Abstract
Antithymocyte globulin (ATG) is an important component of conditioning regimens to prevent graft-versus-host disease (GVHD) in unrelated hematopoietic stem cell transplantation (HSCT), but the optimal dose of ATG remains unknown. We prospectively analyzed 205 unrelated HSCTs in patients with malignant hematological disorders. HSCTs were classified as follows: HLA-matched transplant without ATG (n = 53, group A), HLA-mismatched transplant treated with 6.0 mg/kg thymoglobulin (n = 77, group B), and HLA-matched transplant treated with 4.5 mg/kg thymoglobulin (n = 75, group C). For groups A and B, the 5-year moderate/severe chronic GVHD rates were 31.9% and 24.2%, the 5-year GVHD-free and relapse-free survival (GRFS) rates were 28.3 and 47%, and the 2-year immunosuppressive therapy (IST)-free survival rates were 8.6% and 40.2% (p = 0.0016), respectively. Furthermore, group C had lower incidences of grade II-IV acute GVHD (18.7%) and 5-year moderate/severe chronic GVHD (16.6%) than group A did. Group C had higher 5-year GRFS (52.1% vs 28.3%, p = 0.002), 2-year IST-free survival (51.7% vs 8.6%, p = 0.00004), and 5-year overall survival (OS) (68.3% vs 41.5%, p = 0.007) rates than group A did. Thus, ATG was associated with better GVHD prevention, a higher rate of IST-free survival, lower transplant-related mortality (TRM), and superior OS and GRFS in unrelated HSCTs.
Similar content being viewed by others
Introduction
Unrelated hematopoietic stem cell transplantation (HSCT) has become an important means of treating diseases of the blood system, especially when there is a lack of fully compatible relative donors. The development of HLA typing technology and graft-versus-host disease (GVHD) prevention programs play an important role in the process of unrelated HSCT. However, regardless of sibling or unrelated transplantations, GVHD is one of the most common complications after allogeneic HSCT. In unrelated transplantation, the incidence of grade II–IV acute GVHD (aGVHD) is nearly 40–60% in 100 days [1, 2], and the incidence of 5-year chronic GVHD (cGVHD) is ~40% [3]. Furthermore, GVHD increases transplant-related mortality (TRM), reduces the quality of life of patients, and even leads to a decline in overall survival (OS).
Furthermore, GVHD is more severe in unrelated mismatched HSCT [4]. While some patients lack sibling matched and unrelated matched donors, an unrelated mismatched donor may become the only choice. Numerous large-sample studies have proven that survival outcomes from unrelated mismatched donors were worse than those from HLA-matched donors [4, 5]. Therefore, GVHD prevention should be optimized for unrelated HSCT patients, especially unrelated mismatched pairs.
Antithymocyte globulin (ATG) has been used as part of the conditioning regimen in unrelated allogeneic HSCT to reduce the incidence of acute and chronic GVHD [6, 7]. Previous studies have shown that the use of ATG can reduce GVHD, but high doses of ATG also bring a higher risk of relapse and infections [8,9,10]. To date, the optimal dose of ATG is still unclear.
In the early period of our institution, the incidence of grade II–IV aGVHD reached 43% in unrelated matched patients [11]. To reduce GVHD, we conducted a prospective study to test the efficacy of ATG on unrelated patients. We first added ATG to the conditioning regimen for unrelated mismatched patients since 2007, which was beneficial to reduce GVHD compared with previous outcomes of our center. Next, we expanded the scope to all unrelated HSCT patients, including matched and mismatched patients, since 2010. Therefore, the goal of our research is to compare the outcomes of mismatched-ATG(+), matched-ATG(−), and matched-ATG(+) patients from January 2007 to December 2016.
Materials and methods
Patient characteristics
From January 2007 to December 2016, 205 patients who underwent high HLA-resolution unrelated HSCT for a variety of hematologic diseases were enrolled in this study at the Bone Marrow Transplant Center of The First Affiliated Hospital, Zhejiang University, China. The study was reviewed and approved by the Ethical Committee of Zhejiang University, and informed consent was provided by all patients.
Study design
This prospective study was registered as a clinical trial (registration number: ChiCTR-OCH-12002845), which is shown as Fig. 1. The eligibility criteria included age under 60-years-old, patients diagnosed with malignant hematological disorders, ECOG score 0–2, and provision of an informed consent document. The exclusion criteria included uncontrolled active infection, combination with severe cardiac, hepatic, renal and pulmonary diseases, and pregnancy. All donor-recipient pairs had high resolution typing at HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci. All enrolled patients were divided into three groups: 53 HLA-matched patients without ATG (group A, 2007–2009), 77 HLA-mismatched patients administered 6 mg/kg thymoglobulin (group B, 2007–2016), and 75 HLA-matched patients administered 4.5 mg/kg thymoglobulin (group C, 2010–2016).
Conditioning, GVHD prophylaxis
All patients were given myeloablative conditioning. The myeloablative conditioning regimen involved busulfan-cyclophosphamide (Bu-Cy) and modified Bu-Cy [12]. The use of ATG differs between matched and mismatched patients. The mismatched unrelated recipients received 6.0 mg/kg ATG (thymoglobulin, 1.5 mg/kg daily on days −4 to −1, Sanofi, Paris, France), while the matched unrelated recipients received 4.5 mg/kg ATG (1.5 mg/kg daily on days −3 to −1).
GVHD prophylaxis contained cyclosporin A (CsA), methotrexate (MTX) and low-dose mycophenolate mofetil (MMF). CsA began on day −7 and was administered by successive intravenous infusion. The initial dosage was 2.5 mg/kg daily, and the dose was adjusted to maintain a whole-blood steady-state level of 200–400 ng/mL. The dose was reduced during the second to third months posttransplant according to chimeric status and status of GVHD. MTX was administered at a dose of 10 mg on days + 1, + 3, and +6. MMF was administered at an oral dosage of 250 mg twice daily from day +1 until day +100, and the dose was adjusted depending on the results of routine blood analyses [12].
End points and definitions
The primary end points were the presence of aGVHD and cGVHD, GVHD-free and relapse-free survival (GRFS) and immunosuppressive therapy (IST)-free survival after HSCT. The secondary endpoints were OS, disease-free survival (DFS), relapse, and TRM. aGVHD was defined by the Glucksberg scale [13], and cGVHD was defined according to the NIH Consensus Guidelines and was classified as mild, moderate, or severe. The incidence of cGVHD was evaluated in patients who survived for at least 100 days. GRFS was defined as the time between transplantation and the development of GVHD (grade III–IV aGVHD) or cGVHD requiring systemic IST, disease recurrence, or death [14]. IST-free survival was defined as the status of being alive without IST, but withdrawal of immunosuppression prompted by persistent malignancy, secondary malignancy or imminent death was not counted as a success [15]. In addition, DFS was defined as survival without recurrence of the primary disease.
Biostatistical methods
Cumulative incidence using the competing risk method was used to determine the difference in probabilities of aGVHD, cGVHD, relapse, TRM, and IST-free survival in the three transplantation cohorts. R statistical software (version 3.4.3; http://www.r-project.org) was used for the competing risk analysis. Probabilities for OS, DFS, and GRFS were calculated using the Kaplan–Meier estimator. OS, DFS, and GRFS were performed using SPSS 22.0 software (IBM Corporation, Armonk, NY). Univariate analyses were compared using the log-rank test and multivariate analysis by Cox regression. All variables in the univariate analyses with a p value < 0.2 were included in the multivariate analysis.
Results
Patient and HSCT characteristics
Between January 2007 and December 2016, a total of 205 patients underwent transplantation from unrelated donors using myeloablative conditioning regimens. Fifty-three patients received stem cells from fully matched donors with 10/10 HLA allele loci without ATG (group A, 2007–2009), 77 patients received stem cells from HLA-mismatched donors and 6 mg/kg rabbit-ATG (group B, 2007–2016), and 75 patients received stem cells from fully matched donors with 10/10 HLA allele loci and 4.5 mg/kg rabbit-ATG (group C, 2010–2016). (Table 1) shows the patients, donors, and transplantation characteristics of the study. In group B, 62 patients (80.5%) had a single HLA allele locus mismatched from their donors (9/10 HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci matching), 12 patients (15.6%) had two mismatched allele loci from their donors (8/10 loci matching), and three patients (3.9%) had three mismatched allele loci from their donors (7/10 loci matching).
There were no significant differences in terms of underlying recipient age, donor age, disease type, disease stage, donor-recipient gender relationship, donor-recipient blood type relationship, number of MNCs, median interval of diagnosis and transplantation, or median follow-up time among the three cohorts. There were significant differences among the three cohorts only in terms of the number of CD34+ cells and the source of graft cells.
The end point of the last follow-up for all surviving patients was December 31, 2017. The median follow-up times for surviving patients were 56 (range 1–129) months, 51 (range 1–125) months, and 43 (range 3–95) months in group A, group B, and group C, respectively.
Transplant outcomes
Engraftment
Patients engrafted to absolute neutrophil counts exceeded 0.5 × 109/L in a median time of 12 days (range, 8–19 days) in group A, 12 days (range, 8–17 days) in group B, and 12 days (range, 9–23 days) in group C. Among all patients, one patient in group B failed to engraft and subsequently received a second haploidentical salvage transplantation. After myeloid recovery, all patients achieved sustained, full donor chimerism by day 30 after HSCT except for two patients in group B who died before day 30. Among the patients surviving beyond 30 days, two patients in group C experienced poor platelet engraftment, but both achieved sustained, full donor chimerism later. Except for the patients who died by 30 days and those who had failure to engraft, the median time of platelet engraftment was 13 days (range, 7–19 days) in group A, 13 days (range, 8–18 days) in group B, and 13 days (range, 6–25 days) in group C.
Acute and chronic GVHD
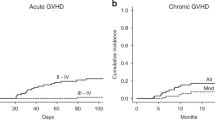
Grade II–IV aGVHD were significantly more frequent in patients in group A than in patients in group C. Cumulative incidences of grade II–IV aGVHD at 100 days were 37.7%, 27.3%, and 18.7%, for groups A, B, and C, respectively (p = 0.004 for group A vs group C, Fig. 2a). The cumulative incidences for grade III–IV aGVHD at 100 days were 13.2%, 10.4%, and 4.0%, respectively, in group A, group B, and group C. The incidence of cGVHD was not significantly different among the three groups. The cumulative incidences of cGVHD at 5 years were 40.4%, 40.4%, and 38.0%, respectively, in group A, group B, and group C. However, the patients in group A had a higher rate of moderate/severe cGVHD at 5 years than those in group C did (31.9% vs 16.6%, p = 0.028) but comparable with the rate of those in group B (31.9% vs 24.2%, p = 0.229) (Fig. 2b).
Multivariate Cox regression analysis for aGVHD in the total cohort of 205 patients (except for two patients who died before day 30), including major known risk factors (Table 2), confirmed that ATG had a positive impact on the risk of grade II–IV aGVHD (p = 0.001, RR = 0.330). In addition, multivariate Cox regression analysis for cGVHD in the cohort of patients who survived more than 100 days confirmed that ATG was associated with the risk of cGVHD (p = 0.170, RR = 0.654) and moderate/severe cGVHD (p = 0.006, RR = 0.336).
Relapse
The 5-year incidence of relapse was not different among the three groups (24.5% in group A, 15.8% in group B, and 27.1% in group C, Fig. 2c). In the multivariate analysis of relapse rate (Table 2), the use of ATG and locus of HLA matching did not influence the risk of relapse among all patients.
Long-term follow-up and survival
Patients in group C had the lowest incidence of TRM compared with those in group A cohort (9.3% vs 35.9%, p = 0.0008) and group B (9.3% vs 25.1%, p = 0.016), whereas patients in group A and group B had comparable incidences of TRM (35.9% vs 25.1%, p = 0.263, Fig. 2d).
For all patients, the five-year OS rate was 57.2%. The five-year OS rates after transplantation were 41.5%, 60.0%, and 68.3% for group A, group B, and group C, respectively. Patients in group A had an inferior OS compared with those in group C (p = 0.007) and group B (p = 0.049). However, the 5-year OS rates were comparable between group B and group C (p = 0.401) (Fig. 3a). In the multivariate analysis for OS (Table 2), the use of ATG (p = 0.005, RR = 0.456) had beneficial effects on OS.
The five-year DFS rates were 37.0%, 56.8%, and 63.6% for group A, group B, and group C, respectively. Patients in group A had an inferior DFS compared with those in group C (p = 0.030). However, the 5-year DFS rates were comparable between group B and group A (p = 0.059) and between group B and group C (p = 0.690) (Fig. 3b).
Furthermore, the 5-year GRFS rates after transplantation were 28.3%, 47.0%, and 52.1% in group A, group B, and group C, respectively. Patients in group A had an inferior GRFS compared with those in group C (p = 0.002) and group B (p = 0.021). However, the 5-year GRFS rates were comparable between group B and group C (p = 0.445) (Fig. 3c). In the multivariate analysis for GRFS (Table 2), the use of ATG (p = 0.002, RR = 0.477) had beneficial effects on GRFS.
IST-free survival
The IST-free survival rates at 2 years were 8.6%, 40.2%, and 51.7% for groups A, B, and C, respectively (p = 0.0016 for group A vs group B, p = 0.00004 for group A vs group C, p = 0.107 for group B vs group C, Fig. 4).
Infection
Most patients suffered a slight infection during the low-cell stage after hematopoietic stem cell transplantation and recovered quickly with the support of antibiotics or antifungal drugs. Finally, a total of 20 patients died due to infection (20/205, 9.8%), including 10 patients (10/53, 24.2%) in group A, 6 patients (6/77, 7.8%) in group B, and 4 patients (4/75, 5.3%) in group C (p = 0.030 for all three groups). Therefore, adding ATG to the treatment regimen did not induce a severe infection in patients.
Discussion
GVHD seriously affects the survival and long-term quality of life of patients after unrelated transplantation. Our study suggests that ATG in combination with CsA, MTX, and MMF leads to a significantly lower cumulative incidence of aGVHD and moderate/severe cGVHD than the lack of ATG treatment does in HLA-matched cohorts. In addition, both the HLA-matched and mismatched patients who received ATG had a significantly higher probability of being alive and free from IST than did patients who did not receive ATG in our series. Furthermore, the HLA-matched patients who received ATG had a significantly superior OS, DFS, and GRFS as well as a lower TRM rate than the HLA-matched patients who did not received ATG did in our series.
To our knowledge, mismatched patients rarely obtain equal survival results as matched patients do, and mismatched loci are a clear risk factor for a low survival rate [4, 5]. However, our study showed that the mismatched patients who received ATG had a similar and even better OS and GRFS than did the matched patients who did not receive ATG. We speculated that the use of ATG could overcome the adverse influence by the mismatched HLA loci. In our study, compared to HLA-matched patients without ATG, the mismatched patients who were treated with ATG had similar aGVHD, cGVHD, relapse, and TRM but better rates of GRFS and IST-free survival.
In some previous studies, a high dose of thymoglobulin was used as part of the conditioning regimen. Bacigalupo et al. [9] observed that the addition of 7.5–15 mg/kg thymoglobulin to cyclosporine/MTX provided protection against extensive cGVHD (15%) and chronic lung dysfunction in unrelated bone marrow HSCTs but resulted in a similar 6-year OS (ATG vs non-ATG, 44% vs 31%, p = 0.80). In another study, Pidala et al. [10] showed that using 7.5 mg/kg thymoglobulin followed by standard tacrolimus plus methotrexate in unrelated mismatched HSCT resulted in 2-year moderate/severe cGVHD, 2-year nonrelapse mortality (NRM), and 2-year OS rates of 28%, 30%, and 45%, respectively. Another type of ATG, ATG-Fresenius (ATG-F) at a dose of 60 mg/kg, was also associated with lower aGVHD and cGVHD in several prospective randomized trials but did not improve the rate of OS [8, 16]. In Soiffer’s study, patients using ATG-F achieved similar cGVHD-free survival but an even worse OS [16]. Moreover, similar results were observed with administration of 60 mg/kg ATG-F in a GVHD prevention regimen of myeloablative unrelated HSCTs in one center of Germany [17], with a nonsignificant 3-year OS in the ATG vs non-ATG groups (55.2% vs 43.3%, p = 0.39). However, in this study, the 3-year IST-free survival rates were 52.9% and 16.9% in the ATG and non-ATG groups, respectively.
Taken together, these data suggest that high-dose ATG was capable of reducing cGVHD and/or aGVHD but did not improve survival rate, which may be attributed to increased rates of relapse or infection, reflecting a directly higher TRM. Therefore, a lower dose of ATG was prospectively used in unrelated HSCTs in our center, which significantly improved OS, GRFS and IST-free survival.
In recent studies, some centers have also chosen a lower dose of ATG for a balance of antileukemia and anti-GVHD effects. In a multicenter randomized study, Walker et al. [15] supported the use of thymoglobulin at a dose of 4.5 mg/kg for unrelated donor hemopoietic cell transplantation and showed a markedly increased IST-free survival at 12 months (37 vs 16%, ATG group versus non-ATG group, p = 0.00060). However, in the above study, a heterogeneous sample of both myeloablative and nonmyeloablative patients were included, as well as HLA matching and mismatching. In another study, Bryant et al. [18] compared 2.5 mg/kg ATG-exposed, matched unrelated HSCTs and ATG-unexposed, matched related HSCTs with GVHD prophylaxis, including tacrolimus plus methotrexate or mycophenolate mofetil. In addition, he obtained similar outcomes of OS in two cohorts, but the ATG-exposed cohort had higher GRFS with a 2-year GRFS of 23 vs 3% (p = 0.003). Considering the usage of 2.5 mg/kg ATG (0.5 mg/kg on day −2, 2 mg/kg on day −1) in this study, we speculated that a closer time of relatively high ATG prior to transplantation may have a positive effect on GRFS. Nevertheless, the addition of ATG did not improve OS in this study, which may be due to the difference of graft source between two cohorts. However, grading of acute and chronic GVHD was not shown in Bryant’s study, influencing the accurate assessment of long-term life quality of patients in unrelated transplantation. Moderate and severe GVHD are indeed the main factors influencing quality of life. In general, until now, there have been few large-sample, clinical trials to confirm the optimum dose of thymoglobulin, especially those could estimate the life quality of the patients.
Our study has limitations due to its single-center design and the need for a larger sample size. In addition, most of our patients were relatively young, which may also be one potential reason why the patients achieved such good results. However, despite these limitations, our data suggest that 4.5 mg/kg and 6 mg/kg thymoglobulin may be optimal at reducing GVHD and TRM in unrelated matched and mismatched HSCTs. Our research indicates that the use of thymoglobulin led to higher OS, DFS, GRFS, and IST-free survival in patients after HSCTs. Furthermore, the use of thymoglobulin in unrelated mismatched patients showed that ATG can overcome reduced survival caused by mismatched HLA loci.
References
Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
Morishima S, Shiina T, Suzuki S, Ogawa S, Sato-Otsubo A, Kashiwase K, et al. Evolutionary basis of HLA-DPB1 alleles affects acute GVHD in unrelated donor stem cell transplantation. Blood. 2018;131:808–17.
Carapito R, Jung N, Kwemou M, Untrau M, Michel S, Pichot A, et al. Matching for the nonconventional MHC-I MICA gene significantly reduces the incidence of acute and chronic GVHD. Blood. 2016;128:1979–86.
Crocchiolo R, Ciceri F, Fleischhauer K, Oneto R, Bruno B, Pollichieni S, et al. HLA matching affects clinical outcome of adult patients undergoing haematopoietic SCT from unrelated donors: a study from the gruppo italiano trapianto di midollo osseo and Italian bone marrow donor registry. Bone Marrow Transplant. 2009;44:571–7.
Furst D, Muller C, Vucinic V, Bunjes D, Herr W, Gramatzki M, et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis. Blood. 2013;122:3220–9.
Czerw T, Labopin M, Giebel S, Socie G, Volin L, Fegueux N, et al. Anti-thymocyte globulin improves survival free from relapse and graft-versus-host disease after allogeneic peripheral blood stem cell transplantation in patients with Philadelphia-negative acute lymphoblastic leukemia: an analysis by the acute leukemia working party of the EBMT. Cancer. 2018;124:2523–33.
Kharfan-Dabaja MA, Parody R, Perkins J, Lopez-Godino O, Lopez-Corral L, Vazquez L, et al. Tacrolimus plus sirolimus with or without ATG as GVHD prophylaxis in HLA-mismatched unrelated donor allogeneic stem cell transplantation. Bone Marrow Transplant. 2017;52:438–44.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–5.
Pidala J, Tomblyn M, Nishihori T, Ayala E, Field T, Fernandez H, et al. ATG prevents severe acute graft-versus-host disease in mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1237–44.
Zhu X, Lai X, Luo Y, Shi J, Tan Y, Zheng W, et al. Combination of low-dose mycophenolate mofetil with cyclosporine and methotrexate as GVHD prophylaxis in unrelated donor allogeneic stem cell transplantation. Leuk Res. 2013;37:1046–51.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: long-term outcomes of a prospective randomized trial. Cancer. 2017;123:2881–92.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase iii clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Bryant A, Mallick R, Huebsch L, Allan D, Atkins H, Anstee G, et al. Low-dose antithymocyte globulin for graft-versus-host-disease prophylaxis in matched unrelated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:2096–101.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC; 81730008), the National Natural Science Foundation of China (NSFC; 81670169), and the Zhejiang Key Research and Development Program (2015C03038). The funders did not influence how the research was conducted or the approval of the manuscript. We thank all patients and transplantation staff.
Author information
Authors and Affiliations
Contributions
HH and YL designed the study. YL and MJ collected, analyzed data, performed statistical analysis, and wrote the manuscript. HH, YL, MJ, YT, YZ, JS, YZ, WZ, XL, and JY contributed to patient care and read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Y., Jin, M., Tan, Y. et al. Antithymocyte globulin improves GVHD-free and relapse-free survival in unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant 54, 1668–1675 (2019). https://doi.org/10.1038/s41409-019-0502-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0502-8
- Springer Nature Limited
This article is cited by
-
Outcomes of haploidentical peripheral blood stem cell transplantation following myeloablative conditioning using two types of rabbit ATG: a propensity score-matched analysis
Annals of Hematology (2024)
-
Different effects of thymoglobulin on acute leukemia with pre-transplant residual blasts in HLA mismatch transplantation
International Journal of Hematology (2023)
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)
-
Effectiveness of prophylactic antiviral therapy in reducing HBV reactivation for HBsAg-positive recipients following allogeneic hematopoietic stem cell transplantation: a multi-institutional experience from an HBV endemic area
Annals of Hematology (2022)
-
The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update
Journal of Hematology & Oncology (2021)