Abstract
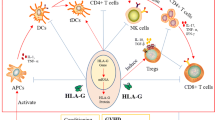
HLA-G is a non-classical class I molecule which induces tolerance in allogeneic situations by inhibition of cytotoxic NK and CD8 + T cells and by induction of regulatory T cells. Concordantly, in solid organ transplantation HLA-G is associated with a lower risk for acute and chronic rejection, whereas its role in allogeneic stem cell transplantation (allo-SCT) is less established. We here present detailed analyses of HLA-G-levels in patients after allo-SCT showing a correlation of elevated soluble HLA-G (sHLA-G) levels with less severe acute (p = 0.06) and chronic GvHD (p = 0.0025) and with a superior overall survival (p = 0.03). Soluble HLA-G levels are also positively correlated with the frequency of regulatory T cells in vivo. These clinical data are corroborated by in vitro analyses showing that patients-derived sHLA-G inhibit allogeneic immune responses. ATG-treatment of patients dominantly affects the sHLA-G levels post allo-SCT. Thus, this study highlights the association of elevated sHLA-G levels with less severe acute and chronic GvHD and provides additional functional analyses elucidating possible tolerance-inducing mechanisms of sHLA-G in the context of allo-SCT.
Similar content being viewed by others
Introduction
Human leukocyte antigen G (HLA-G) is a non-classical class I molecule with tolerogenic properties [1]. The HLA-G gene is located within the major histocompatibility complex on chromosome 6 and is composed of 8 exons and 7 introns. Through alternative splicing of the primary HLA-G transcript seven mature mRNAs can be produced resulting in seven different isoforms: four membrane-bound (HLA-G1, G2, G3, and G4) and three soluble isoforms (HLA-G5, G6, and G7) [2]. Membrane-anchored HLA-G and its soluble forms (sHLA-G) exert multiple functions favoring immune tolerance in allogeneic situations such as pregnancy and transplantation [1]. In contrast to classical HLA class I, HLA-G displays a low polymorphism with only 47 HLA G alleles in the coding region resulting in 15 different proteins, and 2 null alleles [3], but it presents a higher degree of nucleotide variations in the regulatory regions 5′URR (upstream regulatory region) and 3′UTR (untranslated region), which influence the HLA-G expression [4, 5]. Of special interest is the 14 base pair insertion/deletion (14 bp ins/del) polymorphism (rs66554220) in the 3′UTR of the HLA-G gene [6] as low blood levels of sHLA-G molecules could be associated to individuals being homozygous for the 14 bp ins/ins genotype [7, 8]. Furthermore nucleotide variation at position + 3142 influences the binding of specific microRNA, including miR-148a, miR-148b, and miR-152 and thereby the degree of HLA-G expression [9,10,11]. Guanine at the +3142 position increases the affinity of this region to these microRNAs, leading to decreased HLA-G expression due to mRNA degradation and translation suppression. Both, the 14 bp ins/del and the +3142 C/G polymorphism, are considered to be the most important ones with respect to posttranscriptional regulation of HLA-G.
HLA-G differs from classical HLA with respect to the receptors it binds to: HLA-G does not engage T-cell receptors and binds to three predominantly inhibitory receptors: ILT2, ILT4, and KIR2DL4 [1, 12]. Through the interaction with these ligands HLA-G can exert its multiple immunosuppressive functions: inhibition of cytotoxic NK and CD8 + T cells, inhibition of alloreactive CD4 + T cells, inhibition of dendritic and antigen presenting cells, and induction of regulatory T cells [12]. Soluble HLA-G modulates cytokine secretion in CD56bright and CD56dim NK cells and has also influence on TCR gamma/delta T cells [3, 13]. HLA-G plays a central role in pregnancy as the only physiological situation of tolerance towards a semi-allograft: the inhibition of maternal NK cells by HLA-G-expressing fetal cells leading to tolerance of the allogeneic fetus [13, 14]. In the context of pregnancy the production of HLA-G protein is restricted to trophoblasts and is expressed on human embryonic stem cells [2]. Moreover, the immunosuppressive effects of mesenchymal stem cells (MSC) seem to be related to HLA-G. HLA-G5 expression and secretion by MSCs was demonstrated to contribute to the inhibition of NK cells and allogeneic T-cell responses [15, 16]. Physiologically, HLA-G is only marginally expressed on cornea, thymus, pancreatic islets, and blood precursor cells [3, 12]. However, HLA-G is expressed in the allograft after transplantation and in diverse pathological conditions, such as autoimmune diseases, viral infections or tumors [3].
Special attention has been brought to HLA-G in the context of transplantation. In solid organ transplantation various studies have associated HLA-G with a lower risk for the development of acute and chronic rejection [17, 18] suggesting that it regulates the allogeneic response. In kidney or kidney/pancreas transplantation it was shown that low plasma levels of sHLA-G before and after transplantation are associated with acute rejection [19]. In the context of allogeneic stem cell transplantation (allo-SCT) a few studies indicated an association of increased level of sHLA-G with the absence or only a mild manifestation of acute GvHD. Le Maux et al. studied a cohort of 20 patients undergoing allo-SCT and observed a correlation between low sHLA-G levels post allo-SCT and the occurrence of acute GVHD [20]. Liu et al. confirmed these findings in 106 patients after allo-SCT. They specifically observed significantly increased levels of sHLA-G5 on days +15 and +30 after transplantation in patients with grade 0–I acute GVHD (aGvHD) compared to those with grade II–IV aGvHD [21]. However, Waterhouse et al. could not confirm an association between sHLA-G plasma concentration and the occurrence of GvHD [22]. Of course, other factors like HLA disparity or other genetic factors, conditioning regimens, or GvHD prophylaxis might explain these contradictory findings.
Considering the tolerance-mediating features of sHLA-G molecules and its role in solid organ transplantation, the aim of this study was to carve out, whether and under which conditions sHLA-G is a reliable marker predicting clinical outcome after allo-SCT. To this end a systematical analysis of post allo-SCT-derived sHLA-G was undertaken (i) regarding the relationship of sHLA-G and the clinical endpoints severe aGvHD, severe chronic GvHD (cGvHD) and overall survival (OS), (ii) regarding the capability of sHLA-G to modulate an allogeneic immune response in vitro, (iii) regarding the association of sHLA G with regulatory T cells in vivo and (iv) regarding the influence of ATG treatment on sHLA-G levels post allo-SCT.
Materials and methods
Patients’ characteristics and study design
Thirty-two patients, 21 female and 11 male, were enrolled in the study. These patients underwent allo-SCT at the Department of Bone Marrow Transplantation of the University Hospital Essen, Germany. The demographic profile of patients is shown in Table 1. This monocentric study was planned prospectively, approved by the Ethical Board of the University Hospital of Essen and carried out in accordance to the Helsinki Declaration. All patients signed a written consent form to participate in this study. Ethylenediaminetetraacetate (EDTA) plasma samples were serially procured from the patients before and 1, 2, 3, 4, 5, 6, 9, and 12 month(s) after transplantation. EDTA plasma samples were additionally collected from 21 donors before GCSF-treatment.
Conditioning regimens and GvHD prophylaxis
All patients received myeloablative conditioning. 14 of the 32 patients received anti-thymocyte globulin (ATG) as in vivo T-cell depletion. 9 patients received total body irradiation as part of the conditioning regimen. The median follow-up time was 1520 days (range: 38–2004) after allo-SCT.
Quantification of sHLA-G
The determination of sHLA-G was performed as described previously [23]. Plasma samples were diluted 1:2 in PBS and tested in duplicate. Purified sHLA-G5 protein served as standard reagent and 3,3′,5,5′-tetramethybenzidine as substrate solution.
Further information regarding Patients, GvHD diagnosis, isolation of plasma derived HLA-G molecules to microspheres and mixed lymphocyte reaction can be found in Supplementary Materials and Methods.
Cytokine ELISA
Interferon gamma (IFN-γ) levels were quantified in supernatants after mixed lymphocyte reaction (MLR) by using commercial ELISA kits (eBioscience, Inc., San Diego, USA) according to their protocols.
Genotyping of 14 bp and C/G + 3142 polymorphisms in the 3′UTR of the HLA-G gene
Genotyping of the 14 bp and C/G + 3142 polymorphisms of the HLA-G gene was performed as previously described [24,25,26,27] in 31 out of 32 patients and donors.
Flow cytometric analyses
Regulatory T cells (Tregs) were detected using anti-CD3 AlexaFluor700 (BioLegend, USA), anti-CD4 Pacific Blue (BioLegend, USA), anti-CD8 PE-Cy7 (Beckman Coulter, Germany), anti-CD25 ECD (Beckman Coulter), and anti-CD127-APC (eBioscience) antibody mix.
Statistics
Data are presented as mean ± SEM (standard error of mean). After testing for Gaussian distribution, continuous variables were compared by T-test and non-parametric Mann–Whitney or two-way analysis of variance. For categorical data, Fisher’s exact test was used. The cut-off value for sHLA-G was determined regarding optimal values of sensitivity and specificity for the prediction of OS using Stoller’s univariate non-parametric determination analysis. Probabilities of the patients’ OS were analyzed by the Kaplan–Meier method combined with log-rank test. Pearson or nonparametric Spearman correlation was used to correlate the sHLA-G levels to functional data and the presence of CD4 + CD25 + CD127- T cells. Statistical significance was defined as p ≤ 0.05.
Results
Post allo-SCT HLA-G plasma levels and their clinical association to aGvHD, cGvHD, and OS
To study the clinical relevance of sHLA-G levels on allo-SCT outcome, the course of sHLA-G in the first 12 months post SCT was related to aGvHD, cGvHD, and OS. There was a clear trend - although without reaching statistical significance—(p = 0.06, Fig. 1a) of higher sHLA-G levels in patients with aGvHD grade 0–I as compared to patients with aGvHD grade II–IV. The difference in sHLA-G levels was more prominent during the whole observation period, when patients with no or mild cGvHD were compared to patients with severe cGvHD (p = 0.0025, Fig. 1b). A similar observation was made for survival. Patients being alive at the time point of analysis displayed higher sHLA-G levels than patients who did not survive post allo-SCT (p = 0.03, Fig. 1c). Using a sHLA-G level of 35 ng/ml as cutoff, the patients with sHLA-G levels of >35 ng/ml experienced an improved OS compared to patients with sHLA-G levels of <35 ng/ml (p = 0.01, log-rank Hazard Ratio 3.551, 95% CI of ratio 1.39 to 17.28, Fig. 2). No association of the +3142 C/G and the 14 bp ins/del polymorphisms was found regarding acute/chronic GvHD or OS (data not shown). Taken together, it seems that high levels of sHLA-G during the first year post allo-SCT are independent from the +3142 C/G and the 14 bp ins/del polymorphisms. Moreover, sHLA-G levels are associated with an improved clinical outcome in terms of allogeneic immune reaction as it is clinically manifested by the occurrence of severe GvHD and in terms of OS. Plasma levels of sHLA-G of recipients were 3-fold increased one month after allo-SCT [86.8 ± 12.3 SEM (95% confidence interval: 61.5–112) ng/ml] compared with the ones before allo-SCT [30.3 ± 3.9 SEM (95% confidence interval: 22.1–38.5) ng/ml]; p = 0.0002, Suppl. Figure 2, which is mostly caused by the ATG-treated patients, whereas sHLA-G levels of donors did not differ from the ones of recipients before and one month after allo-SCT [50.2 ± 5.6 SEM (95% confidence interval: 38.4–61.9) ng/ml]. Again, enhanced sHLA-G levels post allo-SCT were found to be independent from the +3142 C/G and the 14 bp ins/del polymorphisms (Suppl. Figure 3A and B).
The course of HLA-G levels in relationship to severe acute GvHD (a), chronic GvHD (b), and survival (c) before and during the first year post allo-SCT. The horizontal dashed lines represent the mean sHLA-G value (50.2 ng/ml) with 95% confidential interval (38.4 ng/ml; 61.9 ng/ml) of allo-SCT donors (n = 21). Two-way ANOVA was used for statistical analysis
Patient-derived sHLA-G inhibit allogeneic immune response in vitro
To test the capability of patients-derived sHLA-G to suppress an allogeneic immune response in vitro, microspheres were loaded with sHLA-G molecules derived from patients’ plasma samples 1 month after allo-SCT and co-cultured with PBMC in MLR. As shown in Fig. 3a the allogeneic proliferation response was inversely correlated to the amount of sHLA-G being added via microspheres to the MLR (r = −0.804, p = 0.0009). Additionally, a significant negative correlation was observed between sHLA-G and IFN-γ levels in supernatants obtained after MLR (r = −0.655, p = 0.015, Fig. 3b). These results clearly evidence that circulating sHLA-G molecules in the blood of allo-SCT patients are functionally active in vitro as they impair the allogeneic proliferation and IFN-γ response in a concentration dependent manner.
The capacity of plasma derived sHLA-G molecules to suppress allogeneic immune proliferation and cytokine response in mixed leukocyte reaction (MLR). The vertical dashed lines represent the mean sHLA-G value (50.2 ng/ml) with 95% confidential interval (38.4 ng/ml; 61.9 ng/ml) of allo-SCT donors (n = 21). Pearson correlation was used for statistical analysis
Positive correlation of sHLA-G plasma levels with the frequency of regulatory T cells in vivo
As HLA-G and sHLA-G molecules are reported to be involved in the generation of Tregs and thereby to be involved in the induction of long-term tolerance [1, 28], sHLA-G plasma levels were further correlated with the frequency of Tregs having the phenotype CD4+/CD25+/CD127− on CD3 + T cells post allo-SCT in 14 patients. As shown in Fig. 4, the percentages of Tregs were positively correlated with sHLA-G plasma levels in allo-SCT patients (r = 0.622, p = 0.02, Fig. 4). This suggests that the amount of circulating sHLA-G molecules might influence the frequency of Tregs in the patients post allo-SCT.
Correlation of sHLA-G plasma levels with proportion of regulatory T cells. Plasma samples were taken at various time points: 1, 2, 3, 4, 5, 6 or 7 month(s) (M1, M2, M3, M4, M5, M6, and M7) post allo-SCT. The vertical dashed lines represent the mean sHLA-G value (50.2 ng/ml) with 95% confidential interval (38.14 ng/ml; 61.9 ng/ml) of allo-SCT donors (n = 21). Spearman correlation was used for statistical analysis
The dominant influence of ATG treatment on sHLA-G plasma levels post allo-SCT
We then analyzed whether the conditioning regimens affect sHLA-G levels post allo-SCT. By stratifying the patients into groups of ATG-treated (n = 14) and patients without ATG-treatment (n = 18) it became evident that one month post allo-SCT the mean HLA-G level of ATG treated patients was increased nearly 4-fold (147.7 ± 13.8 SEM ng/ml) compared to patients without ATG-treatment (36.8 ± 4.2 SEM ng/ml, Fig. 5a). The sHLA-G levels of the ATG-treated patients continuously decreased over time, reaching the range of the donors’ sHLA-G on month 10 post allo-SCT. At variance to ATG-treated patients (p < 0.0001), the course of HLA-G of non ATG-treated patients remained in range of the 95% CI of the donors’ sHLA-G levels (n = 21) without any substantial variations during the whole observation period post allo-SCT (Fig. 5a). In contrast, irradiation during conditioning did not reveal a substantial influence on the course of sHLA-G levels (Fig. 5b).
Discussion
The induction of tolerance by HLA-G and its soluble counterparts can be differentiated in short- and long-term tolerance. Short-term tolerance can be achieved via inhibition of T, NK, and B cells via ILT2, by inhibition of cytotoxicity of T and NK cells via ILT2 or CD94/NKG2A, by deletion of T and NK cells via CD8 and by inhibition of antibody production in B cells via ILT2 [1]. Long-term tolerance can be sustained by induction of suppressor T cells, tolerogenic dendritic cells and regulatory T cells. This study confirms the published observation that sHLA-G molecules seem to be effective in prevention of acute GvHD: they were found enriched in plasma of patients without aGvHD, and low sHLA-G levels were associated with the occurrence of aGvHD [20, 21]. Our data confirm a negative correlation between sHLA-G plasma levels and the severity of aGvHD (grade 0–I vs. II–IV). Likewise in patients suffering from severe cGvHD, sHLA-G levels were significantly reduced compared to patients with no or only moderate cGvHD. This to our knowledge is the first study to show this association of sHLA-G and cGvHD.
With respect to functional analyses we could add further evidence on possible tolerance-inducing mechanisms of plasma-derived sHLA-G molecules. In accordance with experimental data, we provide substantial evidence that sHLA-G molecules derived from patients one month post allo-SCT impair the proliferation and IFN-γ response in an allogeneic MLR in a concentration dependent manner in vitro [14, 15, 18]. Our data further demonstrate a positive correlation of sHLA-G plasma levels after allo-SCT with the proportion of regulatory T cells. This observation is in line with experimental data and supports the notion that sHLA-G is involved in the induction and expansion of regulatory T cells in vivo [12, 15, 16, 18, 28,29,30]. Interestingly, a recent report has demonstrated that the application of mesenchymal stem cell derived exosomes containing high concentrations of HLA-G in a patient with steroid-refractory severe acute GvHD has led to a remarkable improvement in GvHD symptoms [31]. In view of this, it is an intriguing notion that HLA-G could be a specific marker for the therapeutic approaches using MSCs or MSC-derived exosomes to treat steroid-refractory severe acute GvHD.
Immunosuppressants appear to have different effects on HLA-G transcription and protein expression: steroids upregulate them, cyclosporine and mycophenolate mofetil appear to have no effect on HLA-G expression, everolimus on the contrary is associated with high HLA-G levels [1, 3, 32]. In this study we elucidated particularly the influence of ATG on sHLA-G and its associated effects after allo-SCT. In general, patients treated with ATG during conditioning showed significantly higher levels of sHLA-G. In contrast, irradiation did not have a consistent influence on sHLA-G. Although further investigations are needed, to our knowledge this study provides the first substantial evidence that ATG treatment strikingly affects circulating amounts of sHLA-G molecules. In this context it is remarkable that ATG is also reported to stimulate the enhanced secretion of interleukin-10 – a cytokine, which selectively induces HLA-G expression by monocytes [33, 34]. Even more, ATG has the capability to promote the expansion of regulatory T cells by so far not clarified pathways [33]. Here it is tempting to speculate that ATG leads to the production of sHLA-G via IL-10, which in turn results in the induction of regulatory T cells.
It has been reported that the genotype of 14 bp polymorphism is significantly associated with sHLA-G expression [7]. Specifically, the 14 bp insertion polymorphism of HLA-G was associated with promoting immune tolerance and preventing aGvHD [35]. Other studies, however, did not confirm a correlation between HLA-G 14 bp polymorphism and the risk of aGvHD occurrence [36]. A recent study showed furthermore no association between the HLA-G 14 bp polymorphism, the soluble HLA-G level and aGvHD, disease recurrence, or death [22]. Hence, the interplay between the 14 bp polymorphism, the presence of soluble HLA-G and ATG requires further analysis, preferably in a larger cohort.
Our study underlines the tolerogenic potential of HLA-G in the context of allogeneic stem cell transplantation. We have added further insights, e.g. the influence of ATG on sHLA-G. This might open new intriguing aspects of HLA-G in allo-SCT. Since our data describe a positive association of high sHLA-G levels with low incidence of severe acute and chronic GvHD, this might be useful to identify patients who might be candidates for a reduction in immunosuppressive treatment, which is particularly important in patients with high relapse risk. Moreover, especially in association with immunosuppressive MSC, HLA-G might be used as a tolerogenic agent or serve as a surrogate parameter to identify MSCs with the highest immunosuppressive potential.
References
Rebmann V, da Silva Nardi F, Wagner B, Horn PA. HLA-G as a tolerogenic molecule in transplantation and pregnancy. J Immunol Res. 2014;2014:297073.
Verloes A, Van de Velde H, LeMaoult J, Mateizel I, Cauffman G, Horn PA, et al. HLA-G expression in human embryonic stem cells and preimplantation embryos. J Immunol. 2011;186(4):2663–71.
Gonzalez A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49(3):63–84.
Martelli-Palomino G, Pancotto JA, Muniz YC, Mendes-Junior CT, Castelli EC, Massaro JD, et al. Polymorphic sites at the 3’ untranslated region of the HLA-G gene are associated with differential hla-g soluble levels in the Brazilian and French population. PLoS ONE. 2013;8(10):e71742.
Castelli EC, Mendes-Junior CT, Veiga-Castelli LC, Roger M, Moreau P, Donadi EA. A comprehensive study of polymorphic sites along the HLA-G gene: implication for gene regulation and evolution. Mol Biol Evol. 2011;28(11):3069–86.
Hviid TV, Hylenius S, Rorbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenetics. 2003;55(2):63–79.
Chen XY, Yan WH, Lin A, Xu HH, Zhang JG, Wang XX. The 14 bp deletion polymorphisms in HLA-G gene play an important role in the expression of soluble HLA-G in plasma. Tissue Antigens. 2008;72(4):335–41.
Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics. 2004;56(3):135–41.
Tan Z, Randall G, Fan J, Camoretti-Mercado B, Brockman-Schneider R, Pan L, et al. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Human Genet. 2007;81(4):829–34.
Veit TD, Chies JA. Tolerance versus immune response—microRNAs as important elements in the regulation of the HLA-G gene expression. Transplant Immunol. 2009;20(4):229–31.
Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, et al. In silico analysis of microRNAS targeting the HLA-G 3’ untranslated region alleles and haplotypes. Hum Immunol. 2009;70(12):1020–5.
Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111(10):4862–70.
Carosella ED. The tolerogenic molecule HLA-G. Immunol Lett. 2011;138(1):22–4.
Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43(2):203–11.
Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem Cells. 2008;26(1):212–22.
Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164(1):1–8.
Deschaseaux F, Delgado D, Pistoia V, Giuliani M, Morandi F, Durrbach A. HLA-G in organ transplantation: towards clinical applications. Cell Mol Life Sci. 2011;68(3):397–404.
Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+T cells suppresses the allo-proliferative response: a CD4+T cell regulatory mechanism. Proc Natl Acad Sci USA. 2001;98(21):12150–5.
Rebmann V, Bartsch D, Wunsch A, Mollenbeck P, Golda T, Viebahn R, et al. Soluble total human leukocyte antigen class I and human leukocyte antigen-G molecules in kidney and kidney/pancreas transplantation. Hum Immunol. 2009;70(12):995–9.
Le Maux A, Noel G, Birebent B, Grosset JM, Vu N, De Guibert S, et al. Soluble human leucocyte antigen-G molecules in peripheral blood haematopoietic stem cell transplantation: a specific role to prevent acute graft-versus-host disease and a link with regulatory T cells. Clin Exp Immunol. 2008;152(1):50–6.
Liu H, Chen Y, Xuan L, Wu X, Zhang Y, Fan Z, et al. Soluble human leukocyte antigen G molecule expression in allogeneic hematopoietic stem cell transplantation: good predictor of acute graft-versus-host disease. Acta Haematol. 2013;130(3):160–8.
Waterhouse M, Duque-Afonso J, Wasch R, Bertz H, Finke J. Soluble HLA-G molecules and HLA-G 14-base pair polymorphism after allogeneic hematopoietic cell transplantation. Transplant Proc. 2013;45(1):397–401.
Rebmann V, LeMaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Quantification and identification of soluble HLA-G isoforms. Tissue Antigens. 2007;69(Suppl 1):143–9.
Hviid TV, Hylenius S, Hoegh AM, Kruse C, Christiansen OB. HLA-G polymorphisms in couples with recurrent spontaneous abortions. Tissue Antigens. 2002;60(2):122–32.
Nuckel H, Castelli EC, Moreau P, Ochsenfarth C, Horn PA, Rebmann V. Simple methods for the detection of HLA-G variants in coding and non-coding regions. Methods Mol Biol. 2012;882:123–42.
Bortolotti D, Gentili V, Melchiorri L, Rotola A, Rizzo R. An accurate and reliable real time SNP genotyping assay for the HLA-G+3142 bp C > G polymorphism. Tissue Antigens. 2012;80(3):259–62.
Zambra FM, Chies JA, Alho CS, Veit TD. Response to Bortolotti et al. 2012--a re-evaluation of our polymerase chain reaction-restriction fragment length polymorphism genotyping method. Tissue Antigens. 2013;82(4):286–7.
Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35(4):1133–42.
Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011;118(25):6499–505.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–44.
Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–3.
Sheshgiri R, Gustafsson F, Sheedy J, Rao V, Ross HJ, Delgado DH. Everolimus but not mycophenolate mofetil therapy is associated with soluble HLA-G expression in heart transplant patients. J Heart Lung Transplant. 2009;28(11):1193–7.
Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+regulatory T cells in vitro. Blood. 2008;111(7):3675–83.
Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11(5):803–11.
La Nasa G, Littera R, Locatelli F, Lai S, Alba F, Caocci G, et al. The human leucocyte antigen-G 14-basepair polymorphism correlates with graft-versus-host disease in unrelated bone marrow transplantation for thalassaemia. Br J Haematol. 2007;139(2):284–8.
Chiusolo P, Bellesi S, Piccirillo N, Giammarco S, Marietti S, De Ritis D, et al. The role of HLA--G 14-bp polymorphism in allo-HSCT after short-term course MTX for GvHD prophylaxis. Bone Marrow Transplant. 2012;47(1):120–4.
Acknowledgements
This study was supported by the Deutsche José Carreras Leukämie-Stiftung e.V. (DJCLS R 07/06 v). Special thanks go to the patients and donors providing the blood samples for this study. We are grateful for the technical support of Sabine Schramm, Monika Collenburg, Ines Krimphoff, Ursel Hill, and Martina Franke as well as other members of the team of the Institute for Transfusion Medicine and the colleagues from the department of Bone Marrow Transplantation, all University Hospital Essen.
Author contributions
VR and LK conceived, designed and performed the experiments, analyzed the data and wrote the manuscript. FSN, BW, MD, TL, ML, FMH, PAH, and DWB confirmed the analyses and assisted in correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kordelas, L., da Silva Nardi, F., Wagner, B. et al. Elevated soluble human leukocyte antigen G levels in patients after allogeneic stem cell transplantation are associated with less severe acute and chronic graft-versus-host disease. Bone Marrow Transplant 53, 1149–1156 (2018). https://doi.org/10.1038/s41409-018-0145-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0145-1
- Springer Nature Limited