Abstract
Posttransplant treatment strategies are narrowed by the vulnerability of bone marrow. Building on immune cells with antitumor activity is a growing field in cancer therapy. Thus, transfer of expanded and preactivated immune cells is a promising intensification of treatment in high-risk tumor patients. We tested ex vivo expanded NK-, γδT-, and CIK cells that were generated by coincubation with irradiated K562-mb15-41BBL and Il2 and compared the expansion conditions of PBMCs versus CD3-depleted PBMCs as well as static versus semi-automated expansion. The median fold expansion was significantly higher using PBMCs and static expansion conditions. Expanded cells were preactivated with a CD56brightCD69high immunophenotype exerting excellent direct cellular cytotoxicity as well as ADCC in various tumor entities. We established a large-scale clinical-grade ex vivo expansion and activation protocol of NK-, γδT-, and CIK cells from donor-derived PBMCs of patients after haploidentical HSCT. In a patient with AML, NK/γδT/CIK cell transfer was associated with MRD response. A significant increase of direct antitumor activity and ADCC post cell transfer was documented. The results that we report here provide the rationale for clinical testing of expanded, preactivated NK/γδT/CIK cells for cancer therapy.
Similar content being viewed by others
Introduction
Natural killer (NK) cells and γδT cells are capable to identify and kill cancerous cells in the absence of prior stimulation and thus should be used for cell-based therapies targeting human malignancies [1, 2]. By cytokine stimulation αβT cells can be driven into MHC non-restricted NK-like function, here referred to as cytokine induced killer (CIK) cells with a CD56bright phenotype [3]. The impact of NK- [4] and CIK- [3] antitumor activity on event-free and overall survival in the context of hematopoietic stem cell transplantation (HSCT) has been demonstrated by several groups in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Based on the analysis of killer cell immunoglobin-like receptors (KIR genes) in HSCT, leukemia patients with favorable KIR activity in graft-versus-host direction showed an improved survival [1, 5]. One decade ago it was proven feasible and beneficial to infuse allogeneic NK cells for antitumor treatment, to induce clinical remission in patients with high-risk AML [6]. NK cell transfer has also been proposed in other cancers to improve the treatment [7]. NK cells represent only about 5–10% of peripheral blood mononuclear cells (PBMCs), hence ex vivo expansion and preactivation of NK/γδT/CIK cells is a prerequisite for clinical application and increased antitumor efficacy [8]. Therapies based on these effector cells will greatly benefit from reliable methods to produce large numbers from available donors on a regular basis to overcome the lack of sustained proliferation and durable engraftment of cells with NK-like properties [8, 9]. Haplo-HSCT could be of advantage for donor recruitment in a time- and cost-saving manner. Moreover, manufacturing should be simplified to facilitate standard production, independent of the manufacturer and especially to save handcraft. Several automated systems have been used to produce clinical-grade NK cell products [10, 11]. One problem in haplo-HSCT is to strictly circumvent the induction of GvHD by profound T-cell depletion [8, 10], as patients face several major HLA disparities. In order to augment the antitumor activity of the patient’s reconstituting immune system and balance the risk for induction of GvHD, we generated ex vivo expanded NK-, γδT- and CIK cells post haploidentical HSCT from the patient’s reconstituting donor-derived PBMCs. Further therapeutic antibodies can be used to redirect effector cells to tumor cells. Post haplo-HSCT ex vivo expanded autologous NK/γδT/CIK cells of donor origin might add to increase antitumor activity with reduced risk for the induction of GvHD.
Materials and methods
A pediatric patient at extremely high-risk for cancer recurrence, who underwent a T- and B-cell depleted family mismatched haplo-HSCT with active disease was considered for posttransplant transfer of autologous patient-derived ex vivo expanded NK/γδT/CIK cells. CIK cells were defined as TcRαβ+CD56bright T-cells. The treatment was based on urgent medical need. Treatment was initiated after informed consent was obtained.
Large-scale clinical-grade expansion of NK/γδ T/CIK cells
Hundred milliliters of peripheral blood from healthy donors (n = 6) and from a haplo-transplanted patient (n = 1) was drawn and processed following current GMP-guidelines. After density gradient centrifugation, MNCs were placed in RPMI1640 complete medium (10% hu-serum and 100 IU/mL huIL2) at 1 × 106 CD56+CD3− cells/mL or underwent pre-expansion CD3-depletion using CD3-microbeads (Miltenyi) according to manufacturer’s instruction. Irradiated (100 Gy) K562-mb15–41BBL cells were added at a 1:10 ratio, CD56+CD3− to K562-mb15–41BBL cells, respectively. Static expansion: Cells were cultured in T175 cm2 flasks (Sigma-Aldrich) in a humidified incubator at 37 °C, 5% CO2. Monitoring and feeding included lactate (<12 mmol/L), glucose (>100 mg/dL). Semi-automated culturing: Dynamic cell culturing in a Z®-RP-bioreactor (Zellwerk/Germany). Cell harvest on day 14. Product release testing included sterility- and endotoxin testing.
Cell count, immunophenotyping, and tracking of cells
Differential blood count was measured on ADVIA®120-Siemens. Flow cytometry was used for immunophenotyping (CD3/CD16/CD56/CD69/TcRαβ/TcRγδ/NKp46/NKG2D) and in-vivo-tracking by CD56brightCD69high phenotype.
Cytotoxicity
Cytolytic activity of NK/γδT/CIK cells was analyzed in a 2h-DELFIA-EuTDA cytotoxicity assay (PerkinElmer/USA) according to the manufacturer’s instructions. LAN-1 (neuroblastoma), K562 (AML), MHH-cALL-4 (BCP-ALL), TC-71 (sarcoma) were used as target cells at indicated E:T ratio with/without Fc-optimized CD19-mAb-4G7SDIE or GD2-mAb-ch14.18 at 1 μg/mL. Fluorescence was measured on a VICTOR-II-multi-label-reader (Wallac/Finland).
Monitoring of bone marrow and MRD detection
MRD was detected by a patient-individual NRAS mutation and CALM-AF-10 fusion transcript. The detection level was 10E-02 for the NRAS mutation and 10E-04 for CALM-AF-10 (nested RT-PCR).
Statistical methods
FCS-Express 5.0 was used for flow cytometric analysis, GraphPad-PRISM 7.0 for statistics.
Results
Comparison of expansion conditions and methods
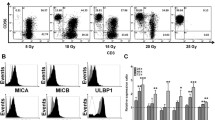
Large-scale clinical-grade ex vivo expansion of NK-γδT-CIK cells using GMP-grade K562-mb15-41BBL and IL2 (100 U/mL) in complete media was tested using PBMCs from healthy donors (n = 6). Unmanipulated PBMCs compared to CD3-depleted PBMCs revealed significant higher cell numbers (Fig. 1a) (n = 6, p < 0.05). Static culturing resulted in higher cell counts than semi-automated culturing in a Z®-RP-bioreactor (Fig. 1b) (n = 6, p < 0.005). Pre-expansion CD3-depletion was proven feasible with low residual T-cell counts in the graft (Fig. 1c). The majority of expanded NK/γδT/CIK cells developed a CD56bright phenotype, commonly observed under IL2/IL15/41BBL culturing (Fig. 1c, d). Additionally, expanded NKs highly expressed FcγRIIIA, required to mediate ADCC. The percentage distribution of CD16−/CD16+ in expanded NK cells was (20%/80%) pre- and post-expansion (Fig. 1d). The highly preactivated immunophenotype was accompanied by bright NKp46/CD69/NKG2D expression (Fig. 1e). Static culturing resulted in higher cytotoxicity of NK/γδT/CIK in various tumor entities (neuroblastoma/AML/BCP-ALL/sarcoma) than in dynamic culturing (n = 30, p < 0.05) (Fig. 1f). Besides excellent direct cytotoxicity NK/γδT/CIK cells exerted high ADCC in LAN-1 and MHH-cALL-4 utilizing the GD2-mAb-ch14.18 and CD19-mAb-4G7SDIE, respectively (Fig. 1g).
Large-scale clinical-grade ex vivo expansion of NK/γδT/CIK cells using GMP-grade K562-mb15–41BBL and IL2 (100 U/mL) in complete RPMI1640 media. a Expansion efficiency of PBMCs compared to CD3-depleted PBMCs (n = 6, p < 0.05). b Expansion efficiency of static versus dynamic expansion (n = 6, p < 0.05). c PBMC versus CD3-depleted PBMC expanded cell product. d Exemplary distribution of CD56 and CD16 in expanded NK cells, from a NK/γδT/CIK cell product. The same CD56/CD16 distribution was observed in CD3-depleted expanded products. e Expanded NK cells express NKp46-, CD69, and NKG2D. f Comparison of cellular cytotoxicity against various targets LAN-1, K562, MHH-cALL-4, TC-71 at E:T 20:1 ratio (n = 30, p < 0.05, pooled results). g NK/γδT/CIK cells exert direct cytotoxicity and ADCC in neuroblastoma and in BCP-ALL (n = 6, p < 0.05 and < 0.005, respectively)
Transfer of autologous, donor-derived ex vivo expanded NK-, γδT-, and CIK cells
A 13-year-old patient with relapsed/refractory NRAS+/CALM-AF-10+ AML at high-risk for relapse underwent a T- and B-cell depleted family haplo-HSCT with active disease. Posttransplant he received ex vivo expanded NK/γδT/CIK cells between day +195–673. For this, donor-derived PBMCs of the patient post haplo-HSCT were used. High absolute number of NK/γδT/CIK cells 3.6E10 was transferred, including 3.5E10 NK and 2.3E09 αβT cells. The highest number of αβT cells/kgBW was 58.4E06, that of γδT cells was 117E06.
The transfer of NK/γδT/CIK cells was well-tolerated with no induction of GvHD. No grade II-V adverse events occurred (CTCAE-v5.0). Only transient coughing and increase of temperature >38 °C for 48 h was observed.
Tracking of transferred preactivated NK cells and functional testing
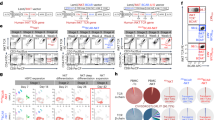
The graft of ex vivo expanded NK/γδT/CIK cells from untouched PBMCs resulted in high NK cells numbers with a mainly CD56bright phenotype. NK and γδT cells outgrow αβT cells T cells (Fig. 2a).
a Density plot of expanded NK/γδT/CIK cells. The majority of cells show a CD56bright phenotype. CIK cells were defined TcRαβ+CD56bright. CD3+CD56bright population from the left plot was distinguished into TcRγδ+ and TcRαβ+ cells (lower right quadrant) in the right plot, defined as CIK cells. b Preactivated NK cells were tracked in the peripheral blood of an AML patient by CD56brightCD69high phenotype. The left plot shows NK phenotype prior to NK/γδT/CIK cell transfer, right plot one hour after infusion. c Pre- and post- NK/γδT/CIK cell transfer, PBMCs were isolated from peripheral blood and spontaneous cytotoxicity/ADCC were tested in a 2h-BATDA-release-assay at indicated E:T ratios versus LAN-1. d The course of CD56+CD3− cells in absolute counts/µl, percentage of lymphocytes and CD56+CD69+ fraction. e The schematic course of leukemic load is shown pre- and post haplo-HSCT. Autologous, donor-derived ex vivo expanded NK/γδT/CIK cells (source: patient’s blood) were transferred on day +195 and +258 and led to complete MRD remission
Flow cytometry allows tracking of preactivated NK cells in the peripheral blood post transfer, by the CD56brightCD69high phenotype. Peripheral blood was drawn prior to NK/γδT/CIK cell transfer and one hour after infusion. Clearly, after infusion of the effector cells, a distinct new CD56brightCD69high population can be identified and quantified (Fig. 2b). Besides in-vivo-tracking of the transferred NK cells in the peripheral blood, PBMC activity post NK/γδT/CIK cell transfer revealed a significant higher cytotoxicity (Fig. 2c).
Prior to and post NK/γδT/CIK cell transfer, CD56+CD3− NK cell count and percentage was measured in the peripheral blood as well as the fraction of CD56brightCD69high. We assumed highly activated NK cells being derived from the transferred NK/γδT/CIK cell graft. CD56brightCD69high cells peaked after infusion and could be tracked for two weeks (Fig. 2d).
MRD reduction in AML by NK-, γδT-, and CIK cells
Posttransplant, NRAS mutation was not detectable. However, CALM-AF-10 fusion transcript was persistent positive at 10E-04 in the bone marrow. After two applications of the NK/γδT/CIK cells on day +195 and day +258, CALM-AF-10 was undetectable. Due to recurring MRD, the patient received another NK/γδT/CIK cell infusion (day +673) plus additional immunotherapy. Five years posttransplant, the patient is in ongoing complete MRD remission (Fig. 2e).
Discussion
Posttransplant generation and transfer of ex vivo expanded NK/γδT/CIK cells from autologous, donor-derived PBMCs is very simple. According to our experience, transfer of high cell counts (>1E10, (>500E06/kgBW) NK and (>1E09, >50E06/kgBW)) αβT cells post haplo-HSCT is feasible without acute and long-term toxicities including GvHD. Our expansion protocol induced sustained and specific proliferation of effector cells from 12 healthy donors and our reported patient [12]. T-cell depleted, expanded NK-cell products have been demonstrated the safe and are not associated with severe adverse events and do not induce GvHD [8]. However, we expect complementary effector function and interaction of NK-, γδT-, and CIK cells and besides NK cells, γδT cells exert ADCC, contributing to targeted therapies with Fc-recruiting mAbs [13]. The generation of donor-derived CIK cells using IL15/IFNγ, holds the risk to induce GvHD in the haplo-setting [3]. Whether generation of posttransplant CIK cells using autologous donor-derived cells from patients will decrease the risk of GvHD and might reduce efficacy is unknown. In patients with no GvHD, expanded T cells originate from tolerant T cells and thus might not induce GvHD. Moreover, continuous cytokine stimulation may contribute to loss of T-cell alloreactivity, due to reprogramming of NK- and T cells [12]. Expanded NK/γδT/CIK cells demonstrated superior cytolysis and ADCC compared to CD3-depleted expanded NK cells. Additionally, primary cellular activity was not restricted to favorable NK-cell targets, like NKG2DLhigh neuroblastoma, but also in BCP-ALL by preactivated expanded NK/γδT/αβT cells. In our testing, static cultured cells were superior in expansion efficiency and in killing capacity. However, by modification of automated production in alternative expansion devices/bioreactors, generation of superior cell products might be achievable [10]. Automated systems simplify, standardize and lower costs by reducing handcraft [10, 11]. In high-risk patients, transfer of NK/γδT/CIK cells might substantially increase or trigger antitumor activity to clear minimal residual tumor cells [3, 14]. The completed NKAML pilot study (NCT00187096) has demonstrated safety of NK-cell infusion in AML and might add to immunologic leukemia control [15]. In our AML patient with posttransplant persistent MRD, we documented a decrease of MRD in the temporal context of the NK/γδT/CIK cell transfer. The efficacy of prophylactic NK transfer is hard to verify. However, prophylactic use [15] or MRD state might be the most reasonable and promising indication to contribute to control residual tumor cells directly or redirected by therapeutic mAb [3, 14, 16]. It is underscored that antitumor activity needs to be balanced to the induction of GvHD [3, 8, 17]. Since the transfer of posttransplant autologous, donor-derived ex vivo expanded NK/γδT/CIK cells probably does not induce GvHD but induces antitumor activity, this approach meets both criteria. As several groups have shown the persistence of transferred NK- and CIK cells in the blood for >2 weeks [3, 16], and since we demonstrated increased antitumor response after NK/γδT/CIK cell transfer, there is a good rationale for the application of preactivated donor-derived effector cells from patients after haplo-HSCT.
References
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100.
Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76.
Rettinger E, Huenecke S, Bonig H, Merker M, Jarisch A, Soerensen J, et al. Interleukin-15-activated cytokine-induced killer cells may sustain remission in leukemia patients after allogeneic stem cell transplantation: feasibility, safety and first insights on efficacy. Haematologica. 2016;101:e153–6.
Lang P, Teltschik HM, Feuchtinger T, Muller I, Pfeiffer M, Schumm M, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165:688–98.
Michaelis SU, Mezger M, Bornhauser M, Trenschel R, Stuhler G, Federmann B, et al. KIR haplotype B donors but not KIR-ligand mismatch result in a reduced incidence of relapse after haploidentical transplantation using reduced intensity conditioning and CD3/CD19-depleted grafts. Ann Hematol. 2014;93:1579–86.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7.
Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94.
Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48:433–8.
Vela M, Corral D, Carrasco P, Fernandez L, Valentin J, Gonzalez B, et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 2018;422:107–17.
Granzin M, Soltenborn S, Muller S, Kollet J, Berg M, Cerwenka A, et al. Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy. 2015;17:621–32.
Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12:1044–55.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7.
Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–33.
Pfeiffer MM, Schumm M, Muller I, Handgretinger R, Lang P. IL-15-stimulated CD3/CD19-depleted stem-cell boosts in relapsed pediatric patients after haploidentical SCT. Leukemia. 2012;26:2435–9.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9.
Davis ZB, Felices M, Verneris MR, Miller JS. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer. Cancer J. 2015;21:486–91.
Rettinger E, Bonig H, Wehner S, Lucchini G, Willasch A, Jarisch A, et al. Feasibility of IL-15-activated cytokine-induced killer cell infusions after haploidentical stem cell transplantation. Bone Marrow Transplant. 2013;48:1141–3.
Acknowledgements
K562-mb15-41BBL was kindly provided by Dario Campana, Singapore.
Funding
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG), CRC685 Immunotherapy, the Bundesministerium für Bildung und Forschung (BMBF-iVac-ALL, BMBF-GO-Bio 0315096/0316070), the Reinhold Beitlich Stiftung, the Stefan Morsch Stiftung, the Förderverein für krebskranke Kinder Tübingen, the German Cancer Consortium (DKTK) and the German Cancer Research Center (DKFZ) Heidelberg, Germany. Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schlegel, P., Lang, AM., Matela, M. et al. Ex vivo expansion of autologous, donor-derived NK-, γδT-, and cytokine induced killer (CIK) cells post haploidentical hematopoietic stem cell transplantation results in increased antitumor activity. Bone Marrow Transplant 54 (Suppl 2), 727–732 (2019). https://doi.org/10.1038/s41409-019-0609-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0609-y
- Springer Nature Limited
This article is cited by
-
Lymphocyte expansion in bioreactors: upgrading adoptive cell therapy
Journal of Biological Engineering (2021)