Abstract
Epstein–Barr virus (EBV) reactivation after allogeneic hematopoietic cell transplantation (allo-HCT) is one of the major concerns that may lead to fatal EBV diseases. However, updated data are needed because of the remarkable evolution of the HCT protocol and donor selection. We conducted a retrospective study that enrolled 890 allo-HCT recipients. Independent risk factors for EBV reactivation were use of antithymocyte globulin, haploidentical donor, and the presence of chronic graft-versus-host disease. The cumulative incidence of EBV reactivation was 2.9%, 11.7%, 27.3%, and 41.9% for patients with 0, 1, 2, and 3 risk factors, respectively (P < 0.001). Posttransplant lymphoproliferative disorders (PTLDs) occurred in seven patients. EBV reactivation was associated with inferior survival in recipients who survived more than 2 years post-HCT (P < 0.001) but might time-dependently benefit those patients with malignancies by decreasing relapse incidence (P = 0.046). A decreased relapse incidence was observed 1 year after HCT for recipients at first or second remission (P = 0.042) and in the first year post-HCT for recipients with advanced diseases (P = 0.032). We concluded that with current management, PTLDs were efficiently controlled, but EBV reactivation still had a multifactorial impact on transplant outcomes. Multicenter prospective studies are warranted to validate these findings.
Similar content being viewed by others
Introduction
Epstein–Barr virus (EBV) is a highly immunogenic latent γ-herpesvirus that has infected >90% of humans worldwide [1, 2]. Regulated by EBV-specific T cells, it can set up an asymptomatic infection for a lifetime in immunocompetent individuals [3]. However, under the immunocompromised circumstances created by hematopoietic cell transplantation (HCT), EBV reactivation is a frequent complication that may lead to uncontrolled B-cell proliferation and result in EBV-related posttransplant lymphoproliferative disorders (PTLDs) and other EBV diseases [1, 4,5,6,7,8]. The reported incidence of EBV reactivation post-HCT ranges from 0.1 to 63% depending on transplant type, antiviral agents, monitoring protocol, and assay sensitivity [9]. The overall incidence of EBV-PTLD varies from 1.2 to 12.9% among different studies [10,11,12], with a high mortality of up to 50–80% [13, 14] and over 90% for advanced patients [15, 16].
With the development of transplant protocols and post-HCT supportive care, the management of EBV reactivation and EBV diseases has markedly improved. In particular, preemptive treatment with rituximab has been widely adopted for the prophylaxis of PTLD [17, 18]. Meanwhile, the increasing number of transplants employing haploidentical donors and antithymocyte globulin (ATG) may potentially increase the risk of EBV reactivation [1]. However, few data have been reported recently describing the current prevalence and features of EBV reactivation post-HCT. Here, we conducted a retrospective study including 890 recipients who underwent allogeneic HCT (allo-HCT) to update the current prevalence, risk factors, and impact on outcomes of EBV reactivation.
Materials and methods
Patients
This was a retrospective study based on data derived from the transplant database in our center, which was established according to the European Society for Blood and Marrow Transplantation registry. The inclusion criteria were as follows: (1) patients who underwent allo-HCT in our center between July 2011 and July 2014 and (2) patients who received regular EBV management after HCT based on an institutional protocol. The study was approved by the Ethics Committee of our center and conducted in accordance with the Helsinki Declaration.
Donor selection, stem cell source, and transplant protocols
The algorithm of donor selection was based on HLA typing, age, donor sex, and ABO compatibility [19]. The preferred donor was an HLA-matched sibling. In the absence of a matched donor, a haploidentical donor could be the prioritized alternative option [20]. Donors were recommended to contribute a bone marrow graft, complemented with peripheral blood stem cells if the CD34+ cell dose failed to achieve the target dose of 2 × 106/kg of recipient body weight. The majority of patients received myeloablative conditioning, including the modified Bu/Cy regimen and the modified TBI/Cy regimen. Patients who were intolerant to intensive chemotherapy received a reduced intensity conditioning (RIC) regimen based on fludarabine, low-dose busulfan, cytarabine or cyclophosphamide according to the primary disease [21].
Management of graft-versus-host disease (GVHD)
The prophylaxis of GVHD included cyclosporin A (CsA) and short-term methotrexate for HLA-matched sibling donor HCT, and mycophenolate mofetil (MMF) combined with ATG (Genzyme, MA, USA) [22] was added for patients receiving grafts from unrelated or haploidentical donors. The diagnosis of acute and chronic GVHD was made according to reference literature [23, 24]. Methylprednisolone at a dose of 1–2 mg/kg/day was given immediately as the first-line treatment in case of overt acute GVHD occurrence. The second-line drugs included tacrolimus, anti-CD25 monoclonal antibody, MMF, and ATG, etc. The first-line treatment of overt chronic GVHD was steroids and/or CsA.
Management of EBV reactivation
Q-PCR was applied to monitor EBV-DNA load in whole peripheral blood weekly from conditioning to +90 days post-HCT in all patients and once every 2 weeks from +90 days until +80 days. Additional detection was performed if symptoms of suspected virus infection were present. Ganciclovir at a dose of 10 mg/kg/day was used from −9 to −2 days to prevent virus infection and then replaced by acyclovir to avoid marrow toxicity. The treatment for EBV-reactivated recipients included tapering of immunosuppressive agents, ganciclovir, foscarnet sodium, and preemptive therapy with rituximab. Preemptive rituximab was prescribed if EBV-DNA reached 105 copies/mL or 104 copies/mL for 2 consecutive weeks.
Definition
EBV reactivation was defined as more than 102 copies/mL EBV-DNA in whole blood by Q-PCR. Person-years at risk were calculated from the date of transplantation to the date of death, last follow-up, or study end, whichever occurred first. The pattern of EBV reactivation occurrence by post-HCT intervals was evaluated by calculating EBV reactivation incidence rates, defined as the number of EBV reactivation cases divided by the number of person-years in each interval. The diagnosis of disease recurrence was based on clinical and pathological criteria. The survival time was calculated starting on the day of transplantation. Overall survival (OS) was calculated with the date of death, last follow-up, or study end, whichever occurred first, as the final date. If the patient was in remission, progression-free survival (PFS) was calculated with the date of death, recurrence, or last follow-up as the final date. Deaths unrelated to the underlying disease were recorded as treatment-related mortality (TRM).
Statistics
Differences in EBV-positive incidence rates among different donor–recipient relationships were compared with independent sample Kruskal–Wallis test. Risk analyses for EBV reactivation were conducted by the Cox regression model, and all risk factors whose P values were below 0.1 in univariate analyses were included in multivariate analyses. OS and PFS were calculated using the Kaplan–Meier method and compared with the log-rank test. The cumulative incidence of relapse (CIR) was calculated by a competing risk model with TRM as a competing risk factor. All P values were two-sided and were defined as statistically significant if <0.05. Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA) and R 3.6.1 software package (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 890 patients were included in this analysis according to the inclusion criteria. The characteristics of recipients with or without EBV reactivation are displayed in Table 1. The median age was 32 (range, 2–63) years old, and 528 patients were male. There were 212 cases of acute lymphoblastic leukemia, 378 of acute myeloid leukemia, 77 of chronic myeloid leukemia, 87 of severe aplastic anemia, 76 of myelodysplastic syndrome, 55 of lymphoma, and 5 of myelofibrosis. Most of the recipients received myeloablative conditioning, and 29.3% received a graft from a haploidentical donor.
Incidence of EBV reactivation and PTLD
One hundred and seventy-five recipients developed EBV reactivation (Fig. 1a), with a median time of 57 (range, 18–1006) days after HCT, and most of these patients (129) developed EBV reactivation within the first 100 days. The incidence of EBV reactivation peaked at 1–2 months after transplantation and then plummeted sharply, except for in haploidentical HCT recipients, in which the incidence of EBV reactivation declined moderately (Fig. 1b). EBV reactivation remarkably decreased 1 year after transplantation and rarely occurred after 2 years (Table 2). The cumulative incidence of EBV reactivation was 18.2% for the first year, 19.3% for the first 2 years, and 19.6% for the first 3 years. There were statistically significant differences in the rate of EBV positivity over time among different donor–recipient relationships (P = 0.005) (Fig. 1b), and the highest incidence in the first year was observed in haploidentical HCT recipients. By the end of follow-up, seven patients developed PTLD.
Risk factor analyses for EBV positivity after HCT
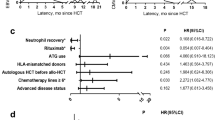
In the univariate analysis, five factors significantly associated with EBV reactivation after HCT were identified, including haploidentical HLA match (P < 0.001), ATG as GVHD prophylaxis (P < 0.001), age younger than 30 years (P = 0.010), and the development of chronic GVHD (P = 0.023). Moreover, grade II–IV acute GVHD had marginal significance (P = 0.078, HR = 1.336, 95% CI: 0.968–1.845) (Table 1), which met the inclusion criteria for the multivariate analysis. The multivariate analysis revealed three independent risk factors for EBV reactivation after HCT (Fig. 2), including ATG as GVHD prophylaxis (P < 0.001), HLA-mismatched donor (P = 0.001) and appearance of chronic GVHD (P = 0.042). The cumulative incidence of EBV reactivation was low (2.9%) among patients with no risk factor but increased to 11.7%, 27.3%, and 41.9% for those with 1, 2, and 3 risk factors, respectively (P < 0.001) (Fig. 3).
Impact of EBV reactivation on transplant outcomes
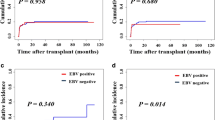
With a median follow-up of 36 months (range, 0–94 months), the estimated 2-year OS was comparable between groups with or without EBV reactivation (60.5% ± 1.9% versus 72.6% ± 3.4%, P = 0.887) (Fig. 4). Meanwhile, there was no statistical difference in PFS (P = 0.905), TRM (P = 0.385), or CIR (P = 0.399) between the two groups. However, it seemed that EBV reactivation had a late-onset impact for recipients who survived more than 2 years. The TRM of EBV-reactivated recipients was significantly higher than that of the EBV-negative group (5-year TRM: 8.9% ± 0.8% versus 3.7% ± 0.1%, P = 0.003), resulting in decreased PFS (5-year PFS: 72.0% ± 6.1% versus 84.4% ± 2.0%, P = 0.035) and OS (5-year OS: 75.9% ± 6.0% versus 91.7% ± 1.6%, P < 0.001) despite a similar CIR (P = 0.818) (Fig. 5). The main causes of death in the EBV-reactivated cohort included relapse (n = 38), severe infection (n = 21), GVHD (n = 11), hemorrhage events (n = 2), and PTLD (n = 1). In EBV-negative recipients, the main causes of death consisted of relapse (n = 151), severe infection (n = 62), GVHD (n = 40), hemorrhage events (n = 5), disseminated intravascular coagulation (n = 1), thrombotic microangiopathy (n = 3), thrombotic thrombocytopenic purpura (n = 1), acute pancreatitis (n = 1), and pulmonary fibrosis (n = 1). There was no statistical difference in causes between the two groups of patients (P = 0.102).
For patients with malignant diseases consisting of acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome, and lymphoma, we found that CIR was significantly reduced in the EBV-reactivated cohort compared with the EBV-negative group (5-year CIR: 16.3% ± 0.1% versus 24.8% ± 0.04%, P = 0.046). In particular, none of the recipients in the EBV-reactivated group relapsed beyond 2 years after HCT, while 4.12% of patients in the EBV-negative group relapsed beyond 2 years after HCT (P = 0.041). For recipients with stable disease (within the 1st or 2nd remission), the CIR dramatically decreased in the EBV-reactivated cohort after 1 year post-HCT (2.7% ± 0.04% versus 11.2% ± 0.04%, P = 0.042) (Fig. 6a), although OS and PFS were not improved. Conversely, for recipients with advanced disease (in progression or at or beyond the 3rd remission), the benefit of EBV reactivation on CIR was observed in the first year post-HCT (14.2% ± 0.4% versus 31.1% ± 0.1%, P = 0.032) (Fig. 6b), leading to superior 1-year OS (68.4% ± 7.5% versus 51.5% ± 4.0%, P = 0.042) as well as PFS (60.5% ± 7.9% versus 45.0% ± 4.0%, P = 0.047) within 1 year after HCT compared with the respective values seen in EBV-negative patients.
Discussion
As a result of the immunocompromised circumstances created under HCT, EBV reactivation is a frequently reported complication posttransplantation that inhibits B-cell apoptosis and induces viral oncogene expression and genetic and epigenetic alterations that lead to B-lymphocyte transformation [8, 25,26,27,28,29,30]. EBV reactivation may be asymptomatic initially but could lead to a series of EBV-related diseases, including pneumonitis, enteritis, and ophthalmitis, without intervention. In addition, EBV reactivation presents in 60–80% of PTLD patients [27, 28], which is usually a fatal malignant complication [5].
Given the inferior outcome caused by EBV reactivation, a large number of studies have explored its risk factors [1, 5, 7, 13, 16, 31] and management strategies, particularly Q-PCR monitoring and preemptive treatment with rituximab [9, 16]. Pooled results from published studies in HSCT recipients suggest that administration of rituximab results in a positive outcome for ~90% of patients treated preemptively and 65% of patients with EBV-PTLD [10, 11, 31,32,33,34,35,36,37]. However, as a validated risk factor, haploidentical HCT has become the preferred alternative option for patients who lack a matched donor and currently accounts for >50% of allogeneic transplants in China [38]. In recent decades, the increasing use of haploidentical transplantation procedures [38,39,40], particularly the Beijing protocol, which employs ATG as GVHD prophylaxis [38, 41], has greatly increased the risk of EBV reactivation. Given the unknown data on EBV reactivation post-HCT in the current treatment environment, studies to update these data are warranted.
Despite the evolution of HCT protocols and antiviral therapies, independent risk factors for EBV reactivation seem to have remained constant, as identified in our study, including use of ATG, grafts from haploidentical donors, and appearance of chronic GVHD [12, 42,43,44,45]. To determine the cumulative impact of these independent risk factors, a risk factor evaluation model was created to compare the incidence of EBV reactivation according to the number of risk factors patients had. As a result, the cumulative incidence of EBV reactivation depended on the number of risk factors involved, which was in line with the research of Uhlin et al. [7]. ATG use is a well-recognized risk factor [12, 43, 44], which was confirmed in our study by both univariate and multivariate analyses. As GVHD prophylaxis, ATG could immunosuppress recipients to pave the way for EBV reactivation by removing T cells from both recipients and donors, which impairs the cellular immune function and/or prolongs the immunosuppressive periods after HCT. The impact of haploidentical donors is usually attributed to unavoidable T-cell depletion in vivo (mainly caused by ATG) or in vitro in various protocols. However, we found that a haploidentical graft was a risk factor independent of ATG, which potentially hinted an alternative pathway that increases the risk of EBV reactivation [46]. GVHD is a profound risk factor resulting from the impairment in specific immune responses due to cytokine storms [42]. Chronic stimulation as well as long-term immunosuppression for treatment may also lead to an increased risk of EBV reactivation [7, 45]. Age is another reported risk factor, and previous studies [1, 47,48,49] indicated that both younger and older (age 50 years or older) patients could have an increased risk of EBV diseases and PTLDs. In our study, age was a risk factor identified in the univariate analysis rather than the multivariate analysis, presumably due to the higher proportion of haploidentical grafts in younger recipients than in older recipients.
With the development of effective virus management strategies and supportive care for HCT in recent years, the outcome of recipients with post-HCT EBV reactivation has obviously improved. Generally, no statistical differences in OS, TRM, PFS, or CIR were found between the groups with or without EBV reactivation, partially in accordance with the findings of Peric et al. [44]. However, controversial data about the impact of EBV reactivation on HCT outcome have also been published. Auger et al. indicated that controlled EBV reactivation in the setting of HCT was associated with superior OS, probably related to a significant increase in circulating NK cells [50]. Intriguingly, a different opinion presented by Li et al. showed that patients with high or very low levels of cell-bound EBV-DNA had a shorter OS than those with moderate EBV load, potentially attributed to the phenomenon of “sneaking through” [51]. According to our data, the impact of EBV reactivation was time-dependent and disease-dependent. We further found that the EBV-reactivated group showed inferior outcomes beyond 2 years after HCT, but CIR decreased in patients with malignancies, starting after 1 year post-HCT for recipients at stable status and immediately after HCT for recipients with progressive disease. A study from Hoegh-Petersen et al. [52] also suggested an improved CIR for EBV-reactivated recipients because of the quick reconstitution of EBV-associated T cells.
It should be noted that the tapering of immunosuppressive agents for EBV-positive patients might also contribute to an enhanced graft-versus-tumor (GVT) effect, although accompanied by an increased risk of GVHD. There are other recognized factors affecting the CIR and the outcomes, particularly the genetic abnormalities in malignancies. Because various entities were included in this study, no stratification system was applicable for all the enrolled patients. Moreover, the risk for patients with or without EBV reactivation was comparable (P = 0.559) when stratified by the NCCN guidelines and had no impact on EBV reactivation in the cohort of our study (P = 0.594).
Nevertheless, the conclusion of our study was restricted by several limitations, including the inherited drawbacks of a single-center retrospective study, the diversity of underlying diseases, deviations in treatments both pre- and post-HCT, disproportionate conditioning regimens, and insufficiency of representativeness. In addition, the impact of biological parameters such as EBV microRNA and immune reconstitution in EBV-reactivated recipients should also be further explored.
In conclusion, our study revealed that with current EBV management, PTLDs were efficiently controlled, although the incidence of EBV reactivation post-HCT remained high in patients with existing risk factors. The majority of EBV reactivation occurred in the first 2 months after transplantation, but haploidentical HCT recipients had longer exposure durations than recipients who received non-haploidentical grafts. The impact of EBV reactivation was multifactorial, depending on the underlying disease and time post-HCT. Our results need to be validated by multicenter prospective studies and further explored to facilitate an optimized EBV management strategy.
References
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001.
Balfour HH Jr, Dunmire SK, Hogquist KA. Infectious mononucleosis. Clin Transl Immunol. 2015;4:e33.
Rayne HR, Chrystal UL, Helen EH. EBV lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol. 2014;21:476–81.
Deeg HJ, Socie G. Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood. 1998;91:1833–44.
Gross TG, Steinbuch M, DeFor T, Shapiro RS, McGlave P, Ramsay NK, et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant. 1999;23:251–8.
Juvonen E, Aalto SM, Tarkkanen J, Volin L, Mattila PS, Knuutila S, et al. High incidence of PTLDs after non-T-cell-depleted allogeneic haematopoietic stem cell transplantation as a consequence of intensive immunosuppressive treatment. Bone Marrow Transplant. 2003;32:97–102.
Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O, et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:346–52.
Cohen JM, Cooper N, Chakrabarti S, Thomson K, Samarasinghe S, Cubitt D, et al. EBV-related disease following haematopoietic stem cell transplantation with reduced intensity conditioning. Leuk Lymphoma. 2007;48:256–69.
Styczynski J, van der VeldenW, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–11.
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802.
Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014;49:397–402.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JAH, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–80.
Sundin M, Le Blanc K, Ringden O, Barkholt L, Omazic B, Lergin C, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91:1059–67.
van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood. 2001;98:972–8.
Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis. 2009;11:383–92.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBVinfections in patientswith hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–70.
Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–8.
Coppoletta S, Tedone E, Galano B, Soracco M, Raiola AM, Lamparelli T, et al. Rituximab treatment for Epstein–Barr virus DNAemia after alternative-donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:901–7.
Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, et al. Donor and recipient age, gender and ABO incompatibility regardless of donor source: validated criteria for donor selection for haematopoietic transplants. Leukemia. 2018;32:492–8.
Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett. 2018;438:63–75.
Chen J, Wang RX, Chen F, Sun AN, Qiu HY, Jin ZM, et al. Combination of a haploidentical SCT with an unrelated cord blood unit: a single-arm prospective study. Bone Marrow Transplant. 2014;49:206–11.
Chen J, Yang L, Fan Y, Xu Y, Han Y, Tang X, et al. Comparison of autologous stem cell transplantation versus haploidentical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant. 2018;24:779–88.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Hirschfeld S, Farrell A, Rizzo JD, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft- versus- host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37–44.
Liu Q, Xuan L, Liu H, Huang F, Zhou H, Fan Z, et al. Molecular monitoring and stepwise preemptive therapy for Epstein-Barr virus viremia after allogeneic stem cell transplantation. Am J Hematol. 2013;88:550–5.
Yoon SO, Yu E, Cho YM, Suh C, Kim KM, Han DJ, et al. Post-transplant lymphoproliferative disorders: clinicopathological analysis of 43 cases in a single center, 1990–2009. Clin Transplant. 2012;26:67–73.
Caillard S, Porcher R, Provot F, Dantal J, Choquet S, Durrbach A, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31:1302–9.
Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–92.
Sanz J, Andreu R. Epstein–Barr virus-associated posttransplant lymphoproliferative disorder after allogeneic stem cell transplantation. Curr Opin Oncol. 2014;26:677–83.
Garcia-Cadenas I, Castillo N, Martino R, Barba P, Esquirol A, Novelli S, et al. Impact of Epstein Barr virus-related complications after high-risk allo-SCT in the era of pre-emptive rituximab. Bone Marrow Transplant. 2015;50:579–84.
Patriarca F, Medeot M, Isola M, Battista ML, Sperotto A, Pipan C, et al. Prognostic factors and outcome of Epstein-Barr virus DNAemia in high-risk recipients of allogeneic stem cell transplantation treated with preemptive rituximab. Transpl Infect Dis. 2013;15:259–67.
van der Velden WJ, Mori T, Stevens WB, de Haan AF, Stelma FF, Blijlevens NM, et al. Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab. Bone Marrow Transplant. 2013;48:1465–71.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M, et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240–5.
Kuriyama T, Kawano N, Yamashita K, Ueda A. Successful treatment of Rituximab-resistant Epstein-Barr virus-associated post-transplant lymphoproliferative disorder using RCHOP. J Clin Exp Hematop. 2014;54:149–53.
Meyer SC, Medinger M, Halter JP, Baldomero H, Hirsch HH, Tzankov A, et al. Heterogeneity in clinical course of EBV-associated lymphoproliferative disorder after allogeneic stem cell transplantation. Hematology. 2014;19:280–5.
Han SB, Bae EY, Lee JW, Jang PS, Lee DG, Chung NG, et al. Features of Epstein-Barr virus reactivation after allogeneic hematopoietic cell transplantation in Korean children living in an area of high seroprevalence against Epstein-Barr virus. Int J Hematol. 2014;100:188–99.
Xu LP, Wu DP, Han MZ, Huang H, Liu QF, Liu DH, et al. A review of hematopoietic cell transplantation in China: data and trends during 2008-16. Bone Marrow Transplant. 2017;52:1512–8.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50:476–82.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811–7.
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124:843–50.
Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multiinstitutional study. Blood. 1999;94:2208–16.
Xuan L, Jiang X, Sun J, Zhang Y, Huang F, Fan Z, et al. Spectrum of Epstein-Barr virus associated diseases in recipients of allogeneic hematopoietic stem cell transplantation. Transplantation. 2013;96:560–6.
Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T, et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25:932–8.
Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transplant. 2011;17:591–7.
Kanda J, Chao NJ, Rizzieri DA. Haploidentical transplantation for leukemia. Curr Oncol Rep. 2010;12:292–301.
Cao XH, Fan ZP, Jiang QL, Zhao J, Yu GP, Wei Q, et al. Risk factors for Epstein-barr virus reactivation after allogeneic hematopoietic stem cells transplantation. J Clin Rehabil Tissue Eng Res. 2011;15:4257–61.
Shimoyama Y, Yamamoto K, Asano N, Oyama T, Kinoshita T, Nakamura S. Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008;99:1085–91.
Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–9.
Auger S, Orsini M, Céballos P, Fegueux N, Kanouni T, Caumes B, et al. Controlled Epstein–Barr virus reactivation after allogeneic transplantation is associated with improved survival. Eur J Haematol. 2014;92:421–8.
Li Q, Rane L, Poiret T, Zou J, Magalhaes I, Ahmed R, et al. Both high and low levels of cellular Epstein-Barr virus DNA in blood identify failure after hematologic stem cell transplantation in conjunction with acute GVHD and type of conditioning. Oncotarget. 2016;7:30230–40.
Hoegh-Petersen M, Sy S, Ugarte-Torres A, Williamson TS, Eliasziw M, Mansoor A, et al. High Epstein-Barr virus-specific T-cell counts are associated with near-zero likelihood of acute myeloid leukemia relapse after hematopoietic cell transplantation. Leukemia. 2012;26:359–62.
Acknowledgements
This work was supported by the National Science Foundation of China (Nos. 81700173 and 81730003), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), National Key R&D Program of China (2016YFC0902800), Innovation Capability Development Project of Jiangsu Province (BM2015004), National Science and Technology Major Project (2017ZX09304021), National Key R&D Program of China (2017YFA0104502), Jiangsu Medical Outstanding Talents Project (JCRCA2016002), and Jiangsu Provincial Key Medical Center (YXZXA2016002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ru, Y., Zhang, X., Song, T. et al. Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant 55, 1754–1762 (2020). https://doi.org/10.1038/s41409-020-0831-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0831-7
- Springer Nature Limited
This article is cited by
-
Evidence of aberrant anti-epstein-barr virus antibody response, though no viral reactivation, in people with post-stroke fatigue
Journal of Inflammation (2024)
-
Early T-cell reconstitution predicts risk of EBV reactivation after allogeneic hematopoietic stem cell transplantation
Clinical and Experimental Medicine (2024)
-
Ruxolitinib versus basiliximab for steroid-refractory acute graft-versus-host disease: a retrospective study
Annals of Hematology (2023)
-
Incidence and impact of Epstein-Barr virus events in the early phase after allogeneic hematopoietic cell transplantation
Annals of Hematology (2021)
-
Epstein–Barr virus and cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation in patients with non–Hodgkin lymphoma: the prevalence and impacts on outcomes
Annals of Hematology (2021)