Abstract
While bone marrow (BM) grafts were initially used for T-replete HLA-haploidentical related donors transplantation (Haplo-SCT) with post-transplantation cyclophosphamide (PT-Cy), the use of peripheral blood stem cell (PBSC) remains debated. We thus conducted a detailed analysis evaluating the incidence, risk factors, and prevalence of GVHD after PBSC Haplo-SCT with PT-Cy. One hundred and eighty-one patients with hematological diseases were included. Median time for neutrophil and platelet recovery was 21 and 30 days, respectively. The cumulative incidence of grade 3–4 acute GVHD and severe chronic GVHD were 8% and 4%, respectively, approaching what was observed after BM Haplo-SCT. NRM at 2 years was 21%, and 41% of the non-relapse deaths were caused by GVHD. The cumulative incidence of relapse at 2 years was 17% in the whole cohort, and 13% among AML patients (n = 54), suggesting a high GVL effect. As surrogate markers for good quality of life, we observed a 2-year GVHD-relapse-free survival probability of 50% and found that 6% and 2% of disease-free patients at 2 years were still living with GVHD and immunosuppressive treatments, respectively. Haplo-SCT with PT-Cy using PBSC grafts results in low incidence GVHD and promising disease control, making PBSCs a valuable alternative to BM graft in this setting.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a curative treatment for many hematological diseases. Donor availability and treatment related morbidity may limit its applications. In 2008, Luznik [1] et al. showed that the use of PT-Cy as GVHD prophylaxis allows for the use of T-replete grafts from HLA-haploidentical related donors (Haplo-HSCT), resulting in a low incidence of GVHD, and thus overcoming previously mentioned hurdles [2, 3]. Several retrospective studies support similar outcomes for Haplo-HSCT using PT-Cy regimen and “canonical” HLA-matched sibling or unrelated donor Allo-HSCT [4,5,6,7,8]. Although initially described using bone marrow as the graft source [1], the use of peripheral blood stem cells (PBSC) has grown and now exceed BM, at least in Europe [9]. In the setting of HLA-identical Allo-HSCT, prospective randomized trials showed that the use of PBSC is associated with faster engraftment kinetics but also with higher incidence of GVHD (notably cGVHD) when compared to BM [10,11,12]. However, no prospective comparison is available in the context of Haplo-HSCT so far, and it is not precisely known to which extend the use of PT-Cy may revert the anticipated higher risk of GVHD of PBSC Haplo-HSCT. Retrospective analyses report contradictory results as to the risk of an increased incidence of GVHD when transplanting PBSC rather than BM [13,14,15]. The heterogeneity in patient characteristics and transplantation procedures (especially in GVHD prophylaxis) in and across these studies do not support robust conclusions, and few data on GVHD prevalence are available. We here present the detailed experience of T-cell replete Haplo-HSCT with PT-Cy in a joint collaborative program at 2 European transplant centers.
Patients and methods
Selection criteria
Inclusion criteria were (1) Haplo-HSCT at the Paoli-Calmettes Institute and Humanitas Cancer Center from 2012 to 2016; (2) PBSC as a graft source; (3) PT-Cy as part of GVHD prophylaxis; and (4) patients with hematologic malignancies.
Non inclusion criteria were (1) previous Allo-HSCT; (2) sequential chemotherapy and conditioning regimen for refractory AML patients. The outcome of those patients is evaluated in other specific studies. Patients gave signed informed consent for the clinical data collection. This study is in accordance with the Helsinki declaration and was approved by the institutional review board of both insitutions.
Transplantation procedures
The Haplo-HSCT program was originally started using non-myeloablative conditioning (NMAC) regimen including fludarabine (Flu), cyclophosphamide (Cy), and 2-Gray total body irradiation (TBI) (Flu-Cy-TBI). In order to improve anti-tumor effect, low dose TBI was progressively replaced with intravenous busulfan (Bu) (at reduced [RIC, ≤260 mg/m²] or myeloablative [MAC, >260 mg/m²] doses according to EBMT criteria [16], while pre-transplant Cy was replaced with thiotepa, 5–10 mg/m² total dose). For the purpose of this study, conditioning regimens were categorized as NMAC (Flu-Cy-TBI), RIC (reduced Bu dose), and MAC (myeloablative Bu dose). All patients received GVHD prophylaxis consisting of PT-Cy 50 mg/kg on days+3 and +4, calcineurin inhibitors (CNI) and mycophenolate mofetil (MMF) starting on day+5. CNI was progressively tapered off starting on day+90 until day+180, while MMF was stopped on day+35, in the absence of GVHD. All patients were given G-CSF starting on day+5. Supportive care is detailed in (Supplemental File). Minimal targeted CD34+ cell dose was 4 × 106/kg (recipient body weight) while no maximal limit was used.
Engraftment and GVHD treatment
Neutrophil engraftment was defined as the first of three consecutive days with absolute neutrophil count (ANC) >0.5 G/L. Platelet recovery was defined as a platelet count (PLT) >20 G/L during three consecutive days, without transfusions for the preceding 7 days. Poor graft function (PGF) was defined by (1) the persistence of cytopenia in at least 2 hematopoietic lineages (ANC < 0.5 G/L, PLT < 30 G/L, Hb < 8.5 g/dL) beyond day+30 post Haplo-SCT or at any time after engraftment; (2) transfusion requirements; (3) presence of full donor chimerism (defined by donor cells >95% among CD3+ sorted PBMC); and (4) absence of GVHD, infection or evidence of hematological disease relapse [17].
Acute and chronic GVHD were classified according to Glucksberg and NIH classification [18, 19], respectively. GVHD treatments are detailed in Supplemental File.
Statistical analyses
Cumulative incidences of neutrophil and platelet recovery, GVHD, relapse, and NRM were calculated taking into account the presence of competing risk while survivals were computed using standard Kaplan–Meier methods (details in Supplemental File). In addition, we evaluated in disease-free patients the prevalence of GVHD and immunosuppressive treatment (IST) at different time points after Haplo-HSCT (months 3, 6, 9, 12, 15, 18, 21, and 24), in order to assess the quality of life of surviving patients. Multivariate Cox model was computed including age (continuous variable), disease risk index (DRI) [20] (low vs. intermediate vs. high/very-high), conditioning regimen (NMAC vs. RIC vs. MAC) [16], CD34+ cell dose (continuous variable) and HCT-CI (0–2 vs. ≥3) [21]. To take into account a potential center effect, we stratified the cox model by center. Statistics were computed using R-project 3.3.2 software (http://www.R-project.org).
Results
Patient, disease, and transplantation characteristics
We analyzed 181 consecutive patients who underwent T-cell replete PBSC Haplo-HSCT for hematologic malignancies from March 2012 to June 2016. Patients characteristics at baseline are detailed in Table 1. Median age was 60 years (range: 19–73), with 60 patients (33%) who were ≥65 years. DRI was high or very high in 47 patients (26%) and HCT-CI was ≥3 in 110 patients (60%). Median CD34+ and CD3+ cell doses were 5.4×106/kg (range: 1.5–18.1) and 280×106/kg (range: 38–704), respectively. Median follow-up was 21 months after Haplo-SCT (range: 6–60).
Hematopoietic recovery
Between 24 and 48 h after Haplo-SCT, 88% of recipients experienced fever with chills (median temperature=40.1 °C). There was no microbiological proof of infection in most patients (91%), while bacterial infection was identified in bloodstream cultures in 9% of patients. In 79% of patients, fever completely disappeared on day+5, 24 h following the last PT-Cy infusion. All but 2 patients (1%) were engrafted. Median time to neutrophil and platelet recovery was 21 (range: 13–112) and 30 (range: 10–394) days, respectively. We observed a slightly but significantly faster ANC recovery (>0.5 G/L) in patients who received CD34+ cell dose above the median value (on D +30: ≤5.4 vs. >5.4 × 106/kg: 85% vs. 94%, p = 0.029, Fig. 1a). No difference in platelet recovery was observed (on D +60: ≤5.4 vs. >5.4 × 106/kg: 70% vs. 75%, p = 0.308; Fig. 1b). At day+30, 173 patients (95%) had blood CD3-sorted complete donor chimerism. Eight patients (4%) experienced PGF (primary: n = 6; secondary: n = 2), without any correlation with the infused CD34+ cell dose. They received CD34-selected stem cell boost in a median time of 197 days after Haplo-SCT (range: 44–224). All but 2 recovered within 32 (range: 20–98) days after CD34-selected stem cell boost infusion.
Acute and chronic GVHD
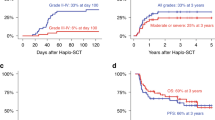
Acute GVHD occurred after a median time of 39 days (range: 16–167) after Haplo-SCT. The cumulative incidence of grade 2–4 and 3–4 acute GVHD at day+100 were 23% [95% CI: 17–29] (day+180: 26% [95% CI: 19–32]) and 8% [95% CI: 4–12] (day +180: 10% [95% CI: 6–15]), respectively (3% of grade 3 and 5% of grade 4) (Table 2). Among patients who developed acute GVHD, skin, gut, and liver were involved in 35 (73%), 17 (35%), and 4 patients (8%), respectively. Forty-one patients (83%) had only 1 organ affected (skin n = 30, gut n = 10, and liver n = 1), whereas six patients had two organs affected and only one had three organs affected. Biopsy proven for isolated gut was given in all but one patient. No biopsy was provided for the patient with isolated acute GVHD liver involvement. We observed no significant difference in the cumulative incidence of acute GVHD, whether patients had received more or less than the median CD34+ cell dose (grade 2–4: ≤5.4 vs. >5.4×106/kg: 20% vs. 27%, p = 0.129; grade 3–4: ≤5.4 vs. >5.4: 8% vs. 9%, p = 0.139; Fig. 2a, b). Although the cumulative incidence of grade 2–4 acute GVHD was similar in both younger and older patients (age <60 vs. ≥60 years: 22% vs. 24%, p = 0.575, Fig. 3a), the severity was higher in older patients with a cumulative incidence of grade 3–4 acute GVHD of 14% compared to 2% in younger patients (p = 0.014, Fig. 3b).
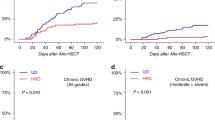
The median time to chronic GVHD occurrence after Haplo-SCT was 172 days (range: 102–644). At 2 years after Haplo-SCT, the cumulative incidence for all grades and moderate+severe chronic GVHD were 17% (95% CI: 12–23) and 9% (95% CI: 5–13; severe=4%), respectively. In seven cases, chronic GVHD followed prophylactic donor lymphocyte infusions (pDLI) that were given in a total of 17 patients. Among patients who developed chronic GVHD, 77% had de novo chronic GVHD. The skin was the most frequently involved organ (77% of patients) before mucosae (n = 19, 61%). Three patients developed severe pulmonary chronic GVHD. CD34+ cell dose did not influence the cumulative incidence of chronic GVHD (all grades: ≤5.4 vs. >5.4×106/kg: 16% vs. 20%, p = 0.739; moderate or severe: ≤5.4 vs. >5.4×106/kg: 11% vs. 7%, p = 0.358; Fig. 2c, d). Multivariate analyses confirmed that CD34+ cell dose did not significantly influence the incidence of both acute and chronic GVHD (Table 3), while age was associated with higher risk of grade 3–4 acute GVHD (p = 0.03). In addition, we did not observed significant impact of conditioning regimen intensity on the risk of both acute (with MAC as reference: p-NMAC = 0.558; p-RIC = 0.675) and chronic GVHD (with MAC as reference: p-NMAC = 0.462; p-RIC = 0.861).
Prevalence of GVHD, immusuppressive treatment (IST), and outcome
Among the 41 patients who developped grade 2–4 acute GVHD, 6 (15%) patients relapsed and 5 of them died from their hematological disease. Twelve (29%) patients died from acute GVHD. The 23 (56%) remaining patients responded to systemic GVHD treatement and were able to discontinue IST (median IST duration: 138 days, range: 79–456). Among the 31 patients who developed chronic GVHD, 2 (6%) patients died from hematological relapse and 3 (10%) patients died from chronic GVHD. Among the 26 (84%) remaining patients, 14 (74%) were able to stop IST at the time of analysis (median IST duration of 134 days, range: 25–475).
At 1 year after Haplo-SCT, 13 and 10% of disease-free patients were living with GVHD and IST, respectively. At 2 years after Haplo-SCT, almost all disease-free patients (>90%) had stopped IST and had no GVHD features (Fig. 4).
NRM and CIR
We observed a cumulative incidence of NRM at 100 days and 2 years of 11% (95% CI, 7–16) and 21% (95% CI, 15–27), respectively (Table 2). Causes of NRM were GVHD (n = 15), infections in absence of GVHD (n = 15), CNS hemorrhage (n = 1), non-well defined neurological complications (n = 2), and 2 hemolytic anemia. Fatal sinusoidal obstruction syndrome (SOS) was observed only in 1 heavily pre-treated patient. Three patients died before engraftment on day+4, +12, and +32.
Two-year CIR was 17% (95% CI, 11–23) (Table 2). The median time from Haplo-HSCT to relapse was 226 days (range: 14–1459). High or very high DRI was the only predictive factor associated with higher risk of relapse (HR = 2.36, 95%CI = [1.08–5.15], p = 0.032). Among the 54 patients transplanted for AML (25 in CR1, 29 in either CR > 1 or refractory disease at the time of Haplo-SCT), the 2-year CIR was 13% (95% CI, 4–21, 12% for patients in CR1 and 14% for those in CR > 1 or refractory disease).
PFS, OS, and GRFS
PFS and OS at 2 years were 62% (95% CI, 54–70) and 66% (95% CI, 59–74), respectively. As a surrogate to evaluate quality of life, we also analyzed the composite endpoint GRFS, which was 50% (95% CI, 43–59) at 2 years (Table 2). By multivariate analysis, high/very-high DRI was the only factor associated with significantly worse OS (HR = 2.26; 95%CI = [1.30–3.93], p = 0.004) and PFS (HR = 1.93; 95% CI = [1.15–3.25], p = 0.013), and with a trend for worse GRFS (HR = 1.48; 95% CI = [0.93–2.37], p = 0.099).
Discussion
The introduction of PT-Cy as GVHD prophylaxis for T-cell replete Haplo-HSCT, as developed by the Baltimore group [1] allowed for achieving good engraftment and low incidence of GVHD with minimal graft processing. Initial reports were using bone marrow grafts [1]. PT-Cy is also an effective GVHD prophylaxis for PBSC Haplo-HSCT, when administering conditioning regimen with different intensities [22, 23]. We previously reported an interim retrospective comparison of BM versus PBSC Haplo-HSCT [14] on 69 patients in which we observed no increased incidence of both acute and chronic GVHD, resulting in similar outcome. Our present study on a larger number of patients confirms the feasibility of PBSC Haplo-HSCT.
The BM versus PBSC graft comparison for Haplo-HSCT with PT-Cy, was assessed in different retrospective studies showing diverging results about the GVHD incidences [23,24,25,26]. Two more recent studies compared BM versus PBSC in the Haplo-HSCT setting. O’Donnell et al. [15] conducted a retrospective matched-pair analysis in patients undergoing PT-Cy Haplo-HSCT using a NMAC regimen (Flu-Cy-TBI). No significant increase in the incidence of acute and chronic GVHD (day-100 grades 2–4 acute GVHD: PBSC 40% vs BM 33% P = 0.50; 2-year all grades chronic GVHD: PBSC 23% vs BM 19% P = 0.63) was observed using PBSC grafts. On the other hand, Bashey et al. [13] showed in a large CIBMTR registry analysis that the use of PBSC was associated with a significantly higher incidence of grades 2–4 acute and chronic GVHD (6-month grades 2–4 acute GVHD: PBSC 42% vs BM 25% P < 0.001; 2-year all grades chronic GVHD: PBSC 41% vs BM 20% P < 0.001).
Two additional European studies on behalf of EBMT showed an increased risk of GVHD using PBSC rather than BM graft [27, 28]. These analysis, conducted on a large registry cohort from EBMT and CIBMTR, included patients from 99 and 350 centers, respectively, leading to a very heterogeneous experience in the field of Haplo-HSCT, different platforms of conditioning regimen and different approaches to GVHD prophylaxis [27, 28]. The last point is especially relevant in the study published by Rubio et al. [27], where PT-Cy as GVHD prophylaxis was used only in 25% of patient received a NMAC and 32% of patients received a MAC regimen. Also in the recent report of Ruggeri et al. [28], some patients (5% and 7% in BM and PBSC group, respectively) received ATG in association with PT-Cy.
Compared to the large registry analyses, our study allow a detailed analysis of GVHD (incidence, organ involvement, and prevalence) on a cohort of patients receiving the same GVHD prophylaxis treatment (PT-Cy+CSA+MMF). In this study, the cumulative incidence of grades 2–4 acute GVHD at 100 days as well as the moderate–severe chronic GVHD at 2 years, continues to be relatively low (23% and 9%, respectively). When compared to the literature, our results with PT-Cy seem to approach those observed after BM [6, 29,30,31] rather than PBSC [13, 23, 24, 28, 32] Haplo-HSCT.
Although the cumulative incidence of GVHD is a common method for evaluating this end point, the co-prevalence analysis of both GVHD and IST allows a better assessment of GVHD and of its treatment after Allo-HSCT. Indeed, we reported the proportion of patients who actually live with GHVD features and/or IST at different time points, giving a clear picture of outcome after Haplo-HSCT. Moreover, although limited by its retrospective nature, our prevalence analysis can provide information about patients’ quality of life. Among the patients alive without evidence of disease progression, 90% and 87% of patients were IST and GVHD-free after 1 year, respectively (98% and 94% at 2 years, respectively).
We were not able to identify any factor associated with an increased risk of developing acute or chronic GVHD. However, we observed that the severity of acute GVHD was higher in older (age ≥60 years) than in younger patients (grades 3–4 at day 100 ≥vs. <60 years: 14% vs. 2%, p = 0.009). In addition, we did not observe any impact of infused CD34+ cell dose on the incidence of GVHD, in both univariate and multivariate analysis. It was initially shown that CD34+ cell dose above 8.3×106/kg was associated with an increase of chronic GVHD. However, this was observed in the setting of HLA matched related Allo-HSCT prepared with MAC regimen and no ATG [33]. Our present study mostly included patients who received RIC or NMAC regimens (88%), making difficult the comparison of previous results to this different context. This was in line with our previous experience in the setting of matched related and unrelated RIC Allo-HSCT showing no impact of CD34+ cell dose [34].
Although the use of PBSC is associated with faster hematological recovery in the setting of both HLA matched related and unrelated donor, we observed a median time from Haplo-SCT to ANC and platelet recovery of 21 and 30 days, respectively. This is similar to what was observed after BM Haplo-SCT [10,11,12]. Also in previous reports on Haplo-SCT with PBSC and PT-Cy, neutrophil engraftment was observed after a median time between 15 and 17 days [23,24,25, 32]. Interestingly, we observed a better ANC recovery in patients receiving higher CD34+ cell dose (≤5.4 vs. >5.4: 85% vs. 94%, p = 0.029), but no effect was found on PLT recovery (≤5.4 vs. >5.4: 70% vs. 75%, p = 0.308).
We observed a relapse incidence of 17% in the whole cohort at 2 years. This is encouraging taking into account the baseline characteristics of the patients (i.e. high or very high DRI in 26% of patients) but the interpretation of this finding is limited by the heterogeneity of our cohort in terms of diagnoses. However, when focused on AML patients (n = 54), we observed a CIR of 13% although more than half of them (n = 29) were transplanted for advanced disease (CR ≥2 or refractory disease). This is in line with previous report from Bashey et al. showing that the use of PBSC is associated with lower CIR compared to bone marrow Haplo-SCT in the setting of AML [13]. Initial reports of bone marrow Haplo-HSCT with PT-Cy showed higher incidence of relapse [1, 30]. Taken together, these results suggest a potential benefit for disease control using PBSC.
We conclude that PT-Cy allows the use of PBSC as graft source for Haplo-HSCT without dramatically increasing the incidence of both acute and chronic GVHD. The overall outcome is promising, with most of disease-free patients (>90%) actually living without IST or GVHD features, suggesting a preserved long-term quality of life. However, severe acute GVHD in older patients remains a concern justifying the optimization of the PT-Cy platform in this specific setting. Beyond the feasibility, the use of PBSC for PT-Cy Haplo-HSCT seems associated with promising antitumor effect. This need to be prospectively evaluated in a disease specific manner.
References
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54.
Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–71.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6.
Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20:1975–81.
McCurdy SR, Fuchs EJ. Comparable outcomes for hematologic malignancies after HLA-haploidentical transplantation with posttransplantation cyclophosphamide and HLA-matched transplantation. Adv Hematol. 2015;2015:431923.
Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811–7.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96.
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81.
Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Société Française de Greffe de Moelle. J Clin Oncol. 2000;18:537–46.
Bashey A, Zhang M-J, McCurdy SR, St Martin A, Argall T, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for Tcell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Castagna L, Crocchiolo R, Furst S, Bramanti S, El Cheikh J, Sarina B, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20:724–9.
O’Donnell PV, Eapen M, Horowitz MM, Logan BR, DiGilio A, Brunstein C, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant. 2016;51:1599–601.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Klyuchnikov E, El-Cheikh J, Sputtek A, Lioznov M, Calmels B, Furst S, et al. CD34(+)-selected stem cell boost without further conditioning for poor graft function after allogeneic stem cell transplantation in patients with hematological malignancies. Biol Blood Marrow Transplant. 2014;20:382–6.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Devillier R, Granata A, Fürst S, Harbi S, Faucher C, Weiller P-J, et al. Low incidence of chronic GVHD after HLA-haploidentical peripheral blood stem cell transplantation with post-transplantation cyclophosphamide in older patients. Br J Haematol. 2017;176:132–5.
Solomon SR, Solh M, Morris LE, Holland HK, Bashey A. Myeloablative conditioning with PBSC grafts for T cell-replete haploidentical donor transplantation using posttransplant cyclophosphamide. Adv Hematol. 2016;2016:9736564.
Bhamidipati PK, DiPersio JF, Stokerl-Goldstein K, Rashidi A, Gao F, Uy GL, et al. Haploidentical transplantation using G-CSF-mobilized T-cell replete PBSCs and post-transplantation CY after non-myeloablative conditioning is safe and is associated with favorable outcomes. Bone Marrow Transplant. 2014;49:1124–6.
Raj K, Pagliuca A, Bradstock K, Noriega V, Potter V, Streetly M, et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:890–5.
Sugita J, Kawashima N, Fujisaki T, Kakihana K, Ota S, Matsuo K, et al. HLA-haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide after busulfan-containing reduced-intensity conditioning. Biol Blood Marrow Transplant. 2015;21:1646–52.
Rubio MT, Savani BN, Labopin M, Piemontese S, Polge E, Ciceri F, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25.
Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018. https://doi.org/10.1002/cncr.31228.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50:S37–39. Suppl 2
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8.
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai H-L, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–61.
Bradstock K, Bilmon I, Kwan J, Blyth E, Micklethwaite K, Huang G, et al. Influence of stem cell source on outcomes of allogeneic reduced-intensity conditioning therapy transplants using haploidentical related donors. Biol Blood Marrow Transplant. 2015;21:1641–5.
Mohty M, Bilger K, Jourdan E, Kuentz M, Michallet M, Bourhis JH, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17:869–75.
Collignon A, Calmels B, Harbi S, Fürst S, Granata A, Faucher C, et al. Impact of CD34-positive cell dose on outcome after peripheral blood stem cell allogeneic transplantation prepared with ATG-based reduced intensity conditioning regimen. Am J Hematol. 2017;92:E57–E59.
Acknowledgements
We thank the patients and the IPC transplantation staff.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Granata, A., Fürst, S., Bramanti, S. et al. Peripheral blood stem cell for haploidentical transplantation with post-transplant high dose cyclophosphamide: detailed analysis of 181 consecutive patients. Bone Marrow Transplant 54, 1730–1737 (2019). https://doi.org/10.1038/s41409-019-0500-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0500-x
- Springer Nature Limited
This article is cited by
-
Peripheral blood haploidentical hematopoietic cell transplantation for patients aged 70 years and over with acute myeloid leukemia or high-risk myelodysplastic syndrome
Bone Marrow Transplantation (2024)
-
Haploidentical stem cell transplantation for patients with lymphoma: a position statement from the Lymphoma Working Party-European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2020)
-
Absence of influence of peripheral blood CD34+ and CD3+ graft cell counts on outcomes after reduced-intensity conditioning transplantation using post-transplant cyclophosphamide
Annals of Hematology (2020)