Abstract
The Web-based One Year Survival Outcomes Calculator developed by the Center for International Blood and Marrow Transplant Research (CIBMTR) applies large-scale registry data to generate individualized estimates of overall survival (OS) probability 1 year after first allogeneic hematopoietic cell transplant (HCT) and can therefore provide a data-driven foundation for personalized patient counseling. We assessed the calibration of the CIBMTR One Year Survival Outcomes Calculator when applied to retrospective data among adult recipients of first allogeneic HCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or myelodysplastic syndrome (MDS) with peripheral blood stem cell transplant (PBSCT) from a 7/8- or 8/8-matched donor from 2000 through 2015 at a single center. Predicted 1 year OS was estimated for each patient using the CIBMTR Calculator. Corresponding observed 1 year OS was estimated for each group by the Kaplan-Meier method. A weighted Kaplan-Meier estimator was used to visually display the average of observed 1 year survival estimates over the continuous range of predicted OS. In the first analysis of its kind, we demonstrated that the CIBMTR One Year Survival Outcomes Calculator could be applied to larger patient cohorts and predicted 1 year prognosis with general agreement between predicted and observed survival.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers a path to cure for many patients with hematologic malignancies, and often the only path. Significant risks of relapse and non-relapse mortality (NRM), however, present barriers to transplant success. Well-validated measures such as the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) [1] and Disease Risk Index (DRI) [2] provide a basis to estimate such risks, but these instruments rely on an ultimately limited number of variables to assess patient- and disease-specific risks, respectively. In the face of myriad nuances that distinguish an individual patient, transplant planning often hinges on clinicians’ subjective assessments of disease-, comorbidity-, and transplant-related factors.
The 1-Year Survival Outcomes Calculator developed by the Center for International Blood and Marrow Transplant Research (CIBMTR) applies regression techniques to large-scale registry data to generate individualized estimates of overall survival (OS) probability. The CIBMTR uses aggregate data from US transplant centers to provide authorized users an estimate of 1-year OS for individual patients who will receive a first allogeneic HCT, in a Web-based tool available on the CIBMTR Portal. Analytic results from the center-specific survival analysis produced annually by the CIBMTR inform the tool. Users can enter specific values for patient-, disease-, and transplant-related characteristics and generate a predicted probability of 1-year survival with 95% confidence limits. Odds ratios for each statistically significant characteristic from the final multivariate risk adjustment survival model that supports the center-specific survival analysis are used by the calculator (methodology for the center-specific analysis are available at https://www.cibmtr.org/ReferenceCenter/SlidesReports/csafaq/Pages/default.aspx). The calculator is updated annually and reflects the most recent 3-year time period used in the center-specific survival analysis. Limitations include a time frame of 3 years, reliance on first allogeneic HCT performed in the US, and the risk factors limited to those variables collected by CIBMTR and found to be significant in the multivariate risk adjustment model used to support the center-specific survival analysis.
The CIBMTR One Year Survival Calculator can therefore serve as a valuable tool to inform decision-making for the clinician to weigh the risks and benefits of allogeneic HCT in real time, and provide a data-driven foundation for patient counseling. We hypothesized that the CIBMTR One Year Survival Calculator could also be applied to multipatient data sets and thereby serve as a benchmark to compare observed survival outcomes within a given patient cohort with those predicted by registry data. We thus sought to employ the Calculator to evaluate survival among allograft recipients at our center.
Subjects and methods
Patient population
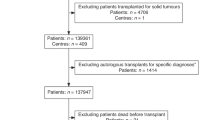
The analysis included adult recipients of first allogeneic HCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or myelodysplastic syndrome (MDS) with peripheral blood stem cell transplant (PBSCT) from a 7/8- or 8/8-matched related or unrelated donor at Memorial Sloan Kettering Cancer Center from January 2000 through December 2015. All patients and donors provided written informed consent for treatment. Pre-transplant characteristics and clinical outcomes were captured in real time per standard clinical practice and stored in an institutional database prior to analysis for this study. The MSKCC Institutional Review and Privacy Board approved this retrospective protocol.
Statistical analysis
Predicted 1 year OS was determined for each patient using the CIBMTR One Year Survival Calculator (version accessed October 2016). Patients were divided into groups in intervals of 5% based on their predicted OS probabilities (e.g., predicted OS 41–45%, 46–50%) (±2%). Corresponding observed 1 year OS was then estimated for each group with 10 or more patients by the Kaplan-Meier method. A smoothed estimate across the predicted values of 1 year OS was also estimated using a weighted Kaplan-Meier function, which utilized a Gaussian density as a kernel function [3]. The Brier score between observed survival at 1 year and predicted 1 year survival was also calculated using inverse probability of censoring weights.
Results
Patient characteristics
A total of 685 patients met inclusion criteria. For 67 patients, missing data from our center precluded calculation of predicted 1 year OS; this left a study cohort of 649 patients. Table 1 shows patient, disease, and transplant characteristics included in the CIBMTR One Year Survival Calculator. No patients were lost to follow-up before 1 year post-HCT. Median age was 55 years (range 18–73). The majority (n = 470, 72%) received ex vivo CD34+ cell–selected grafts without further graft-versus-host disease (GvHD) prophylaxis, with TBI- (n = 170) or chemotherapy-based (n = 300) myeloablative conditioning regimens as previously described [4,5,6]. The remaining 179 patients (28%) received unmodified, T cell–replete grafts, 106 (59%) with myeloablative (TBI-based in 24 [13%], chemotherapy based in 82 [46%]), 63 (35%) with reduced intensity, and 10 (6%) with nonmyeloablative conditioning. All patients undergoing unmodified PBSCT received calcineurin inhibitor–based GvHD prophylaxis.
CD34+ Cell-selected PBSCT
Given the preponderance of patients who underwent CD34+ cell–selected HCT, we analysed this group separately. Table 2 shows probability of 1 year OS predicted by the CIBMTR One Year Survival Calculator versus observed 1 year OS. We removed 16 patients with predicted 1-year OS at the lower (<38%) and upper (>87%) extremes of predicted 1 year OS as there were too few patients within each 5% stratum. Subsequently 454 patients remained. While the observed survival tended to increase as the predicted survival increased, the observed survival tended to be higher than predicted (Table 2, Fig. 1). The observed 1-year Brier score was 0.188.
Comparison of 1 year overall survival predicted by the CIBMTR Calculator with observed 1 year OS in recipients of CD34+ cell–selected PBSCT from 8/8- or 7/8-matched donors for AML, MDS, or ALL from 2000 to 2015. Numerals indicate the number of patients in each group. Vertical bars indicate 95% confidence intervals.
In one group of six patients with predicted 1 year OS probability of 85 ± 2%, observed OS was lower, though not significantly so, at 76% (95% CI 62–93%). We therefore evaluated this group in more detail: Median age was 38 years (range 21–58). HCT-CI score was 0 in 13 patients (43%), 1–2 in 13 (44%), and 3 in 4 (13%); no patient in this group had an HCT-CI score > 4. The majority (n = 22, 73%) were CMV-seronegative. Almost all (n = 29, 97%) had an 8/8-matched related or unrelated donor; 1 (3%) received a 7/8-matched unrelated donor graft. This group was notable for intermediate or high Disease Risk Index (DRI) [2] in 29/30 patients (97%). The majority (27/30, 90%) had AML or ALL in CR1 or CR2, and of these, 59% had poor prognostic disease by ELN/NCCN criteria (n = 12, 44%) or other adverse features such as minimal residual disease pre-HCT or extramedullary disease (n = 4, 15%). The most common cause of 1 year mortality in this subset was relapse, all in patients with acute leukemia.
Unmodified PBSCT
We next evaluated the CIBMTR One Year Survival Calculator among recipients of unmodified PBSCT (Table 2, Fig. 2). There were a total of 179 patients in this group, from which we again removed those at the lower (<28%, n = 8) and upper (>77%, n = 8) extremes of predicted OS. For the remaining 163 patients, estimated 1 year OS tended to increase with that predicted by the CIBMTR One Year Survival Calculator, though there was some deviation when the predicted survival was 0.35–0.40 with higher than predicted survival observed (Table 2, Fig. 2). The observed 1-year Brier score was 0.230.
Comparison of 1 year overall survival predicted by the CIBMTR Calculator with observed 1 year OS in recipients of unmodified PBSCT from 8/8- or 7/8-matched donors for AML, MDS, or ALL from 2000 to 2015. Numerals indicate the number of patients in each group. Vertical bars indicate 95% confidence intervals.
While too few to be included in the figures, there were six patients who had 25% ± 2% predicted likelihood of survival. Of these 6 patients, 5 survived to 1 year, but only 1 survived to the 2-year mark. Median age in this group was 60 years (range 33–66). Median KPS was 70 (range 50–80), and all patients had HCT-CI scores ≥3 (range 3–8). Five patients were CMV-seropositive. All 6 had acute leukemia (AML in 5, ALL in 1) with relapsed/refractory disease without remission prior to HCT. Three received ablative conditioning (TBI 1375 cGy, thiotepa, cyclophosphamide in 1; busulfan and fludarabine in 2), and the other three received fludarabine and melphalan. Two patients received grafts from matched sibling donors, in both cases age >60, and the remaining patients from <8/8-matched unrelated donors.
An additional two patients had still lower predicted 1 year OS, <20%. Both underwent transplant for refractory AML from an 8/8-matched donor. Patients were 55 and 67 years of age, with KPS of 70, and HCT-CI score 7–8. Both were CMV seropositive. One received ablative conditioning with busulfan and melphalan; the other received fludarabine and melphalan. Neither survived to 1 year post-HCT. Among all eight of these patients with 25% likelihood of 1 year survival or lower, two were treated on clinical trials. Cause of death was relapse in two patients, GvHD in two patients, infection in one patient, GI bleed in one patient, and unknown (without relapse) in one patient.
AML
As the majority of patients underwent HCT for AML (n = 380), we also analyzed these patients in isolation regardless of graft manipulation or other characteristics (Table 3; Fig. 3). For this cohort, the 1-year Brier score was 0.197. One group had 1 year OS lower than predicted (predicted: 50% ± 2%; observed: 28% [13, 73%]), though this was not statistically significant. Detailed characteristics of this group, as well as those groups with predicted 1 year OS one stratum above and below, are in Table 4. Among these 18 patients, 11 underwent CD34+ cell–selected PBSCT (all in CR1 or CR2), and the remaining 7 received unmodified grafts (4 in CR1 or CR2; the other 3 with refractory disease). There were seven relapses within 1 year of transplant, three after CD34+ cell–selected and three after unmodified HCT. Relapse occurred at a median of 103 days post-transplant (range, 33–190); all seven relapsed patients died before the 1-year mark. No other single cause predominated among the other six deaths in this group.
Discussion
In the first analysis of its kind, we demonstrated that the CIBMTR One Year Survival Calculator could feasibly be applied to multipatient samples and could serve as a unique tool for transplant centers. The CIBMTR One Year Survival Calculator generally predicted the trend of 1 year prognosis, with the mean deviation from the predicted values of between 0.08 and 0.09 with, in general, higher rates of observed OS than predicted. During this time interval, this center’s survival rate was designated as “above expected” in the Center-Specific Survival Analysis that generated the regression model used for the CIBMTR One Year Survival Calculator, so this degree of deviation could be due to a center effect, or possible unmeasured confounders. The general agreement between the predicted and observed rates was high, however. This was seen both in a relatively homogeneous cohort of myeloablative CD34+ cell–selected PBSCT, in which the majority of recipients entered the transplant procedure with acute leukemia in CR, and in more heterogeneous groups of patients undergoing HCT for AML and those undergoing unmodified HCT across a spectrum of disease states and conditioning regimen intensities. Among more heterogenous populations, there was a broader range of predicted OS, and smaller numbers within each stratum did compromise precision. Expansion of this analysis to larger cohorts will be important to determine whether the trends we noted are significant.
Discordant results in some strata compelled us to investigate our institutional outcomes in focused and granular fashion. In this single-center cohort, for instance, CD34+ cell–selected PBSCT was associated with 1 year OS generally comparable to that predicted by registry data with higher predicted survival corresponding to higher observed survival, though some deviation was observed: Notably, in a small cohort whose predicted 1-year OS was very high, 85 ± 2%, we observed a possible trend toward lower survival at our center. Further investigation of this cohort revealed a relatively high proportion of patients with markers of aggressive disease biology not included in the CIBMTR One Year Survival Calculator estimates at the time of this analysis, such as cytogenetic profile (since added to the Calculator), genomic features, and minimal residual disease status, all of which might allow for further refinement of survival prediction. We thereby found that, in this way, the CIBMTR One Year Survival Calculator could pave the way for individual center observations to reflect back on registry data collection and highlight areas of unmet need for future data capture.
We closely evaluated patients with AML with predicted 1-year survival of 50%, whose observed OS (give number) appeared to be an outlier. Although the sample size was relatively small for this group, we did note that this group appeared enriched for less fit patients, with 78% entering transplant with KPS < 90 and 89% with HCT-CI score ≥3. At the same time, more than 80% of patients in this group received ablative conditioning, many with CD34+ cell–selected HCT. If similar results are found on follow-up analyses with larger samples, it may warrant changes in practice at our center—in this case, specifically at our center, a reconsideration of our criteria for myeloablative conditioning, including in the CD34+-selected setting; this is especially true in light of findings from BMT CTN 1301 (NCT02345850), a randomized trial comparing CD34+ selection with post-transplant cyclophosphamide and tacrolimus/methotrexate for GvHD prophylaxis, which showed increased NRM and reduced OS among recipients of CD34+-selected HCT [7]. Ultimately, even in cases where observed OS does not differ significantly from what the CIBMTR One Year Survival Calculator predicts, assessments of this kind can encourage centers to remain vigilant for needed practice changes before such trends become magnified.
The CIBMTR One Year Survival Calculator also allowed us to reflect on unmet clinical and research needs. Several patients for whom registry data predicted 1 year survival likelihood ≤25% underwent HCT during the period we reviewed, with most surviving to 1 year. However, outcomes for these patients at 2 years were extremely poor. Such estimates are invaluable for clinical decision-making and counseling with patients at high risk of mortality. Moreover, our finding that only 25% of those patients with the poorest prognosis received transplant on research protocols called attention to a gap in our research portfolio. Moving forward, the CIBMTR One Year Survival Calculator could be utilized as a triage tool to identify patients who would be best served by clinical trials, such as investigations of innovative approaches to reduce relapse and toxicity risk or novel cell-based therapies. In turn, Calculator-derived OS predictions could foreseeably serve as novel clinical trial benchmarks in themselves.
We acknowledge the limitations of our analysis. First, it was retrospective in nature and applied a single year’s Calculator predictions, according to the 2016 version of the calculator, across a patient cohort that spanned 15 years. The inclusion of transplants from such a broad time period, while necessary to achieve a sample of sufficient size, likely underestimated the effect of enhanced supportive care and practice since 2000 [8], as well as advances in salvage therapy that may prolong survival even in patients who relapse early after allograft. Prospective collection of CIBMTR One Year Survival Calculator predictions would circumvent this limitation and strengthen future analyses, as would regularly updated analyses as the Calculator is updated annually. Other prognostic models such as the HCT-CI and DRI have been well validated in large cohorts, and new models such as the Simplified Comorbidity Index and the Disease Risk Stratification System, respectively, continue building on those earlier instruments to hone their predictions and align with more contemporary data [9, 10]. Forming a unique complement to such tools, the CIBMTR One Year Survival Calculator similarly mirrors ongoing improvements in transplant outcomes and modifications to variables of interest but, also, evolves perpetually over time.
In addition, while the application of the CIBMTR One Year Survival Calculator on the scale of this analysis was feasible, application to larger sample sizes would certainly be more onerous, though still possible, as the CIBMTR publishes all necessary coefficients as well as center-specific analysis methodology. Future directions for centers could also include analyses using the CIBMTR One Year Survival Calculator alongside other innovative models, such as machine learning techniques that incorporate the relative contribution of patient variables as well as interactions among them [11] and multistate models that encompass additional clinical outcomes such as relapse status and GvHD [12, 13]. It should also be noted that the CIBMTR center-specific survival analysis, which forms the basis of the One Year Survival Calculator’s estimates, is intended to compare centers’ observed performance to what is predicted and not for prognostication; thus variables that may inform outcome, such as conditioning regimen and GvHD prevention method, are not included, which may affect the accuracy of predictions.
In summary, the CIBMTR One Year Survival Calculator has myriad potential applications in the clinic and beyond. Our analysis documents its utility in estimating prognosis in a reliable way that can be used for clinical decision making in specific patient cohorts. The Calculator also deftly elucidated areas where there is room to improve. The Calculator can serve as means of efficient, continuous self-assessment by transplant centers to guide quality improvement, programmatic development, and research efforts.
Data availability
The datasets generated during and analyzed during the current study are not publicly available due to patient privacy considerations but are available in deidentified form from the corresponding author on reasonable request.
References
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Gentleman R, Crowley J. Graphical methods for censored-data. J Am Stat Assoc. 1991;86:678–83.
Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–90.
Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–9.
Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transpl. 2008;14:458–68.
Pasquini MC, Luznik L, Logan B, Soiffer R, Wu J, Devine S, et al., editors. Calcineurin inhibitor-free graft-versus-host disease (GVHD) prophylaxis in hematopoietic cell transplantation (HCT) with myeloablative conditioning regimens (MAC) and HLA-matched donors: results of the BMT CTN 1301 Progress II Trial. Oral presentation at: Transplant and Cellular Therapy Meetings of ASTCT and CIBMTR, February 2021; virtual meeting. https://tct.confex.com/tandem/2021/meetingapp.cgi/Paper/18048. Accessed 1 Nov 2021.
McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med. 2020;172:229–39.
Shouval R, Fein JA, Cho C, Avecilla S, Ruiz JD, Alarcon Tomas A, et al. The Simplified Comorbidity Index (SCI)—a new tool for prediction of non-relapse mortality in allogeneic HCT. Blood Adv. 2022;6:1525–35.
Shouval R, Fein JA, Labopin M, Cho C, Bazarbachi A, Baron F, et al. Development and validation of a disease risk stratification system for patients with haematological malignancies: a retrospective cohort study of the european society for blood and marrow transplantation registry. Lancet Haematol. 2021;8:e205–e15.
Shouval R, Labopin M, Bondi O, Mishan-Shamay H, Shimoni A, Ciceri F, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a european group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33:3144–51.
Holtan SG, Zhang L, DeFor TE, Bejanyan N, Arora M, Rashidi A, et al. Dynamic graft-versus-host disease-free, relapse-free survival: multistate modeling of the morbidity and mortality of allotransplantation. Biol Blood Marrow Transpl. 2019;25:1884–9.
Gerstung M, Papaemmanuil E, Martincorena I, Bullinger L, Gaidzik VI, Paschka P, et al. Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat Genet. 2017;49:332–40.
Acknowledgements
This work was supported in part by National Institutes of Health/National Cancer Institute grant P01 CA23766 and National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748.
Author information
Authors and Affiliations
Contributions
CC, SD, SAG, and MAP designed the study. SD performed the biostatistical analyses. MM served as the data manager. CC curated the data. CC and SD wrote the manuscript. MMH, BL, JDR, BS, SAG, and MAP critically reviewed the manuscript and made substantive contributions to the text and interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
CC, SD, MM, MMH, BL: None. JDR: Optum Stem Cell - Compensation as participant on expert panel to provide input/information about the center-specific survival analysis; compensation directed to Medical College of Wisconsin rather than personally. SAG: Amgen, Actinium, BMS, Celgene, GSK, Kite, Janssen, Jazz Pharma, Johnson & Johnson, Novartis, Pfizer, Sanofi, Spectrum Pharma, Takeda - Membership on board of directors or advisory committees. Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi, Omeros - Institutional research support. M-AP—Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, Vor Biopharma - Honoraria. Cidara Therapeutics, Medigene, Sellas Life Sciences - DSMB. NexImmune - Membership on advisory committee. NexImmune, Omeros, OrcaBio - Ownership interests. Other. Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, Novartis - Institutional research support.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, C., Devlin, S., Maloy, M. et al. Application of the CIBMTR One Year Survival Outcomes Calculator as a tool for retrospective analysis. Bone Marrow Transplant 58, 1089–1095 (2023). https://doi.org/10.1038/s41409-023-02031-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02031-2
- Springer Nature Limited