Abstract
Alternative donor transplantation with the haplo-cord platform allows the use of a lower-dose single umbilical cord blood unit (CBU) by co-infusion of third-party CD34+-selected cells from a haploidentical relative, which provides early transient engraftment while awaiting durable CBU engraftment. In our experience, ~15% of patients lack a suitable haploidentical donor. Here we report 26 patients who underwent haplo-cord transplant using CD34+-selected partially matched unrelated donor grafts. Twenty-four were conditioned with fludarabine/melphalan +/− low-dose TBI (n = 16). Twenty-five received ATG and all received posttransplant tacrolimus and mycophenolate mofetil. Median time to neutrophil and platelet recovery was 11 and 18 days. CBU engraftment, with CD33 and CD3 >5% cord chimerism in the myeloid/lymphoid compartment by day +60, occurred in 20 of 24 patients (83%). Incidence of grade 2–4 acute graft-versus-host disease (GVHD) was 27% at day +100, and chronic GVHD was 4% at 1 year. Overall survival at 1 year was 54%. For patients in need of an alternative transplant who lack a haploidentical donor, haplo-cord transplantation using CD34+-selected partially matched unrelated donor grafts results in rapid engraftment with no increased rate of cord graft failure or GVHD.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a curative treatment for hematological malignancies. For patients without a human leukocyte antigen (HLA) identical sibling or a suitably matched unrelated donor (URD), alternative sources of hematopoietic components have been used. Umbilical cord blood is a rapidly available hematopoietic progenitor cell source, associated with an increased graft-versus-malignancy effect, with lower incidence of relapse, and with low rates of chronic graft-versus-host disease (GVHD) [1,2,3,4,5,6]. Widespread adoption in adults has been limited by the low cell dose infused that can result in delayed engraftment and poor immune cell reconstitution, with early posttransplant morbidity and mortality. We have circumvented this by using haplo-cord transplants, combining a lower dose single umbilical cord blood unit (CBU) and third-party CD34+-selected cells from a haploidentical-related donor, which provides early transient neutrophil recovery, a “myeloid bridge” from the initial haplo engraftment, while awaiting durable CBU engraftment [7,8,9,10,11]. We have recently shown how this approach results in faster neutrophil recovery with lower rates of chronic GVHD than the more widely used haploidentical transplantation with posttransplant cyclophosphamide [8]. Over the years we have encountered patients lacking a suitable haploidentical 1st or 2nd degree relative for stem cell donation. For such patients we have utilized partially matched URDs from the donor registry as third-party donors. Here we report the outcomes, particularly the incidence of cord graft engraftment, patterns of chimerism, and incidence of GVHD, for the patients undergoing cord transplant supplemented by CD34+-selected partially matched URD grafts. We labeled such transplants “unrelated haplo-cord,” which though semantically incorrect, evokes the similarity to true haplo-cord transplants.
Patients and methods
Patients referred for transplant and who lacked HLA-identical sibling donors (12/12) or fully matched (12/12)/permissively mismatched (10/12 or 11/12 DP permissive) URDs, were offered participation in studies of haplo-cord transplantation. To this purpose we encouraged typing of first- and second-degree family members. For patients lacking suitable haploidentical-related donors, or who had excessive levels of donor-specific anti-HLA antibodies (DSA) against such donors, we identified unrelated haploidentical donors in the donor registry. Such donors were matched at no more than 5 or 6 HLA alleles in HLA-A, -B, -C, or -DQ and in no less than 4/8 alleles. Most patients were treated on a prospective study of reduced intensity conditioning and haplo-cord transplantation (NCT01810588). For five patients, CBUs were obtained through NCT01351545, a multicenter access and distribution protocol for unlicensed cryopreserved CBUs. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Weill Cornell Medical College. All patients provided written informed consent.
CBUs were selected based on HLA typing and cell count. We utilized high-resolution HLA typing for HLA-A, -B, -C, and -DR for cord graft selection [7]. In contrast to common practice, we prioritized matching (at least four of eight HLA loci by the standard criteria) over cell dose and established a minimum cell count of 1.2 × 107 total nucleated cells (TNC)/kg of the recipient’s body weight. After collection, partially matched URD grafts underwent CD34+ cell enrichment and T-cell depletion using the Miltenyi CliniMACS device under an Investigational New Device permit from the United States Food and Drug Administration (IND 15912). The infused cell dose of the adult donor graft was fixed at a minimum of 3 × 106 CD34+ cells/kg and a maximum of 5 × 106 CD34+ cells/kg of recipient weight. Adult donor cells were infused on day 0, and CBU units were infused either later on the same day or on the following day.
Definitions and study endpoints
Neutrophil recovery was defined as the first of three consecutive days with an absolute neutrophil count of at least 500/μL, and platelet recovery was defined as the first of seven consecutive days with platelet count of at least 20,000/μL without receiving platelet transfusion [12].
CBU engraftment was the primary endpoint, defined as umbilical cord chimerism >5% at day 60 in the myeloid and lymphoid compartments irrespective of hematopoietic function [7]. Patients who died before day 60 were excluded from the primary CBU engraftment analysis because cord engraftment may be either lost or gained at time points before day 60. Secondary outcomes included neutrophil recovery by day 30; platelet recovery by day 60; primary CBU graft failure, with CBU chimerism of <5% in the myeloid and lymphoid compartment by day 60; secondary CBU failure defined as loss of CBU chimerism (<5%) in both CD3 and CD33 compartments without evidence of relapse at the time of analysis; cumulative incidences of acute and chronic GVHD; cumulative incidences of relapse and NRM; progression-free survival (PFS); and overall survival (OS).
Statistical analysis
Patient and disease characteristics were tabulated. Statistical analysis used to determine association between CBU engraftment and predictor variables were the Fisher’s exact test for dichotomous and Kruskal–Wallis for continuous predictor variables. The limited number of events precluded multivariable models. The cumulative incidences of neutrophil and platelet engraftment were estimated at 30 days and 60 days, respectively. Donor chimerism was evaluated at day 30, day 60, day 180, and 1 year. The cumulative incidences of relapse and NRM were analyzed together in a competing risk framework [13]. The probability of developing acute GVHD or chronic GVHD was calculated using the cumulative incidence function, with death or relapse, as competing risks. For neutrophil engraftment and platelet engraftment, the competing events were graft loss, relapse, and death before any of these events. PFS, OS, and GVHD-free/relapse-free survival (GRFS) were estimated by the Kaplan–Meier method. Events for GRFS included grade 3–4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death in the first post-HCT year. P values reported reflect 2-sided tests with an alpha of 0.05 considered significant. All estimates are reported with 95% confidence intervals (95% CI). Statistical analyses were performed using Stata v13.0 and R Studio.
Results
Between December 2014 and June 2018, 126 patients with hematological malignancies were candidates for haplo-cord transplant. Twenty-six patients (21%) had no suitable haploidentical relative and partially matched URDs were selected. The most common reasons were: no first or second degree partially matched related donors or unavailable relative (n = 23), high titers of DSA against all relatives (n = 1) and evidence of a familial hematological condition (n = 2). The latter included one patient with primary myelofibrosis who had two siblings with the disease and one young patient with myelodysplastic syndrome (MDS) who had a matched sibling with pancytopenia and evidence of EZH2 deletion on myeloid molecular panel.
Patient characteristics are summarized in Table 1. There were eighteen patients with acute myeloid leukemia (AML)/MDS including one with post primary myelofibrosis (MF)-AML and one patient with MDS and chronic phase chronic myeloid leukemia (CML), five with acute lymphoblastic leukemia (ALL), and one each with primary MF, non-Hodgkin lymphoma (NHL), and plasma cell leukemia (PCL). For two patients, this was the second allo-HCT after graft failure from previous double cord or related haplo-cord. Median age was 57 (range 24–75), with 7 (27%) older than 60. CIBMTR Disease Risk Index (DRI) was intermediate in 46%, high in 38%, and very high in 8%, and HCT-CI was 0–2 in 31%, 3–5 in 35%, and >5 in 35% patients. Twenty-four patients (92%) were conditioned with Fludarabine and Melphalan, 16 of them with additional low-dose total body irradiation (TBI) (400 cGy in 15 and 200 cGy in one). One ALL patient was conditioned with high-dose etoposide and TBI 1200 cGray and one AML patient with fludarabine/cyclophosphamide for second transplant. All patients received ATG 4.5 mg/kg except for one patient with aplastic anemia who had previously received ATG and therefore received alemtuzumab (60 mg). All received posttransplant tacrolimus and mycophenolate mofetil as previously reported [11]. HLA matching for URDs was 9/12 (4%), 8/12 (31%), 7/12 (35%), 6/12 (27%), and 4/12 (4%). For URDs, median CD34+ collected cell dose was 5 (range 3.03–6.06) × 106/kg. Infusion was limited to a maximum of 5 × 106/kg. CBU HLA matching was 8/8 (4%), 7/8 (27%), 6/8 (31%), 5/8 (23%), or 4/8 (15%). For CBU, median cells collected was 2.1 (range 1.1–4.1) × 107 TNC/kg and the median CD34+ cell dose was 0.6 (range 0.1–1.9) × 105/kg. No patients had DSA against the unrelated adult graft and two patients had DSA against CBU, peak 2016 MFI, and 2853 MFI. The latter was treated with desensitization protocol with bortezomib and velcade [14]. At the time of infusion DSA were <2000 MFI for both patients.
Count recovery, chimerism, and graft failure
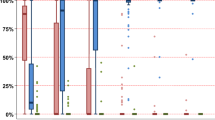
Median time to neutrophil recovery was 11 days (range 9–35). The cumulative incidence of neutrophil recovery was 96% (95% CI 88–100%) at day 30 (Fig. 1a). The median time to platelet recovery was 18 days (range 11–124); three patients died prior to platelet recovery. At day 30 and day 60, the cumulative incidence of platelet recovery was 65% (95% CI 47–83%) and 81% (95% CI 65–96%) (Fig. 1b).
Myeloid (CD33) and lymphoid (CD3) compartment chimerism results at day +60, +100, +180, and +365 are presented in Fig. 2. At day +60, for 24 assessable patients, myeloid chimerism patterns were as follows; 13 patients (54%) had mixed URD-cord chimerism, 4 patients (17%) had 100% cord donor chimerism, 4 patients (17%) remained with 100% adult URD chimerism, and 3 patients (12%) had mixed recipient chimerism. For lymphoid chimerism, 8 patients (54%) had mixed URD-cord chimerism, 7 patients (29%) had 100% cord chimerism, 1 patient (4%) remained with 100% URD chimerism, 7 patients (12%) had mixed recipient chimerism, and for one patient CD3 chimerism failed.
By day 100 for 20 evaluable patients, CD33 and CD3 chimerism showed increasing cord dominance. For the myeloid component: eight patients (40%) with mixed URD-cord chimerism, seven patients (35%) with 100% cord donor chimerism, two patients (10%) remained with 100% URD chimerism, and three patients (15%) had mixed recipient chimerism. For lymphoid chimerism, two patients (10%) with mixed URD-cord chimerism, ten patients (50%) with 100% cord donor chimerism, seven patients (35%) had mixed recipient chimerism, and for one patient, CD3 chimerism failed. By day 180 for 15 evaluable patients; 7 patients (50%) had 100% cord chimerism in the myeloid and lymphoid compartment.
Primary CBU graft failure, with cord donor chimerism of <5% in the myeloid and lymphoid compartment by day 60, occurred in 4 out of 24 assessable patients (17%), three with AML and one with aplastic anemia.
Predictors of CBU engraftment at day 60
We were unable to identify significant predictors for CBU engraftment at day 60, although small numbers limited the power of this analysis. Table 2 lists individual HLA matching, cell dose of the CBU and level of DSA against the CBU. Fourteen of fifteen patients receiving TBI-based conditioning had CBU engraftment vs. six of nine patients receiving chemotherapy only (p = 0.13). Engraftment of better matched CBU (≥6/8) was similar to that of less well-matched ones (p = 0.85). CBU cell dose by TNC and CD34+ cells was similar for those with CBU engraftment and for patients with CBU graft failure [1.96 × 107 TNC/kg vs. 2.51 × 107 TNC/kg (p = 0.12), and 0.6 × 106/kg vs. 0.4 × 106/kg (p = 0.57), respectively]. None of the four patients with CBU graft failure at day 60 had DSA against the CBU and both patients with DSA against the CBU had adequate cord engraftment.
Acute and chronic graft-versus-host disease
The estimated cumulative incidence of grade 2–4 acute GVHD was 27% (95% CI 9–45%) at day +100 and 31% (95% CI 13–49%) at day +180 (Fig. 1c). Grade 3 acute GVHD occurred in five patients: four with stage II–III GI involvement and one patient with stage III liver involvement. There was one patient with stage-IV lower GI GVHD. The estimated cumulative incidence of chronic GVHD was 4% (95% CI 0–12%) at 1 year and 8% (95% CI 0–20%) at 2 years (Fig. 1d).
Non-relapse morbidity, mortality, relapse, GRFS, and survival
Table 2 details characteristics and outcomes of individual patients. Of the four patients with primary graft failure, two subsequently relapsed. One died from his disease and the second is still alive receiving ruxolitinib for MPN. The latter patient had delayed detection of the cord blood graft after day 180. Over time the cord blood graft accounted for up to 50% of CD33 cells and 7% of CD3 cells, and it has persisted at the time of last follow-up, more than 4 years after transplant. One patient died from infectious complications and one patient with AML had autologous hematopoietic reconstitution and is still alive and in remission more than 5 years after transplant. There were no cases of secondary CBU graft failure.
The cumulative incidence of NRM was 15% (95% CI 0–31%) at day +100 and 38% (95% CI 20–56%) by 1 year. Most non-relapse deaths by 1 year were due to infections including CMV (1) adenovirus (2), EBV-related PTLD (1), and fungal infection (1). One was attributed to CBU graft failure and one to early intracranial hemorrhage.
The cumulative incidence of relapse was 23% at 1 year (95% CI 5–41%). The patients who relapsed within 1 year included one patient each with PCL (high DRI); NHL (high DRI), MDS with concomitant CML, post MF-AML (high DRI), and three AML patients [two high and one intermediate DRI], for one of whom, this was the second transplant.
With a median follow of 29 months (range 11–53), the estimated PFS and OS was 38% (95% CI 20–53%) and 54% (95% CI 33–71%), respectively at 1 year. The 1-year GRFS was 28% (95% CI 13–46%).
Discussion
Advances in transplant methodologies have resulted in steady improvements in outcome, particularly in the area of alternative donor transplantation [6]. We have used haploidentical donors as donors of third-party CD34+-selected cells to support engraftment of single unit CBU grafts resulting in rapid count recovery and low rates of chronic GVHD [7,8,9,10]. This method has relied on the use of haploidentical family members as donors of third-party adult stem cells. Here we confirm the observation that up to 17% of patients—because of family structure (smaller families, older patients, international patients, etc.) [15, 16], high titers of donor-directed HLA antibodies [14, 17], or familial hematological disorders with underlying germline predisposition [18]—lack adequate haploidentical-related donors and would thus also not be candidates for the widely used haploidentical transplantation with a posttransplant cyclophosphamide platform.
For these patients, we used a CD34+ cell-enriched T-cell-depleted graft from a partially matched adult URD to support the engraftment of a CBU graft. This strategy has also been used by Spanish centers for haplo-cord transplantation using alternative conditioning with fludarabine, cyclophosphamide, IV Busulfan, or 10 Gy TBI, with rabbit ATG [19, 20]. Most patients achieved very early neutrophil recovery with a median time of 11 days and 96% attaining neutrophil recovery by day 13. This compares favorably with other CBU approaches [21,22,23]. Patterns of chimerism were also similar to haplo-cord transplant using family donors and in the majority of patients, early dominance of the adult graft was ultimately replaced by persistence of the umbilical cord blood graft [10, 24]. CBU engraftment, defined as more than 5% CBU chimerism in the CD3 and CD33 compartments at day +60, occurred in 20 out of 24 (83%). The rate of CBU graft failure of 17% was similar to our previously reported rate of CBU graft failure for haplo-cord transplant using family donors of 15% [7] and to other platforms of CBU transplant with different conditioning intensities and without use of ATG, where it ranges from 10 to 20% [25, 26]. Lastly, rate of acute and chronic GVHD were low and similar to those observed after haplo-cord transplant. In our analysis, CBU HLA matching, CBU cell dose, and intensity of the conditioning with additional TBI were not predictors for CBU engraftment.
Based on the limitations of our report, mainly the small number of high-risk patients with significant heterogeneity, our results can only be hypothesis generating and this strategy needs to undergo further validation and optimization, but it is reassuring that 1-year GRFS of 28% (95% CI 13–46%), is similar to 31% (95% CI 27–35%) at the University of Minnesota over a 12-year period for recipients of single and double UCB transplants [27]. In a previous comparison of haplo-cord transplants vs. double UCB grafts reported to CIBMTR, haplo-cord transplants had better engraftment, lower incidence of GVHD, improved relapse-free survival, and better GRFS (38% with haplo cords vs. 21% with double cords) [28]. OS, however, was the same and suggests different long-term complications. In the current study, the main cause of non-relapse mortality was late infections occurring after myeloid engraftment and the risk might have been exacerbated by ATG [29,30,31]. Preliminary analysis of immune reconstitution patterns after haplo-cord transplants using ATG show early normalization of B- and NK-lymphocytes, but a delay T-cell immune recovery that might affect incidence of viral reactivations and incidence of relapse [32, 33]. Minimization and/or replacement of ATG is currently being investigated as a means to further improve outcomes.
Conclusion
Haplo-cord transplant using CD34+-selected partially matched URD grafts resulted in rapid engraftment. Transition to durable CBU grafts occurred in the majority of cases, and the rate of CBU engraftment was 84%. This is similar to our experience using CD34-selected cells from related haploidentical donors as a myeloid bridge. The rates of acute GVHD were acceptable, and incidence of chronic GVHD was very low. Further studies should aim at decreasing non-relapse mortality and infections complications.
References
Barker JN, Kurtzberg J, Ballen K, Boo M, Brunstein C, Cutler C, et al. Optimal practices in unrelated donor cord blood transplantation for hematologic malignancies. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2017;23:882–96. https://doi.org/10.1016/j.bbmt.2017.03.006.
Gutman JA, Ross K, Smith C, Myint H, Lee CK, Salit R, et al. Chronic graft versus host disease burden and late transplant complications are lower following adult double cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transplant. 2016;51:1588–93. https://doi.org/10.1038/bmt.2016.186.
Choe HK, van Besien K. Against the odds: haplo-cord grafts protect from GvHD and relapse. Bone Marrow Transplant. 2017;52:1590–1. https://doi.org/10.1038/bmt.2017.102.
Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–900. https://doi.org/10.1038/leu.2015.98.
Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N. Engl J Med. 2016;375:944–53. https://doi.org/10.1056/NEJMoa1602074.
Shouval R, Fein JA, Labopin M, Kroger N, Duarte RF, Bader P, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6:e573–84. https://doi.org/10.1016/S2352-3026(19)30158-9.
Tsai SB, Liu H, Shore T, Fan Y, Bishop M, Cushing MM, et al. Frequency and risk factors associated with cord graft failure after transplant with single-unit umbilical cord cells supplemented by haploidentical cells with reduced-intensity conditioning. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016;22:1065–72. https://doi.org/10.1016/j.bbmt.2016.02.010.
van Besien K, Artz A, Champlin RE, Guarneri D, Bishop MR, Chen J, et al. Haploidentical vs haplo-cord transplant in adults under 60 years receiving fludarabine and melphalan conditioning. Blood Adv. 2019;3:1858–67. https://doi.org/10.1182/bloodadvances.2019000200
van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H, et al. Haplo-cord transplant: HLA-matching determines graft dominance. Leuk lymphoma. 2017;58:1512–4. https://doi.org/10.1080/10428194.2016.1248964.
van Besien K, Hari P, Zhang MJ, Liu HT, Stock W, Godley L, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016;101:634–43. https://doi.org/10.3324/haematol.2015.138594.
Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–45. https://doi.org/10.1182/blood-2011-08-372508.
CIBMTR. Instructions for post-transplant essential data (Post-TED) form. CIBMTR; 2012.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. https://doi.org/10.1080/01621459.1999.10474144.
Choe H, Gergis U, Hsu J, Phillips A, Shore T, Christos P, et al. Bortezomib and immune globulin have limited effects on donor-specific HLA antibodies in haploidentical cord blood stem cell transplantation: detrimental effect of persistent haploidentical donor-specific HLA antibodies. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2019;25:e60–4. https://doi.org/10.1016/j.bbmt.2018.10.018.
Canaani J, Savani BN, Labopin M, Huang XJ, Ciceri F, Arcese W, et al. Donor age determines outcome in acute leukemia patients over 40 undergoing haploidentical hematopoietic cell transplantation. Am J Hematol. 2018;93:246–53. https://doi.org/10.1002/ajh.24963.
Kosuri S, Wolff T, Devlin SM, Byam C, Mazis CM, Naputo K, et al. Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: practical implications for donor selection. Biol Blood Marrow Transplant. 2017;23:965–70. https://doi.org/10.1016/j.bbmt.2017.03.001.
Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19:647–52. https://doi.org/10.1016/j.bbmt.2013.01.016.
Trottier AM, Bannon S, Bashir Q, Carraway HE, Hofmann I, Godley LA. When should transplant physicians think about familial blood cancers? Adv Cell Gene Ther. 2019;2:e68. https://doi.org/10.1002/acg2.68.
Sanz J, Kwon M, Bautista G, Sanz MA, Balsalobre P, Piñana JL, et al. Single umbilical cord blood with or without CD34(+) cells from a third-party donor in adults with leukemia. Blood Adv. 2017;1:1047–55. https://doi.org/10.1182/bloodadvances.2017006999.
Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2014;20:2015–22. https://doi.org/10.1016/j.bbmt.2014.08.024.
Ballen KK, Logan BR, Chitphakdithai P, Spellman S, Adams AJ, Drexler RJ, et al. Excellent outcomes in 1589 patients receiving umbilical cord blood transplantation using unlicensed units from a centralized cord blood registry. Biol Blood Marrow Transplant. 2017;23:S170. https://doi.org/10.1016/j.bbmt.2016.12.282.
Barker JN, Fei M, Karanes C, Horwitz M, Devine S, Kindwall-Keller TL, et al. Results of a prospective multicentre myeloablative double-unit cord blood transplantation trial in adult patients with acute leukaemia and myelodysplasia. Br J Haematol. 2015;168:405–12. https://doi.org/10.1111/bjh.13136.
Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19:799–803. https://doi.org/10.1016/j.bbmt.2013.02.007.
van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H, et al. Cord blood chimerism and relapse after haplo-cord transplantation. Leuk Lymphoma. 2017;58:288–97. https://doi.org/10.1080/10428194.2016.1190970.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8. https://doi.org/10.1182/blood-2011-03-344853.
Kwon M, Martinez-Laperche C, Balsalobre P, Serrano D, Anguita J, Gayoso J, et al. Early peripheral blood and T-cell chimerism dynamics after umbilical cord blood transplantation supported with haploidentical cells. Bone Marrow Transplant. 2014;49:212–8. https://doi.org/10.1038/bmt.2013.177.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8. https://doi.org/10.1182/blood-2014-10-609032.
van Besien K, Hari P, Zhang M-J, Liu H-T, Stock W, Godley L, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016;101:634. https://doi.org/10.3324/haematol.2015.138594.
Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–51. https://doi.org/10.1182/blood-2007-05-092130.
Martin-Donaire T, Rico M, Bautista G, Gonzalo-Daganzo R, Regidor C, Ojeda E, et al. Immune reconstitution after cord blood transplants supported by coinfusion of mobilized hematopoietic stem cells from a third party donor. Bone Marrow Transplant. 2009;44:213–25. https://doi.org/10.1038/bmt.2009.15.
Castillo N, Garcia-Cadenas I, Barba P, Canals C, Diaz-Heredia C, Martino R, et al. Early and long-term impaired T lymphocyte immune reconstitution after cord blood transplantation with antithymocyte globulin. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2017;23:491–7. https://doi.org/10.1016/j.bbmt.2016.11.014.
Orfali N, Mokhtar AE, Guarneri D, Hsu J, Phillips A, Gergis U, et al. Seven years of haplo-cord transplantation: immune reconstitution and outcomes using anti-thymocyte globulin. Biol Blood Marrow Transplant. 2020;26:S309. https://doi.org/10.1016/j.bbmt.2019.12.410.
Jain N, Liu H, Artz AS, Anastasi J, Odenike O, Godley LA, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54:1242–9. https://doi.org/10.3109/10428194.2012.739688.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KvB has the following relevant financial relationship(s) to disclose: unrestricted grant from Miltenyi Biotec. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomez-Arteaga, A., Orfali, N., Guarneri, D. et al. Cord blood transplants supported by unrelated donor CD34+ progenitor cells. Bone Marrow Transplant 55, 2298–2307 (2020). https://doi.org/10.1038/s41409-020-0959-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0959-5
- Springer Nature Limited
This article is cited by
-
Haploidentical hematopoietic cell transplantation with or without an unrelated cord blood unit for adult acute myeloid leukemia: a multicenter, randomized, open-label, phase 3 trial
Signal Transduction and Targeted Therapy (2024)