Abstract
Extracorporeal photopheresis (ECP) is a therapy that combines the collection of mononuclear cells by apheresis, the addition of a photosensitizer (8-methoxisoralen), the illumination of the product with ultraviolet A light, and the immediate infusion of the product to the patient. Initially developed and approved to treat T-cell cutaneous lymphomas, soon started to be used to treat graft versus host disease (GvHD) developed after allogeneic hematopoietic-cell transplantation. The high response rate of ECP in skin, ocular, oral, pulmonary, and liver forms of chronic GvHD, the steroid-sparing effect, and the improved overall survival of treated patients, made ECP one of the second-line treatments used to treat steroid-resistant acute and chronic GVHD. Recently, the development of new drugs for treating GVHD has changed the position of ECP in the therapy of GVHD and has started to be used in combination with drugs for increasing the response rate to the treatment in severe or resistant forms of acute and chronic GVHD. ECP remains an essential therapeutic resource in the management of patients with refractory acute and chronic GVHD.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic-cell transplantation (allo-HCT) is widely used in the management of acquired and inherited disorders such as hematopoietic malignancies and in recent years for autoimmune and metabolic disorders [1]. In spite of the introduction of new efficacious and safe strategies to prevent it [2], graft versus host disease (GVHD) continues to be one of the major complications of the procedure that affects significantly survival and quality of life of the patients. As a consequence of the small number of well-designed clinical trials, with enough number of patients, there is significant variability in the treatment of the two forms that the GVHD can develop after allo-HCT, acute GVHD (aGVHD) and chronic (cGVHD).
In the late 80s of the last century, extracorporeal photopheresis (ECP) was introduced in therapeutics for the management of cutaneous T-cell lymphomas [3]. Since then, it has been increasingly used in the treatment of other severe and refractory conditions such as aGVHD and cGVHD and rejection of transplanted solid organs such as lungs and heart [4].
In this manuscript, we will review what is known about ECP and its current position in the management of acute and chronic GVHD after allo-HC.
Extracorporeal photopheresis
The modern use of photosensitizers and light started in the 1970s when at the Massachusetts General Hospital, in Boston a team of dermatologists and pharmacologists treated severe psoriasis using orally administered 8-methoxypsoralen (8-MOP) and exposure of the skin to ultraviolet A ((UVA), 320–400 nm) radiation [5]. The finding in the 1980s that in mice and rats, the infusion of lethally damaged T-cell clones could prevent the induction of autoimmune diseases produced by subsequent administration of viable autoreactive T cells, led to a group of dermatologists at Yale University School of Medicine, in New Haven to use that approach to treat cutaneous T-cell lymphomas [3]. The patients underwent a mononuclear cell (MNC) collection using an apheresis system after taking 8-MOP orally. The collected cells were exposed to UVA (1 to 2 J/m2) and then returned to the patient. Twenty-seven of 37 patients with refractory cutaneous T-cell lymphomas responded to the treatment.
However, some patients had intolerance to the ingestion of the 8-MOP including nausea and vomiting that together with the differences in the gastrointestinal absorption due to individual variability, resulted in inconsistent blood concentration of 8-MOP and the need of monitoring plasma levels during the treatment. To avoid those problems the 8-MOP was added directly to the collected product [6]. The modern ECP was born.
Current technologies for extracorporeal photopheresis
The principle described earlier continues to be what we are using 30 years after its development. We perform an MNC collection using apheresis, we add between 80 and 100 µg of 8-MOP to the bag, we illuminate the product with UVA, and finally, we reinfuse the product to the patient. What has changed significantly, is the way how we perform it.
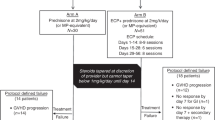
The first technology in the market that allowed the performance of all the steps of ECP using single needle access, in the same platform was the Therakos UVAR (Therakos, at that time a Johnson & Johnson company, PA, USA) introduced in the market in 1987. The system allowed the collection of the MNC cells, the addition of the 8-MOP and the UVA illumination, and finally, the illuminated product was infused to the patient. This system underwent different upgrades and the current model in the market is the Therakos Cellex Plus (Therakos, Mallinckrodt Pharmaceuticals) [7]. Currently, the usual procedure for treating GVHD patients is to collect the MNC present in 1500 mL of blood per session. In case of cGVHD, the recommended schedule is two consecutive sessions every week (one cycle) for the first 3 months followed by one cycle twice per month and then tapered depending on clinical response. For aGVHD the recommended schedule is two or three sessions per week until complete response [8].
In Europe, another technique for performing ECP was developed because one of the problems of the Therakos UVAR single needle design, was the high extracorporeal volume (that made the application of the treatment to pediatric patients challenging). Medical doctors of the Pitié-Salpétrière-Hôtel-Dieu and Cochin Hospitals in Paris created a two steps technique, later known as off-line or disconnected ECP [9, 10]. The first step was the MNC collection performed in a cell separator (initially, Spectra, Cobe, Co, USA) that provided flexibility regarding the volume to process and the amount of MNC collected. After, 8-MOP was added to the collected product, and the mixture was illuminated in an ethylene vinyl acetate bag transparent to UV, in an external UV illuminator.
In many countries of Europe disconnected ECP is the technology more commonly used. For instance, according to the Italian registry of therapeutic apheresis, in 2015 78% of the 6606 ECP procedures gathered by the registry, were performed using the disconnected technology [11].
Since 2019, in Europe there has been available another technology for ECP that combines the collection of MNC in the Amicus separator (Fresenius Kabi, Bad Homburg, Germany) with a photoactivation device (Phelix, Fresenius Kabi), creating an online, closed system to perform ECP. There is a single-use disposable kit combining the collection of the cells and their illumination in the photoactivation device, so the cells are continuously connected to the patients until their infusion. The system allows to process up to 8 L of blood of the patient in each session [12].
Currently, the usual schedule of the disconnected ECP for treating GVHD patients is to process 1 blood volume in the apheresis platform per session, two sessions in separate days the first 2–4 weeks, followed by one session per week, every 2 weeks for a minimum of 6 months [13].
The ninth edition of the American Society for Apheresis Guidelines on the use of therapeutic apheresis recommends for aGVHD two or three treatments weekly until response obtained (minimum of 8 weeks) and for cGVHD, one cycle (i.e., two treatments in 1 week) weekly or every other week for up to 3 months, then, if responding, taper to one cycle per month to clinical response [14].
There are differences in the cost between the different technologies currently available for performing ECP in Europe. For reference, we have available the cost in Spain of the three technologies currently available to perform ECP [15]. In case of ECP using Therakos technology the cost of a round of treatment (treatment for 6 months, in total 28 sessions of processing 1.5 L) was higher (850 euros per kit for 28 sessions, 23,800 euros in total) than for the disconnected strategy (14 sessions processing a total blood volume per session, using each time a collection and an illumination kit, representing 500 euros both, in total 7.000 euros) or the new connected technology also processing a total blood volume per session (665 euros per kit, in total 9.310 euros). However, costs can vary widely depending on the country considered. for example, a recent study reported that the cost of the ECP using Therakos in the USA was calculated to be 37,744 US $, around 34,742 euros, 46% more expensive than in Spain [16].
There are several studies that evaluated the cost-effectiveness of ECP in the management of GVHD in different jurisdictions [16,17,18,19]. All the studies have concluded that ECP is a cost-effective option for steroid-refractory cGVHD.
Mechanism of action of extracorporeal photopheresis
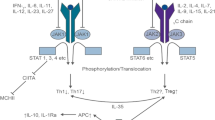
It is well known what we provoke with the collection of the MNC, its illumination in the presence of 8-MOP and the reinfusion to the patient, the apoptosis of the cells, however at different rates. Up to 15% of the reinfused cells will develop immediate apoptosis with a flip-flop of phosphatidylserine to the outer membrane [20] and a second wave of apoptosis (caused by several mechanisms) that ends up with absolute killing of exposed cells on in vitro cultures between 48 and 72 h after ECP [21]. The susceptibility to ECP-induced apoptosis varies depending on the type of cells. B-cells, T cells, NK cells, and monocytes are very sensitive to the treatment, while regulatory T cells (Tregs) are more resistant to ECP with apoptosis levels (annexin 5A positive cells) below 30% at 24 h and levels of 30–65% 48 h after treatment [22].
However, what we do not yet know are the mechanisms by which this effect ends up producing its therapeutic effects, some of them apparently contradictory. According to the latest edition of the Guidelines on the Use of Therapeutic Apheresis of the American Society for Apheresis ECP is recommended as category I (apheresis is accepted as first-line therapy, either as a primary standalone treatment or in conjunction with other modes of treatment) in erythrodermic mycosis fungoides and Sézary syndrome, and category II (apheresis is accepted as second-line therapy, either as a standalone treatment or in conjunction with other modes of treatment) in case of GVHD acute and chronic, rejection, heart transplantation rejection (cellular, recurrent and prophylaxis), and lung transplantation (chronic lung allograft dysfunction, bronchiolitis obliterans syndrome) [14].
So ECP has shown its efficacy for inducing an immune response against cutaneous lymphoma cells (since its therapeutic effect cannot be attributed to the destruction of the malignant cells during the treatment since only 5% to 10% of the body lymphocytes are treated during a cycle of treatment [23]) and for inducing an immunotolerance against the transplanted solid organs or from the hematopoietic cells transplanted against the recipient. Interestingly, Xipell et al. have recently reported that two patients undergoing ECP due to kidney transplantation rejection that concomitantly had viral infections (cytomegalovirus and BK virus), viral infections were successfully controlled during the treatment, so according to the authors an immunogenic effect of ECP in kidney transplant patients might exist [24]. Currently, in the ground of allo-HCT and solid organ transplantation, the concept is that ECP is an efficacious treatment inducing immunotolerance without immunosuppressing the recipient so there is no increase in infectious complications associated to its use [25]. Actually, the safety profile of ECP is excellent, with minimal side effects and no long-term complications, particularly in comparison with other immunosuppressive therapies currently available for GVHD [4]. In those patients carrying implantable ports for performing the treatments, vein thrombosis and infection of the ports can be an issue. However, the use of ultrasonography for cannulation of peripheral veins reduces the need of central lines [26]. For instance, in our institution, 98% of the ECP procedures performed in 2023 were done using peripheral veins.
As stated previously the ultimate mechanisms by which ECP exerts its therapeutic effects is not known but the evidence accumulated so far indicates that multiple events occur that contribute to the result of the treatment. These include contact of cells with external plastic surfaces (apheresis and illumination kits), exposure to 8-MOP and UVA that activates platelets, monocytes, and other myeloid cells, the release of damage-associated molecular patterns, and differentiation of monocytes into dendritic cells. Once reinfused to the patient, the ECP product generates and presents numerous antigens after the phagocytosis of apoptotic cells, increases the frequency and activity of Tregs [27], shift the systemic cytokine balance, and promotes extravasation of immune cells that together, they are responsible for the therapeutic effect of ECP [28]
Clearly, more research is needed, ideally exploring new fields [29] to gain more insight into the mechanisms involved in the therapeutic effect of ECP in the different medical conditions that we are currently treating with it.
Extracorporeal photopheresis in acute graft versus host disease
Acute GVHD is a major, life-threatening complication of allo-HCT. Immune effector cells of the donor will recognize and attack tissues and organs of the recipients provoking the typical signs and symptoms of the disease [30]. The more commonly affected organs include the skin, gastrointestinal tract, and liver [31, 32]. Depending on the severity of the symptoms each organ receives a score, and the combinations of these three scores will lead to a classification of the aGVHD into four different levels being I the mildest and IV the most severe [30]. For many years it was considered aGVHD when any of those symptoms developed before day 100 post-transplantation and cGVHD when symptoms developed after day 100 [33]. However, in 2005, and revised in 2014, the National Institutes of Health Consensus Conference proposed new diagnostic criteria for GVHD based only on clinical manifestations without any reference to the time of commencement [34, 35].
In the early days, about 30–60% of patients who underwent an allo-HCT would develop aGVHD [36]. Fortunately, the introduction of the administration of high-dose post-transplant cyclophosphamide (PTCY) as prophylaxis of GVHD has decreased those figures. In a recent retrospective study of 272 adult patients, Salas et al. reported that PTCY and tacrolimus reduced the cumulative incidence of aGVHD grade II–IV at day +180 to 14.7% in comparison to 41.8% in the group receiving other types of GVHD prophylaxis [2].
The general consensus is that when a patient develops grade II or higher aGVHD after allo-HCT, corticosteroids (methylprednisolone or prednisone) in a dose of 1–2 mg/kg/24 h should be initiated. If corticosteroid resistance or dependence occurs, a second-line of treatment is recommended. And this second-line is a major challenge as can be deduced from the fact that a recent review listed 14 different therapeutic options to choose from [36].
Currently among the main options that the clinicians select from that list are ECP and JAK inhibitors such as ruxolitinib. Ruxolitinib proved its efficacy and safety in managing of aGVHD in a randomized, controlled clinical trial of 309 patients. One hundred fifty-four patients were allocated to the ruxolitinib arm and 155 were assigned to the control group, according to investigator’s choice (in 31% of the patients ECP was selected). Durable overall response at day 56 was higher in the ruxolitinib group than in the control group (40% vs 22%, p < 0.001) [37].
Since the publication of this pivotal study and the approval by FDA and EMA, ruxolitinib has gained importance in the treatment of GVHD primarily due to the ease of administration compared to ECP, and currently, ruxolitinib is considered the treatment of choice in aGVHD-resistant to steroids (SR-aGVHD) [36]. In a recent retrospective study performed by the EBMT Transplant Complications Working Party, they compared patients receiving ECP or ruxolitinib in 31 centers as treatment for SR-aGVHD. At 90 days after starting treatment, there were no statistical differences in overall response, in overall survival, progression-free survival, non-relapse mortality, and relapse incidence. However, ruxolitinib efficacy does not come without a toll. Up to day 28, in 33% of the patients on the ruxolitinib group thrombocytopenia was observed while in the control groups, the incidence was 18% [38, 39].
Since the introduction of ruxolitinib, a third approach tried to overcome the dilemma of selecting ECP or ruxolitinib as a second-line treatment for SR-aGVHD: the combination of both. Modemann et al. reported a single-center experience of combining ruxolitinib with ECP in 18 patients with severe SR-aGVHD of lower gastrointestinal tract. The treatment was well tolerated and no severe cytopenia was observed. Complete and partial responses were observed in 44% and 11% patients, respectively. Corticosteroids were tapered rapidly with a median time of 2 days for halving of dosage, avoiding additional steroid-associated side effects [40].
In summary, nowadays in case of SR-aGVHD, the standard practice has become to use ruxolitinib as a second-line treatment, leaving ECP as a third line in case of poor response to the ruxolitinib or combining ruxolitinib to ECP in case of severe manifestations or the existence of a clinically significant cytopenia.
Extracorporeal apheresis in chronic graft versus host disease
Chronic GVHD is the major cause of non-relapse mortality and severely impairs the quality of life in long-term survivors of allo-HCT. As in aGVHD, immune effectors cells of the donor recognize and attack tissues of the recipient, although the biological mechanisms involved are not yet as well understood as those leading to aGVHD [41]. Symptoms usually develop within 3 years after allo-HCT and often follow a history of aGVHD. cGVHD frequently involves skin, liver, eyes, mouth, upper respiratory tract, esophagus, and less frequently serosal surfaces, lower gastrointestinal tract, female genitalia, and fascia and presents features reassembling autoimmune and other immunologic diseases [35].
When allo-HCT is carried out using the standard prophylaxis regimen with a calcineurin inhibitor and an antimetabolite, cGVHD develops in 30% to 70% of patients [42]. However, as in the case of aGVHD, the introduction of PTCY has significantly changed the landscape. Bolaños-Meade et al. recently reported a study where patients with hematologic cancers receiving allo-HCT from an HLA-matched related donor or a matched or 7/8 mismatched (mismatched at only one of the HLA-A, HLA-B, HLA-C and HLA-DRB1 loci) unrelated donor, with reduced-intensity conditioning, were randomized to receive cyclophosphamide–tacrolimus–mycophenolate mofetil (experimental prophylaxis) or tacrolimus–methotrexate (standard prophylaxis). The authors reported that the cumulative incidence of cGVHD at 1 year in the experimental prophylaxis group was 21.9% while in the standard prophylaxis group, the incidence was 35.1%. Patients in the experimental prophylaxis group appeared to have less severe acute or chronic GVHD and a higher incidence of immunosuppression-free survival at 1 year. Overall disease-free survival, relapse, transplantation-related death, and engraftment did not differ substantially between the groups [43].
Unfortunately, in spite of the improvement seen in the prophylaxis of cGVHD after allo-HCT, some patients still develop the disease and require treatment. Currently, the first line of treatment is corticosteroids at a dose of 0.5–1 mg/kg/day prednisone dose equivalent. Although prior randomized controlled trials demonstrated that addition of another immunosuppressor at the start of corticosteroids is not beneficial, in severe cGvHD expert opinions suggest that addition of another immunosuppressive agent is of value, such as calcineurin inhibitors [44].
In case of SR-cGVHD, defined as a clinical progression on more than 1 mg/kg/day, or stability on more than 0.5 mg/kg/day, or inability to taper to less than 0.25 mg/kg/day on two separate occasions, or steroid intolerance, a second-line treatment is recommended. And again, as in the aGVHD, the list of potential treatments to be used as second-line is extensive. A recent review listed 20 different options with overall response rates varying from 28% to 81% and wide range of adverse effects [44].
However, nowadays, most centers performing allo-HCT select two drugs depending on availably and cost considerations, ruxolitinib and belumosudil (already approved by FDA but not yet EMA). In the case of ruxolitinib, a phase III, randomized controlled trial, randomized 329 SR-cGVHD patients to receive ruxolitinib 10 mg twice daily or therapy chosen by the investigators (34.8% ECP, 22.2% mycophenolate mofetil, and 17.1% ibrutinib). Overall response at week 24 was greater in the ruxolitinib group than in the control group (49.7% vs. 25.6%, p < 0.001). The most common adverse events of grade 3 or higher, up to week 24 were thrombocytopenia appearing in 15.2% in ruxolitinib group and 10.1% in the control group and anemia (12.7% and 7.5%, respectively) [45].
The role of belumosudil in treating refractory cGVHD was evaluated in phase 2, randomized multicenter study that compared two doses of belumosudil (200 mg daily vs 200 mg twice daily) in 132 patients with cGVHD that had received two to five prior lines of therapy. The best overall response rate (the primary endpoint of the study) was 74% (200 mg daily) and 77% (200 mg twice daily) at a median follow-up of 14 months [42].
ECP use in the treatment of cGVHD is a case in point of therapeutics that have been developed by the medical community without a direct support of the industry [46, 47], in contrast to the use of ECP in the treatment of cutaneous T-cell lymphomas that was developed and licensed by regulators having a private company as sponsors. Reported, there are two randomized controlled trials with 148 patients [48, 49] and 8 controlled trials with 228 patients [14] showing the efficacy (particularly in skin and oral (83%) while visceral (53%) or lung (27%) involvements, responds to a lesser extent to ECP [50]) and safety of ECP in the treatment of cGVHD. Another important effect of ECP in cGVHD is the well-documented steroid-sparing effects [51]. However, some limitations of the therapy have precluded a more general use such as frequent (one or two times a week, depending on the type of technology used for ECP) and lengthy visits to the hospital for 3–6 months and often longer depending on response; need for a vascular access (although ultrasonography guided venous canalization has reduced the need of placing a central venous catheter [26]); and the relatively slow onset of the action (1–3 months).
For many years it has been considered ECP as one of second-line treatment of cGVHD [52], however nowadays, due to the availability new drugs for the treatment of SR-GVHD, in many centers, the role of ECP has moved to the consideration of salvage therapy. Actually, the updated version of the consensus recommendations of the European Society for Blood and Marrow Transplantation (EBMT), for the management of GVHD, lists ECP in the group of strategies “beyond” second-line treatment [53]. A recent publication by the EBMT Transplant Complications Working Party compared 57 patients treated with ruxolitinib with 84 patients treated with ECP for SR-cGVHD. At day +180 days after initiation of treatment, there were no statistically significant differences in overall response, overall survival, progression-free survival, non-relapse mortality, and relapse incidence [54].
As in aGVHD, another observed tendency is the combination of ruxolitinib with ECP to increase the effectiveness of both treatments. Maas-Bauer et al. reported a retrospective analysis of 23 patients with SR-GVHD treated with ruxolitinib and ECP. In this group of heavily pretreated patients, the best overall response (complete or partial response) at any time point during the treatment was 74% and the 24-month survival was 75% [54].
Conclusions
The favorable profile of the ECP in the treatment of aGVHD and cGVHD made that for many years this treatment was deemed one of the therapies to be considered in the treatment of SR-acute and chronic GVHD as a second-line therapy. However, the development of new medications for treating GVHD has changed the position of ECP and currently is considered more as a salvage therapy. Nevertheless, interest has emerged in combining ECP with medications such as ruxolitinib in search of a higher response rate in severe or resistant forms of acute and chronic GVHD.
References
Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transpl. 2020;55:1604–13. https://doi.org/10.1038/s41409-020-0826-4.
Salas MQ, Pedraza A, Charry P, Suarez-Lledo M, Rodriguez-Lobato LG, Brusosa M, et al. Post-transplantation cyclophosphamide and tacrolimus for graft-versus-host disease prevention after allogeneic hematopoietic cell transplantation from HLA-matched donors has more advantages than limitations. Transplant Cell Ther. 2023. https://doi.org/10.1016/j.jtct.2023.11.020.
Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. https://doi.org/10.1056/NEJM198702053160603.
Knobler R, Berlin G, Calzavara-Pinton P, Greinix H, Jaksch P, Laroche L. et al. Guidelines on the use of extracorporeal photopheresis. J Eur Acad Dermatol Venereol. 2014;28:1–37. https://doi.org/10.1111/jdv.12311.
Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291:1207–11. https://doi.org/10.1056/NEJM197412052912301.
Knobler RM, Trautinger F, Graninger W, Macheiner W, Gruenwald C, Neumann R, et al. Parenteral administration of 8-methoxypsoralen in photopheresis. J Am Acad Dermatol. 1993;28:580–4. https://doi.org/10.1016/0190-9622(93)70077-7.
Therakos ECP Immunomodulation. 2024. https://therakos.eu/company/. Accessed 27 March 2024.
Knobler R, Arenberger P, Arun A, Assaf C, Bagot M, Berlin G. et al. European Dermatology Forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34:2693–716. https://doi.org/10.1111/jdv.16890.
Andreu G, Leon A, Heshmati F, Tod M, Menkes CJ, Baudelot J, et al. Extracorporeal photochemotherapy: evaluation of two techniques and use in connective tissue disorders. Transfus Sci. 1994;15:443–54. https://doi.org/10.1016/0955-3886(94)90178-3.
Heshmati F, Andreu G. Extracorporeal photochemotherapy: a historical perspective. Transfus Apher Sci. 2003;28:25–34. https://doi.org/10.1016/S1473-0502(02)00097-6.
De Silvestro G. The Italian registry of therapeutic apheresis—2015. Transfus Apher Sci. 2017;56:75–81. https://doi.org/10.1016/j.transci.2016.12.024.
Extracorporeal Photopheresis. Amicus Blue. 2024. https://www.amicusblue-fresenius-kabi.com/wp-content/themes/amicus-blue/documents/Amicus_ECP_Brochure.pdf. Accessed 20 May 2024.
Cid J, Carbasse G, Suarez-Lledo M, Moreno DF, Martinez C, Gutierrez-Garcia G, et al. Efficacy and safety of one-day offline extracorporeal photopheresis schedule processing one total blood volume for treating patients with graft-versus-host disease. Transfusion. 2019;59:2636–42. https://doi.org/10.1111/trf.15384.
Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the writing committee of the American Society for Apheresis: the ninth special issue. J Clin Apher. 2023;38:77–278. https://doi.org/10.1002/jca.22043.
Boluda B, Solana-Altabella A, Cano I, Acuna-Cruz E, Rodriguez-Veiga R, Ballesta-Lopez O, et al. Extracorporeal photopheresis vs standard therapies for steroid-refractory chronic graft-vs-host disease: pharmacoeconomic assessment of hospital resource use in Spain. J Clin Apher. 2021;36:612–20. https://doi.org/10.1002/jca.21901.
Yalniz FF, Murad MH, Lee SJ, Pavletic SZ, Khera N, Shah ND, et al. Steroid refractory chronic graft-versus-host disease: cost-effectiveness analysis. Biol Blood Marrow Transpl. 2018;24:1920–7. https://doi.org/10.1016/j.bbmt.2018.03.008.
Crespo C, Perez-Simon JA, Rodriguez JM, Sierra J, Brosa M. Development of a population-based cost-effectiveness model of chronic graft-versus-host disease in Spain. Clin Ther. 2012;34:1774–87. https://doi.org/10.1016/j.clinthera.2012.06.029.
de Waure C, Capri S, Veneziano MA, Specchia ML, Cadeddu C, Di Nardo F, et al. Extracorporeal photopheresis for second-line treatment of chronic graft-versus-host diseases: results from a health technology assessment in Italy. Value Health. 2015;18:457–66. https://doi.org/10.1016/j.jval.2015.01.009.
Peacock A, Dehle FC, Mesa Zapata OA, Gennari F, Williams MRI, Hamad N, et al. Cost-effectiveness of extracorporeal photopheresis in patients with chronic graft-vs-host disease. J Health Econ Outcomes Res. 2024;11:23–31. https://doi.org/10.36469/001c.92028.
Gerber A, Bohne M, Rasch J, Struy H, Ansorge S, Gollnick H. Investigation of annexin V binding to lymphocytes after extracorporeal photoimmunotherapy as an early marker of apoptosis. Dermatology. 2000;201:111–7. https://doi.org/10.1159/000018472.
Bladon J, Taylor PC. Extracorporeal photopheresis in cutaneous T-cell lymphoma and graft-versus-host disease induces both immediate and progressive apoptotic processes. Br J Dermatol. 2002;146:59–68. https://doi.org/10.1046/j.1365-2133.2002.04560.x.
Budde H, Berntsch U, Riggert J, Legler TJ. In vitro effects of different 8-methoxypsoralen treatment protocols for extracorporeal photopheresis on mononuclear cells. Cent Eur J Immunol. 2017;42:1–9. https://doi.org/10.5114/ceji.2017.67312.
Knobler R, Barr ML, Couriel DR, Ferrara JL, French LE, Jaksch P, et al. Extracorporeal photopheresis: past, present, and future. J Am Acad Dermatol. 2009;61:652–65. https://doi.org/10.1016/j.jaad.2009.02.039.
Xipell M, Molina-Andujar A, Cid J, Pineiro GJ, Montagud-Marrahi E, Cofan F, et al. Immunogenic and immunotolerogenic effects of extracorporeal photopheresis in high immunological risk kidney recipients. A single center case series. J Clin Apher. 2022;37:197–205. https://doi.org/10.1002/jca.21958.
Perotti C, Sniecinski I. A concise review on extracorporeal photochemotherapy: where we began and where we are now and where are we going! Transfus Apher Sci. 2015;52:360–8. https://doi.org/10.1016/j.transci.2015.04.011.
Mustieles MJ, Lozano M. Vascular access for apheresis: state of the art. Transfus Apher Sci. 2023;62:103669. https://doi.org/10.1016/j.transci.2023.103669.
Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–21. https://doi.org/10.1182/blood-2007-11-125542.
Vieyra-Garcia PA, Wolf P. Extracorporeal photopheresis: a case of immunotherapy ahead of its time. Transfus Med Hemother. 2020;47:226–35. https://doi.org/10.1159/000508479.
Bozzini S, Del Fante C, Morosini M, Berezhinskiy HO, Auner S, Cattaneo E, et al. Mechanisms of action of extracorporeal photopheresis in the control of bronchiolitis obliterans syndrome (BOS): involvement of circulating miRNAs. Cells. 2022;11. https://doi.org/10.3390/cells11071117.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:27 https://doi.org/10.1038/s41572-023-00438-1.
Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N. Engl J Med. 2017;377:2167–79. https://doi.org/10.1056/NEJMra1609337.
Holtan SG, Yu J, Choe HK, Paranagama D, Tang J, Naim A, et al. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review. Bone Marrow Transpl. 2022;57:1581–5. https://doi.org/10.1038/s41409-022-01764-w.
Akahoshi Y, Spyrou N, Hogan WJ, Ayuk F, DeFilipp Z, Weber D, et al. Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. 2023;7:4479–91. https://doi.org/10.1182/bloodadvances.2023009885.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56. https://doi.org/10.1016/j.bbmt.2005.09.004.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21:389–401 e1. https://doi.org/10.1016/j.bbmt.2014.12.001.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e67. https://doi.org/10.1016/S2352-3026(19)30256-X.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800–10. https://doi.org/10.1056/NEJMoa1917635.
Penack O, Peczynski C, Boreland W, Lemaitre J, Afanasyeva K, Kornblit B, et al. ECP versus ruxolitinib in steroid-refractory acute GVHD - a retrospective study by the EBMT transplant complications working party. Front Immunol. 2023;14:1283034. https://doi.org/10.3389/fimmu.2023.1283034.
Penack O, Peczynski C, Boreland W, Lemaitre J, Reinhardt HC, Afanasyeva K, et al. ECP versus ruxolitinib in steroid-refractory chronic GVHD—a retrospective study by the EBMT transplant complications working party. Bone Marrow Transpl. 2024;59:380–6. https://doi.org/10.1038/s41409-023-02174-2.
Modemann F, Ayuk F, Wolschke C, Christopeit M, Janson D, von Pein UM, et al. Ruxolitinib plus extracorporeal photopheresis (ECP) for steroid refractory acute graft-versus-host disease of lower GI-tract after allogeneic stem cell transplantation leads to increased regulatory T cell level. Bone Marrow Transpl. 2020;55:2286–93. https://doi.org/10.1038/s41409-020-0952-z.
Teshima T, Hill GR. The Pathophysiology and Treatment of Graft-Versus-Host Disease: Lessons Learnt From Animal Models. Front Immunol. 2021;12:715424. https://doi.org/10.3389/fimmu.2021.715424.
Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278–89. https://doi.org/10.1182/blood.2021012021.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48. https://doi.org/10.1056/NEJMoa2215943.
Baumrin E, Loren AW, Falk SJ, Mays JW, Cowen EW. Chronic graft-versus-host disease. Part II: disease activity grading and therapeutic management. J Am Acad Dermatol. 2024;90:19–36. https://doi.org/10.1016/j.jaad.2022.12.023.
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–38. https://doi.org/10.1056/NEJMoa2033122.
Rossetti F, Zulian F, Dall’Amico R, Messina C, Montini G, Zacchello F. Extracorporeal photochemotherapy as single therapy for extensive, cutaneous, chronic graft-versus-host disease. Transplantation. 1995;59:149–51. https://doi.org/10.1097/00007890-199501150-00029.
Greinix HT, Volc-Platzer B, Rabitsch W, Gmeinhart B, Guevara-Pineda C, Kalhs P, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–104.
Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–74. https://doi.org/10.1182/blood-2008-03-141481.
Jagasia M, Scheid C, Socie G, Ayuk FA, Tischer J, Donato ML, et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019;3:2218–29. https://doi.org/10.1182/bloodadvances.2019000145.
Sakellari I, Gavriilaki E, Batsis I, Mallouri D, Panteliadou AK, Lazaridou A, et al. Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: prospective single-center study. J Clin Apher. 2018;33:654–60. https://doi.org/10.1002/jca.21660.
Ussowicz M, Musial J, Mielcarek M, Tomaszewska A, Nasilowska-Adamska B, Kalwak K, et al. Steroid-sparing effect of extracorporeal photopheresis in the therapy of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplant Proc. 2013;45:3375–80. https://doi.org/10.1016/j.transproceed.2013.07.053.
Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–15. https://doi.org/10.1182/blood-2014-08-551994.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e59. https://doi.org/10.1016/S2352-3026(23)00342-3.
Maas-Bauer K, Kiote-Schmidt C, Bertz H, Apostolova P, Wasch R, Ihorst G, et al. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56:909-16. https://doi.org/10.1038/s41409-020-01122-8.
Author information
Authors and Affiliations
Contributions
ML, PC, MPM and CM wrote the manuscript. MQS, MSL, FAA, MR, and JC revised the manuscript. All authors have read and approved the final version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
ML on behalf of his institution, Clinic Research Foundation, has received research support from Terumo BCT, Fresenius-Kabi, Macopharma, and Sanofi-Genzyme as a consultant ML has received fees from Macopharma. The other authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lozano, M., Charry, P., de Pablo-Miró, M. et al. Role of extracorporeal photopheresis in the management of acute and chronic graft versus disease: current status. Bone Marrow Transplant 59, 1209–1214 (2024). https://doi.org/10.1038/s41409-024-02360-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02360-w
- Springer Nature Limited