 0.10, 95% CI 0.01–0.78) and cN0-ypN0 and complete nodal response predicted reduced systemic recurrence (HR 0.08, 95% CI 0.01–0.61 and HR 0.39, 95% CI 0.20–0.76). Complete multivariable analysis of locoregional and systemic recurrence-free survival can be seen in Supplementary Table 2.
CRM positivity
CRM positivity (Table 2) improved with nodal response across both pathological response groups. In the Mandard 4 and 5 patients, CRM positivity was 22.95% in the complete nodal response group and 68.11% in the group with persistent nodal disease (p < 0.001).
Tumour volume
Two hundred and fifty-six patients from the main cohort had pre- and post-neoadjuvant chemotherapy CT estimated tumour volume available for analysis (Table 4). Forty (15.62%) patients experienced an increase in tumour volume. The majority of patients, 216 (84.38%), were found to have tumour volume reduction following chemotherapy. The mean overall tumour volume change in the 256 patients was a volume reduction of 10.1 cm3. There was no significant difference between the volume change of a tumour and its subsequent Mandard score (p = 0.709). There was a statistically significant increased volume reduction in all patients (Mandard 1–5) with a complete nodal response (16.3 cm3) compared to patients with persistent nodal disease (7.7 cm3) (p = 0.03).
Discussion
This study demonstrates a discordance between primary tumour and locoregional and systemic chemotherapy responses. Eighteen percent of patients with a poor response to chemotherapy in their primary tumour (Mandard 4 and 5) showed a complete nodal response to chemotherapy and these patients experienced significantly improved survival and reduced rates of systemic and locoregional recurrence. The majority of patients benefitted from a tumour volume reduction following chemotherapy, although the magnitude of volume reduction did not correspond with Mandard tumour regression grade. Patients with a complete nodal response showed a significantly greater tumour volume reduction. Taken together, these findings suggest that Mandard tumour regression fails to identify a subset of patients who may actually have responded to chemotherapy, manifested either as a lymph node response or a reduction in tumour volume that is insufficient to change T status. This may be important in clinical decision making, for example, the recommendation for continuing adjuvant chemotherapy.
Other studies have highlighted an improved survival of patients with a nodal response to chemotherapy despite a poor response in the primary tumour.10,13,14 One multicentre study found 21.3% of patients with Mandard 3–5 tumours had a complete nodal response to chemotherapy (cN+ to ypN0).13 This group had equivalent survival to patients with a good or complete response in their primary tumours (Mandard 1 and 2). This current study grouped responders and non-responders differently according to the survival analysis in Supplementary Table 1. This may reflect possible subjective differences to how Mandard 2–4 are classified in different institutions. However, this current study supports the findings of the above-mentioned study and adds a detailed analysis of recurrence patterns and tumour volume changes within these groups.
There are several methodological limitations to this study that deserve attention. This was a retrospective analysis of prospectively collected data and therefore the results should be interpreted with some caution. The accuracy of preoperative staging based on CT, PET and EUS still remains suboptimal.15 One possibility is that the inaccuracies of clinical nodal staging mean that the survival advantages of nodal downstaging in this study simply represent clinical overstaging at the outset. To compensate for this, naturally node-negative and complete nodal responders were analysed as separate groups. If it were the case that the survival advantage seen in Mandard 4/5 tumours with nodal downstaging was simply the result of overstaged disease, then there would be little or no difference between naturally node-negative and complete node-negative groups, which was not the case. Furthermore, the first multivariable model included two nodal variables (ypN status and nodal response) in keeping with an analysis performed in a similar study.13 Although It is likely that there is overlap between the two nodal variables, complete nodal response became a significant prognostic variable despite this in the model examining patients with Mandard 4 and 5 tumours. It was for this reason that the models examining recurrence contained nodal response as the single nodal variable. Neoadjuvant treatment changed during the study period. While this followed randomised trial evidence available at the time with the majority of patients receiving epirubicin, cisplatin and 5-FU or capecitabine, the lack of overall uniformity with neoadjuvant regimens may have been a source of bias.
Pathological analysis of the primary tumour has remained the gold standard of neoadjuvant treatment assessment since the Mandard tumour regression grade was first described in 1994. This was described using 93 patients, mainly with squamous cell carcinoma, undergoing neoadjuvant chemoradiotherapy.6 Many studies have since used the Mandard tumour regression grade as an outcome measure to test clinical and radiological predictors of chemotherapy and chemoradiotherapy response in both CT16,17,18 and 18F fluorodeoxyglucose positron emission tomography.19,20,21 Using radiological metrics (e.g. PET SUV) to identify non-responders to neoadjuvant treatment has been a strategy used in some studies to divert patients onto alternative treatment pathways.7,22 However, the discordance between tumour and lymph node regression may have affected the outcome of these studies. The MUNICON I study showed no survival disadvantage in the early termination of chemotherapy in patients who showed no PET response in the primary tumour. The study did not determine the extent to which any therapeutic advantage was lost in patients with a nodal response to chemotherapy despite a poor response in their primary tumour.
In clinical experience, patients can have an obvious radiological tumour and nodal size reduction following chemotherapy yet have a poor response in the primary tumour on pathological analysis. Studies have assessed CT estimated dimensional changes in the primary tumour and have shown little or inconsistent correlation between volume change and pathological tumour response.9,16,17,23 This is perhaps unsurprising as response to chemotherapy in the primary tumour is a heterogeneous process involving a complex interaction of inflammation, oedema, fibrosis and chemotoxicity in the tumour microenvironment.24,25,26,27,28 A recent study, however, showed a composite assessment of tumour volume change and PET metabolic activity correlated with histological response and survival.29 The present study showed that crude tumour volume reduction corresponded more closely with lymph node downstaging as opposed to pathological tumour regression. Another interesting finding was that CRM positivity rates in the Mandard 4 and 5 tumours were lower in the nodal downstaged group. This suggests that the mechanisms that predispose to nodal downstaging and tumour shrinkage may have a bearing on the resectability of the primary tumour, despite a lack of tumour fibrosis.
Studies assessing lymph node response to chemotherapy have found correlation between changes in node size, PET avidity, pathological nodal regression and overall survival. One study in patients with mainly squamous cell carcinoma found CT estimated lymph node size reduction correlated more closely with survival than measurements of the primary tumour.30 A study involving pathological analysis of lymph node regression found 40% of patients with a lymph node response had a poor response in the primary tumour. Lymph node regression predicted a survival advantage independently of response in the primary tumour.10 Another recent study has demonstrated the discordance between metabolic nodal response and metabolic tumour response on PET CT and pathological tumour response and has advocated the use of metabolic nodal response in chemotherapy response assessment.14 It has been suggested that these nodal metastases represent an aggressive subpopulation of cancer clones that have a separate and complex genetic and phenotypic evolution separate from the primary tumour.14,31 Lymph node metastases remain the most important prognosticator in oesophageal adenocarcinoma and accurate measurement of lymph node response to chemotherapy will be an essential component of designing patient-specific treatments.
Previous studies have shown that naturally node-negative patients (cN0 to ypN0) experience improved survival compared to patients with a complete nodal response.32 This study only demonstrated this relationship in patients with Mandard 1–3 tumours and not in the overall cohort. Patients with Mandard 4 and 5 tumours, with naturally node-negative disease had much less favourable survival and recurrence rates than in patients with a complete nodal response. This maybe because this group includes patients with chemoresistant tumours and nodal clones that have yet to metastasise and were simply diagnosed and treated when they were clinically node negative. This may be why this cohort had smaller pre-chemotherapy tumour volumes. These patients are likely to have a chemoresistant micrometastatic disease, which will manifest as poor survival despite node-negative disease.
The discordance between lymph node and primary tumour chemotherapy response has implications for adjuvant and neoadjuvant treatment strategies. Studies have demonstrated a survival benefit to adjuvant chemotherapy, especially in patients who have already shown a chemotherapy response.33 This benefit may be underestimated if the Mandard tumour regression grade is the only marker used to measure chemotherapy response. Adenocarcinoma of the oesophagus has a tendency to metastasise early and systemically, especially with established nodal disease.34 Any neoadjuvant treatment strategy, which aims to divert patients onto treatment pathways based on a radiological response to chemotherapy in the primary tumour only, will potentially disadvantage the 20% of patients who are systemically chemosensitive but have not demonstrated a local response.
In conclusion, response to chemotherapy is a complex phenomenon. Patients can exhibit discordance between a systemic and local response to neoadjuvant treatment, which if overlooked could mean patients could forgo treatment that may be beneficial. The findings of this paper suggest that composite scores involving volumetric, metabolic and pathological assessment of both primary tumour and lymph nodes will improve an overall estimation of true chemotherapy response.
References
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358, 36–46 (2008).
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I. & Langley, R. E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 27, 5062–5067 (2009).
Al-Batran, S.-E., Homann, N., Schmalenberg, H., Kopp, H.-G., Haag, G. M., Luley, K. B. et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J. Clin. Oncol. 35(Suppl.), 4004–4004 (2017).
van Hagen, P., Hulshof, M., van Lanschot, J., Steyerberg, E., van Berge Henegouwen, M., Wijnhoven, B. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer for the CROSS Group*. N. Engl. J. Med. 22366, 2074–2084 (2012).
Noble, F. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J. Gastroenterol. 19, 9282 (2013).
Mandard, A.-M., Dalibard, F., Mandard, J.-C., Marnay, J., Henry-Amar, M., Petiot, J.-F. et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73, 2680–2686 (1994).
zum Büschenfelde, C. M., Herrmann, K., Schuster, T., Geinitz, H., Langer, R., Becker, K. et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J. Nucl. Med. 52, 1189–1196 (2011).
Li, R., Tian-wu Chen, M., Hu, J., Guo, D., Xiao-ming Zhang, M., Deng, D. et al. Tumor volume of resectable adenocarcinoma of the esophagogastric junction at multidetector cT: association with regional lymph node metastasis and N Stage 1. Radiology 269, 130–138 (2013).
Tamandl, D., Gore, R. M., Fueger, B., Kinsperger, P., Hejna, M., Paireder, M. et al. Change in volume parameters induced by neoadjuvant chemotherapy provide accurate prediction of overall survival after resection in patients with oesophageal cancer. Eur. Radiol. 26, 311–321 (2016).
Davies, A. R., Myoteri, D., Zylstra, J., Baker, C. R., Wulaningsih, W., Van Hemelrijck, M. et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br. J. Surg. 105, 1639–1649 (2018).
Mapstone, N. P. Dataset for the Histopathological Reporting of Oesophageal Carcinoma (The Royal College of Pathologists, 2007).
Rosset, A., Spadola, L. & Ratib, O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 17, 205–216 (2004).
Noble, F., Lloyd, M. A., Turkington, R., Griffiths, E., O’Donovan, M., O’Neill, J. R. et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br. J. Surg. 104, 1816–1828 (2017).
Findlay, J. M., Bradley, K. M., Wang, L. M., Franklin, J. M., Teoh, E. J., Gleeson, F. V. et al. Predicting pathologic response of esophageal cancer to neoadjuvant chemotherapy: the implications of metabolic nodal response for personalized therapy. J. Nucl. Med. 58, 266–275 (2017).
Yip, C., Cook, G. J. R., Landau, D. B., Davies, A. & Goh, V. Performance of different imaging modalities in assessment of response to neoadjuvant therapy in primary esophageal cancer. Dis. Esophagus 29, 116–130 (2016).
van Heijl, M., Phoa, S. S. K. S., van Berge Henegouwen, M. I., JMT, Omloo, Mearadji, B. M., Sloof, G. W. et al. Accuracy and reproducibility of 3D-CT measurements for early response assessment of chemoradiotherapy in patients with oesophageal cancer. Eur. J. Surg. Oncol. 37, 1064–1071 (2011).
Griffith, J. F., Chan, A. C., Chow, L. T., Leung, S. F., Lam, Y. H., Liang, E. Y. et al. Assessing chemotherapy response of squamous cell oesophageal carcinoma with spiral CT. Br. J. Radiol. 72, 678–684 (1999).
Konieczny, A., Meyer, P., Schnider, A., Komminoth, P., Schmid, M., Lombriser, N. et al. Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur. Radiol. 23, 2492–2502 (2013).
Weber, M.-A., Bender, K., von Gall, C. C., Stange, A., Grünberg, K., Ott, K. et al. Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction. J. Gastrointestin. Liver Dis. 22, 45–52 (2013).
Malik, V., Lucey, J. A., Duffy, G. J., Wilson, L., McNamara, L., Keogan, M. et al. Early repeated 18F-FDG PET scans during neoadjuvant chemoradiation fail to predict histopathologic response or survival benefit in adenocarcinoma of the esophagus. J. Nucl. Med. 51, 1863–1869 (2010).
Gillham, C. M., Lucey, J. A., Keogan, M., Duffy, G. J., Malik, V., Raouf, A. A. et al. 18FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br. J. Cancer 95, 1174–1179 (2006).
Lordick, F., Ott, K., Krause, B.-J., Weber, W. A., Becker, K., Stein, H. J. et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 8, 797–805 (2007).
Tullie, L. G. C., Sohn, H.-M., Zylstra, J., Mattsson, F., Griffin, N., Sharma, N. et al. A role for tumor volume assessment in resectable esophageal cancer. Ann. Surg. Oncol. 23, 3063–3070 (2016).
Senthebane, D. A., Jonker, T., Rowe, A., Thomford, N. E., Munro, D., Dandara, C. et al. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int. J. Mol. Sci. 19, 2861 (2018).
Siddik, Z. H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279 (2003).
Nakamura, K. & Smyth, M. J. Targeting cancer-related inflammation in the era of immunotherapy. Immunol. Cell Biol. 95, 325–332 (2017).
Jain, R. K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013).
Lin, E. W., Karakasheva, T. A., Hicks, P. D., Bass, A. J. & Rustgi, A. K. The tumor microenvironment in esophageal cancer. Oncogene. Nat. Publ. Group 35, 5337–5349 (2016).
Makino, T., Yamasaki, M., Tanaka, K., Masuike, Y., Tatsumi, M., Motoori, M. et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann. Surg. 270, 1090–1095 (2019).
Urakawa, S., Makino, T., Yamasaki, M., Tanaka, K., Miyazaki, Y., Takahashi, T. et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000003445 (2019).
Jones, S., Chen, W. D., Parmigiani, G., Diehl, F., Beerenwinkel, N., Antal, T. et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA 105, 4283–4288 (2008).
Zanoni, A., Verlato, G., Giacopuzzi, S., Motton, M., Casella, F., Weindelmayer, J. et al. ypN0: does it matter how you get there? Nodal downstaging in esophageal cancer. Ann. Surg. Oncol. 23, 998–1004 (2016).
Saunders, J. H., Bowman, C. R., Reece-Smith, A. M., Pang, V., Dorrington, M. S., Mumtaz, E. et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J. Surg. Oncol. 115, 821–829 (2017).
Knight, W. R. C., Zylstra, J., Van Hemelrijck, M., Griffin, N., Jacques, A. E. T., Maisey, N. et al. Patterns of recurrence in oesophageal cancer following oesophagectomy in the era of neoadjuvant chemotherapy. BJS Open 1, 182–190 (2017).
On behalf of the Guy’s & St Thomas’ Oesophago-Gastric Research Group
O. Hynes1, G. Tham1, C. Iezzi1, R. Bott1, N. Maisey5, A. Gaya5, S. Ngan5, A. Qureshi5, M. Green6, A. Jacques2, V. Goh7, H. Deere8, F. Chang8, U. Mahadeva8, B. Gill-Barman8, S. George8, J. Dunn9, S. Zeki9 and J. Meenan9
Author information
Authors and Affiliations
Consortia
Contributions
Study concepts: W.R.C.K., C.R.B. and J.A.G. Study design: W.R.C.K., C.R.B., N.G., M.K., A.R.D. and J.A.G. Data acquisition: W.R.C.K., N.G. and A.R.D. Quality control of data and algorithms: W.R.C.K., W.W. and J.A.G. Data analysis and interpretation: W.R.C.K., W.W. and J.A.G. Statistical analysis: W.R.C.K., W.W. and J.A.G. Manuscript preparation: W.R.C.K. Manuscript editing: W.R.C.K., C.R.B., M.K., A.R.D. and J.A.G. Manuscript review: W.R.C.K, C.R.B., N.G., M.K., A.R.D., J.A.G. and W.W.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval for use of the database was obtained through the Integrated research application system (IRAS reference: 12-NW-0511). Appropriate consent was gained from all patients.
Consent to publish
Not applicable.
Data availability
Data were available on request from the authors.
Competing interests
The authors declare no competing interests.
Funding information
No funding was used for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Knight, W.R.C., Baker, C.R., Griffin, N. et al. Does a high Mandard score really define a poor response to chemotherapy in oesophageal adenocarcinoma?. Br J Cancer 124, 1653–1660 (2021). https://doi.org/10.1038/s41416-021-01290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01290-4
- Springer Nature Limited