Abstract
Cardiac inflammation contributes to heart failure (HF) induced by isoproterenol (ISO) through activating β-adrenergic receptors (β-AR). Recent evidence shows that myeloid differentiation factor 2 (MD2), a key protein in endotoxin-induced inflammation, mediates inflammatory heart diseases. In this study, we investigated the role of MD2 in ISO-β-AR-induced heart injuries and HF. Mice were infused with ISO (30 mg·kg−1·d−1) via osmotic mini-pumps for 2 weeks. We showed that MD2 in cardiomyocytes and cardiac macrophages was significantly increased and activated in the heart tissues of ISO-challenged mice. Either MD2 knockout or administration of MD2 inhibitor L6H21 (10 mg/kg every 2 days, i.g.) could prevent mouse hearts from ISO-induced inflammation, remodelling and dysfunction. Bone marrow transplantation study revealed that both cardiomyocyte MD2 and bone marrow-derived macrophage MD2 contributed to ISO-induced cardiac inflammation and injuries. In ISO-treated H9c2 cardiomyocyte-like cells, neonatal rat primary cardiomyocytes and primary mouse peritoneal macrophages, MD2 knockout or pre-treatment with L6H21 (10 μM) alleviated ISO-induced inflammatory responses, and the conditioned medium from ISO-challenged macrophages promoted the hypertrophy and fibrosis in cardiomyocytes and fibroblasts. We demonstrated that ISO induced MD2 activation in cardiomyocytes via β1-AR-cAMP-PKA-ROS signalling axis, and induced inflammatory responses in macrophages via β2-AR-cAMP-PKA-ROS axis. This study identifies MD2 as a key inflammatory mediator and a promising therapeutic target for ISO-induced heart failure.
Similar content being viewed by others
Introduction
Heart failure (HF) is the ultimate outcome of most cardiovascular disorders with high prevalence and poor prognosis [1]. Generally, the HF patients show a hyper-active sympathetic nervous system (SNS) with elevated plasma catecholamine levels that correlate with disease severity [2]. The SNS hyperactivity is initially a compensatory mechanism to stimulate contractility and maintain cardiac output. Unfortunately, this chronic stimulation becomes detrimental and causes decreased cardiac function, reduced inotropic reserve, and increased cardiac hypertrophy, fibrosis, and arrhythmias [3]. In addition to these pathological processes, increasing evidences have indicated the importance of SNS-induced inflammatory responses in mediating HF. The chronic β-adrenergic receptor (β-AR) stimulation in myocardium using β-AR agonist, isoproterenol (ISO), is sufficient to induce expression of several myocardial proinflammatory cytokines, including interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), which contribute to myocardial damage and long-term pathological remodelling [4, 5]. It has also been reported that treatment with β-AR blockers was negatively correlated with circulating levels of inflammatory cytokines in patients with chronic HF [6, 7]. There are several subtypes of β-ARs including β1-AR, β2-AR, and β3-AR. β1-AR and β2-AR are vital in the regulation of excitation-contraction coupling of a myocardium. The β1-AR comprises 75%–80% of β-ARs found in the heart, and β2-AR comprises 20%–25% of cardiac β-ARs [8]. It has been reported that β1-AR highly expressed in cardiomyocytes and β2-AR highly expressed in macrophages mediate ISO-induced inflammatory activation in HF [9]. Blocking the over-activation of inflammatory responses is proposed as a promising strategy to protect against ISO-induced cardiac remodelling [10]. However, the detailed mechanisms by which β-AR activation induces cardiac inflammation remains elusive. Illustrating the pro-inflammatory mechanism of ISO-β-AR signalling in hearts may provide new therapeutic strategy and target for the treatment of HF.
Myeloid differentiation factor 2 (MD2) is a key protein, which mediates lipopolysaccharide (LPS)-induced innate immune and inflammatory response [11]. Upon LPS challenge, MD2 is required for the activation of toll-like receptor 4 (TLR4) in the form of dimerization complex, which further recruits myeloid differentiation primary response-88 (MyD88) and then activates TGFβ-activated kinase-1 (TAK1) and nuclear factor-kappa B (NF-κB) to upregulate the expression of inflammatory cytokine genes [12]. Recent studies have reported that MD2-TLR4 signalling also regulates chronic and sterile inflammation in the pathogenesis of some non-infectious disorders, including heart diseases [13, 14]. In patients with dilated cardiomyopathy, a positive correlation was found between the serum MD2 level and mortality rate [15]. Our previous studies showed that MD2 mediates angiotensin II-, palmitic acid-, and high-concentration glucose-induced cardiac inflammation and remodelling via activating TLR4-Myd88-TAK1-IKKβ-NF-κB signalling cascade to up-regulate the gene expression of pro-inflammatory cytokines in both cardiomyocytes and macrophages [13, 16, 17]. However, it remains unknown whether MD2 is involved in ISO-β-AR-related cardiac inflammation and HF.
In this study, we found that cardiomyocyte MD2 and bone marrow-derived cell MD2 were significantly up-regulated in ISO-challenged mouse hearts. MD2 deficiency improved ISO-induced cardiac inflammation, hypertrophy and dysfunction. We also presented a mechanism by which ISO triggers inflammatory genesis and injuries in both cardiomyocytes and macrophages, which underscores the importance of MD2 as a new and potential therapeutic target for HF caused by adrenergic stimulation.
Methods and materials
General reagents
A list of reagents used in this study is provided in Supplementary Table S1. Compound L6H21, a selective MD2 inhibitor, was synthesized, validated, and prepared with a purity of 98.9% as described by our report [18].
Animal experiments
All animal care and experimental procedures were approved by the Wenzhou Medical University Animal Policy and Welfare Committee (Approval Document No. wydw2021-1016). All animals received humane care according to the NIH guidelines (Guide for the care and use of laboratory animals). Male C57BL/6 (wild-type, WT) mice were obtained from the Animal Centre of Wenzhou Medical University. Male MD2 knockout (MD2KO) mice (B6.129P2-Ly96 KO) with C57BL/6 background were provided by Riken BioResource Centre of Japan (Tsukuba, Ibaraki, Japan). MD2KO mice and WT mice were maintained in a SPF environment with 12 h of light and adequate access to food and water. The animals were acclimatized to the laboratory for at least 2 weeks before initiating the studies. ISO was dissolved in 0.002% ascorbic acid and administered using osmotic mini-pumps that delivered 30 mg·kg−1·d−1 ISO (Alzet MODEL 1002, CA; USA) for 2 weeks, as described previously [19]. All animal experiments were performed and analyzed by blinded experimenters. Randomization were used when dividing the groups. At the end of the study, the mice were sacrificed to collect serum and heart tissue samples. We used 2 mouse models of ISO-induced cardiac dysfunction.
Model 1: Eight-week-old C57BL/6 mice and MD2KO mice were randomly divided into 6 groups: (1) untreated C57BL/6 mice (WT; n = 7); (2) ISO-infused C57BL/6 mice (ISO; n = 7); (3) untreated MD2KO mice (MD2-/-; n = 7); (4) ISO-infused MD2KO mice (MD2-/- + ISO; n = 7); (5) L6H21 reconstituted in 1% CMC-Na solution was administered by oral gavage at 10 mg/kg every 2 days. ISO-infused C57BL/6 mice with L6H21 treatment (L6H21 + ISO; n = 7); and (6) propranolol reconstituted in sterile water was administered by oral gavage at 40 mg/kg every day. ISO-infused C57BL/6 mice with propranolol treatment (Prop+ISO; n = 7).
Model 2: One week before transplantation, five-week-old C57BL/6 mice and MD2KO mice were put in a clean environment and given acidified water containing neomycin (1.1 mg/L) and polymyxin B sulphate (1000 U/L). Twelve hours prior to transplantation, mice were subjected to total body irradiation (800 cGy X-rays). For transplantation, C57BL/6 mice or MD2KO mice were injected intravenously with 5 × 106 bone marrow cells from pools of bone marrow from six-week-old C57BL/6 mice or MD2KO mice, as described previously [20]. Three weeks later, the chimeric mice were infused with 30 mg·kg−1·d−1 ISO for 2 weeks to induce cardiac remodelling. At the end of the study, the isolated primary peritoneal macrophages (MPMs) and heart tissues were harvested and RT-qPCR was performed to confirm the desired combinations in our studies.
Echocardiography
Mice underwent transthoracic echocardiography examination using a Vevo 3100 (Fujifilm VisualSonics) ultrasound system using a 30-MHz linear array ultrasound transducer. Heart rate (HR) of anesthetized mice was maintained at 400–500 beats per minute (bpm). After anesthetized with oxygen and 1%–2% isoflurane, the mice underwent ultrasound analysis for cardiac function. All derived measures by echocardiography were obtained by averaging the readings of three consecutive and complete cardiac cycles.
Histological analysis
The heart tissue was fixed with 4% formaldehyde, followed by dehydrated, transparent, and embedded with paraffin. Finally, the heart tissue was cut into 5 μm thick samples for subsequent experiments. Afterwards, the slices were stained with hematoxylin and eosin (H&E) for histopathological assessment. Sections were also stained with Masson’s Trichrome and Sirius Red to evaluate the cardiac fibrosis content. The stained images were observed by a light microscope (Nikon, Japan). The frozen heart tissues were cut into 5-μm thickness. After permeabilized and blocked, the slices were stained with WGA-FITC stain to evaluate the hypertrophy degree of myocardium. The stained images were observed by an epifluorescence Nikon microscope (Nikon, Japan).
Enzyme-linked immunosorbent assay
IL-6 and TNF-α levels of conditioned medium and serum were measured by ELISA kits. Total amount of proteins was calculated using the standard curve method. The ANP level in the serum was determined by an ELISA method. All experiments followed the instructions.
Cell isolation and culture
Embryonic rat heart-derived H9c2 cells were obtained from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco/BRL life Technologies, Eggenstein, Germany) containing 4.5 g/L glucose. Media was supplemented with 10% foetal bovine serum (FBS; Thermo Fisher Scientific, Massachusetts, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C in a humidified 5% CO2 incubator.
Isolation and culture of neonatal rat cardiomyocytes was performed as described previously [21]. Briefly, heart tissues from 0 to 2 days neonatal Sprague-Dawley (SD) rats were harvested and dissociated with trypsin. The primary cardiomyocytes were removed from fibroblasts by applying the method of differential adherent culture. Cell suspension was plated for 1 h on culture dishes in DMEM supplemented with 10% FBS. This pre-plating cells were basically fibroblasts. Non-adherent cells were then collected, counted and plated on culture plates. The adherent cells (fibroblasts) were cultured, passaged 2–3 times and then collected, counted and plated on culture plates. Primary cardiomyocytes and fibroblasts were cultured in the same growth medium as H9c2 cells.
The MPMs were isolated according to the experimental method described in a previously published study [14]. Briefly, mice received a single intraperitoneal injection of 6% thioglycollate solution (0.6 g beef extract, 2 g tryptone, 1 g sodium chloride dissolved in 200 mL ddH2O, then filtrated through 0.22-μm filter membrane). Two days later, mice were euthanized, and peritoneal cavity was flushed with RPMI-1640 medium (Gibco/BRL life Technologies, Eggenstein, Germany). Samples were collected, counted and plated in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. Nonadherent cells were removed 1 h after seeding the cell suspension.
Real-time quantitative PCR
Total RNA from heart tissue and cells was isolated and purified using Trizol reagent and reverse-transcribed to cDNA using PrimeScript™ RT reagent kit (cat# RR037A, Takara, Japan). Quantitative polymerase chain reaction (PCR) was carried out using SYBR Green reagent kit (cat# 1708882AP, Bio-Rad, CA, USA) on a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, MA, USA). The primers of target genes are listed in the Supplementary Table S2 and were obtained from Invitrogen (Shanghai, China).
Western blotting and co-immunoprecipitation
Lysates from mouse myocardial tissue and cultured cells were extracted with RIPA buffer (cat# P0013C, Beyotime Biotechnology, Shanghai, China) and protein concentrations were determined by the Bradford assay (cat# 5000205, Bio-Rad, CA, USA). Samples were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and electro-transferred to PVDF membranes. Membranes were then blocked for 1 h at room temperature in 5% non-fat dry milk. Primary antibody incubations were carried out at 4 °C overnight. Then the membranes were incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. The immune complexes were visualized using enhanced chemiluminescence reagents (cat# 1705062, Bio-Rad, CA, USA). The band densities were quantified using ImageJ analysis software.
Protein complexes were evaluated by co-immunoprecipitation (IP), coupled with immunoblotting (IB). Cell lysates were incubated with precipitating antibody at 4 °C overnight. Samples were immunoprecipitated with protein A + G-Sepharose beads with shaking at room temperature for 2 h. Protein-bead complexes were washed three times with PBS, electrophoresed, and transferred to PVDF membranes and detected with IB antibody. The immune complexes were visualized using enhanced chemiluminescence reagents (cat# 1705062, Bio-Rad, CA, USA). The band densities were quantified using ImageJ analysis software.
Immunofluorescence staining
The cardiac tissues were collected and embedded in optimum cutting temperature compound. Serial 5 mm-thick cryosections of heart from each mouse were obtained. Frozen sections were used for α-actinin, F4/80, Vimentin, and MD2 staining. H9c2 cells and MPMs cultured on gelatin-coated glass slides were also stained. Cells were used for NF-κB P65 staining. Frozen sections and cells were fixed in cold methanol and permeabilized using 0.25% Triton-X. Sections and cells were then blocked with 5% fetal bovine serum for 1 h, incubated with the primary antibody for overnight at 4 °C. After washing, the sections and cells were incubated with fluorescent-labelled secondary antibodies for 1 h, and counterstained with nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. The stained sections were observed and photographed under a fluorescence microscope (Nikon, Japan).
Cell transfections for gene knockdown
MD2 were silenced in H9c2 cells and primary neonatal rat cardiomyocytes by siRNA transfections. The siRNA sequences were purchased from Gene Pharma Co. Ltd. (Shanghai, China) and shown in Supplementary Table S3. Negative control transfections included scrambled siRNA sequences. The transfections were carried out using Lipofectamine 2000. Knockdown of genes in the transfected cells was confirmed by Western blot analysis.
TRITC Phalloidin staining
H9c2 cells and primary cardiomyocytes were seeded in glass-bottom dishes, cleaned twice with sterile PBS, and then fixed with 4% formaldehyde solution at room temperature for 10 min. After the excess formaldehyde with PBS was removed, the cells were permeated with 0.5% Triton X-100 solution for 10 min. Then, cardiomyocytes were incubated with TRITC-labelled Phalloidin working solution at room temperature in the dark for 30 min. Finally, the nuclei were stained with DAPI solution, followed by fluorescence observation under a fluorescence microscope (Nikon, Japan).
Surface plasmon resonance (SPR) analysis
The potential binding of ISO to MD2 was determined using Biacore T200 Protein Interaction Assay system (GE Healthcare, MA, USA) with a CM7 sensor chip. Recombinant human MD2 protein (rhMD2) was dissolved in acetate acid buffer. Amine coupling kit (cat# BR100050, GE Healthcare, MA, USA) was used to immobilize rhDM2 on the chip. Different concentrations of ISO, including 500, 250, 125, 62.5, 31.25, 15.625, 7.8125, 3.90625, 1.95, 0.975, 0.4875, 0 μM were prepared with running buffer (PBS). Sensor and sample plates were placed in the instrument. The interactions were determined at a flow rate of 30 μL/min for 180 s during the association phase, followed by 250 s for the dissociation phase at 25 °C. The data were analyzed with Biacore T200 manager software. Binding kinetic parameters were calculated by global fitting of the kinetic data from various concentrations of MD2 using a 1:1 Langmuir binding model.
Measurement of ROS levels
ROS production in cardiomyocytes and macrophages was determined by Dihydroethidium (DHE) staining. Primary cardiomyocytes and macrophages were incubated with DHE (5 μM) for 30 min at 37 °C under 5% CO2 in air, followed by fluorescence observation under a fluorescence microscope (Nikon, Japan) with a 100 ms exposure time. DHE fluorescence was quantified using ImageJ analysis software. The level of ROS is expressed as the mean fluorescence intensity (MFI).
Statistical analysis
All experiments were randomized and blinded. Data presented in this study are representative of at least 3 independent experiments and are expressed as mean ± SEM. The exact group size (n) for each experiment is provided and ‘n’ refers to independent values, not technical replicates. Statistical analysis was performed with GraphPad Prism 8.0 software (San Diego, CA, USA). Comparisons between two groups were analyzed by Student’s t test. We used one-way ANOVA followed by Tukey post-hoc test when comparing more than two groups. P ˂ 0.05 was considered statistically significant. Post-tests were run only if F achieved P < 0.05 and there was no significant variance in homogeneity.
Results
MD2 was elevated in cardiomyocytes and cardiac macrophages of ISO-challenged mice
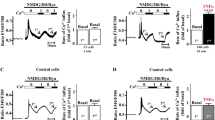
We determined whether MD2 is associated with ISO-induced cardiac injury in mice. We found that ISO challenge markedly enhanced both Md2 mRNA and MD2 protein levels in mouse hearts (Fig. 1a, b). In addition, ISO treatment increased the mRNA and protein levels of MD2 expression in a time-dependent manner from 3rd day to 14th day after ISO treatment (Supplementary Fig. S1). A further analysis of cardiac tissues revealed a significant increase in the combination of MD2 and TLR4 following the onset of ISO-induced cardiac injury, indicating the activation of MD2 (Fig. 1c). To investigate the cellular source of increased MD2 in ISO-induced models, we examined the MD2 expression in cultured neonatal rat primary cardiomyocytes (NRCMs), neonatal rat primary fibroblasts (NRCFs), and mouse MPMs. Western blot assay showed that MD2 was mainly expressed in cardiomyocytes and macrophages (Fig. 1d). Besides, immunostaining showed that elevated colocalized MD2 immunoreactivity with α-actinin (a marker of cardiomyocytes) and F4/80 (a marker of macrophages) was identified under ISO perfusion, while low MD2 location was observed in fibroblasts (Fig. 1e–g). These results indicated that cardiomyocytes and macrophages MD2 may play an essential role in cardiac remodelling induced by ISO.
C57BL/6 mice were administered 30 mg·kg−1·d−1 ISO or an equal volume of 0.002% ascorbic acid for two weeks. a The mRNA levels of Md2 in heart tissues based on real-time qPCR. Actb mRNA was used as a loading control. b Representative blots showing the levels of MD2 in heart tissue. GAPDH was used as a loading control. c Representative immunoblots showed the co-IP of MD2 and TLR4 in heart tissue. Endogenous MD2 was immunoprecipitated by anti-MD2 antibody. IgG, immunoglobulin G. d Representative Western blot analysis for MD2 protein levels in NRCMs, NRCFs and MPMs. GAPDH was used as a loading control. Representative immunofluorescence staining of mouse heart tissues for MD2 (red) and α-actinin (green, e), Vimentin (green, f), or F4/80 (green, g), respectively. Sections were counterstained with DAPI (blue). Arrows indicate MD2-positive cells (scale bar = 50 μm). Quantifications are shown respectively. Mean ± SEM; for (a–c), n = 6; for d, n = 3; for (e–g), n = 5; *P < 0.05, **P < 0.01, ns not significant.
MD2 deficiency prevented ISO-induced ventricular remodelling
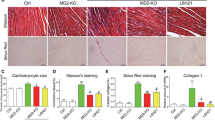
MD2KO mice and WT mice were utilized to study the role of MD2 in ISO-induced cardiac injury. A small-molecule inhibitor of MD2, L6H21, was also used to examine the effects of MD2 pharmacological inhibition [18]. In addition, a β-AR antagonist, propranolol, was used as a positive comparison. Echocardiography results showed that MD2 deficiency inhibited severe deterioration of cardiac function under ISO stimulation (Table 1). The ratios of heart weight/body weight (HW/BW) and heart weight/tibia length (HW/TL) did decrease significantly in MD2-/- mice and L6H21-treated WT mice compared with ISO-stimulated WT mice (Table 1). Analysis of the harvested heart tissues showed the degree of the structural and hypertrophic changes were lower after MD2 deficiency or pharmacological inhibition (Fig. 2a, b). Sirius Red and Masson’s Trichrome staining also showed suppressive activity of MD2 deficiency on fibrotic responses (Fig. 2c–f). WGA staining indicated MD2 blockage attenuated ISO-induced cardiac hypertrophy (Fig. 2g, h). Consistent with the echocardiography and pathological examinations, the serum atrial natriuretic peptide (ANP) level and the mRNA and protein expression of hypertrophy-associated factors (beta-Myosin heavy chain (β-MyHC) and ANP) and fibrosis-associated factors (Collagen type I (COL-1) and transforming growth factor-beta1 (TGF-β1)) in heart tissue were significantly reduced by MD2 knockout in ISO-challenged mice (Fig. 2i–k and Supplementary Fig. S2a). Meanwhile, the inflammatory responses in ISO-related cardiac remodelling were found to be inhibited by MD2 deficiency. The ISO-increased levels of serum IL-6 and TNF-α and the expression levels of Il6 and Tnf genes were suppressed by MD2 blockade (Fig. 2l, m). Determination of NF-κB p65 phosphorylation and IκB degradation in heart tissues confirmed that MD2 deletion normalized ISO-induced NF-κB activation (Fig. 2n and Supplementary Fig. S2b). Like MD2 gene knockout, both MD2 inhibitor L6H21 and β-AR antagonist propranolol reversed ISO-induced cardiac inflammation, remodelling, and dysfunction in mice (Fig. 2 and Supplementary Fig. S2).
MD2KO mice and WT mice were administered 30 mg·kg−1·d−1 ISO or an equal volume of 0.002% ascorbic acid for two weeks. a Representative H&E staining (longitudinal) of cardiac tissues (scale bar = 100 μm). b Representative H&E staining (transverse) of cardiac tissues (scale bar = 100 μm). c–f Representative staining images and quantification of mouse heart sections stained with Masson’s Trichrome (c, d) and Sirius Red (e, f) (scale bar = 100 μm). g, h Representative staining images and quantification of heart tissues with FITC-WGA (scale bar = 50 μm). i ANP level in the serum based on ELISA. j The mRNA levels of Myh7, Nppa, Col1a1, and Tgfb1 in the heart tissues based on real-time PCR. Actb mRNA was used as loading control. k Representative immunoblots showing cardiac tissue β-MyHC, ANP, COL-1, and TGF-β1. GAPDH was used as a loading control. l IL-6 and TNF-α levels in the serum based on ELISA. m The mRNA levels of Il6 and Tnf in the heart based on real-time PCR. Actb mRNA was used as loading control. n Activation of the NF-κB pathways was assessed by probing for the degradation of IκBα and phosphorylated NF-κB P65. Total P65 and GAPDH were used as controls. Mean ± SEM, for (a–h), n = 6, **P < 0.01; for (i–n), n = 7, *P < 0.05, **P < 0.01.
Both cardiomyocyte MD2 and bone marrow-derived macrophage MD2 contributed to ISO-induced cardiac remodelling
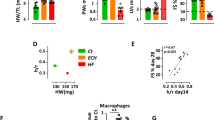
To determine the contribution of cardiomyocyte MD2 and macrophage MD2 to the mediation of ISO-induced cardiac injuries, we irradiated WT mice and MD2KO mice to deplete their marrows and then reconstituted them with either WT or MD2KO marrow-derived cells, respectively. We confirmed the high efficiency of bone marrow depletion and reconstitution through examining the levels of Md2 in heart tissues and MPMs from four groups (Supplementary Fig. S3). Thereafter, the chimeric mice were subjected to ISO challenge for two weeks to induce cardiac dysfunction (Fig. 3a). Cardiac functional tests revealed improved EF%, FS%, LVPWd and left ventricular posterior wall end-systolic dimension (LVPWs) in WT → KO, KO → WT and KO → KO groups (Table 2). Further, cardiac histological analysis, including hematoxylin and eosin (H&E) staining, Masson trichrome staining, and Sirius Red staining, indicated myocardial disorganization and excessive fibrosis in the WT → WT group, but not in the WT → KO, KO → WT and KO → KO groups (Fig. 3b–g). Based on the values of LV mass, HW/BW, HW/TL, the WGA staining and the level of serum ANP, both cardiomyocyte and bone marrow-derived macrophage MD2 mediated cardiac hypertrophy induced by ISO (Table 2, Fig. 3h–j). Such findings were confirmed by the protein and mRNA levels of hypertrophy-associated factors (β-MyHC and ANP) and fibrosis-associated factors (COL-1 and TGF-β1) (Fig. 3k, l and Supplementary Fig. S4a). Finally, cardiac inflammation was evaluated in mice. The levels of serum inflammatory IL-6 and TNF-α, the expression of Il6 and Tnf, and the activation of NF-κB were decreased in both WT → KO, KO → WT, and KO → KO mice (Fig. 3m–o and Supplementary Fig. S4b). Such findings indicate that both cardiomyocyte and bone marrow-derived cell MD2 predominantly mediated ISO-induced cardiac inflammation and remodelling.
WT mice or MD2KO mice were irradiated and administered bone marrow cells from either WT and MD2KO mice, WT → WT: marrow-derived cells from WT mice were transplanted in irradiated WT mice, WT → KO: marrow-derived cells from WT mice were transplanted in irradiated MD2KO mice, KO → WT: marrow-derived cells from MD2KO mice were transplanted in irradiated WT mice, KO → KO: marrow-derived cells from MD2KO mice were transplanted in irradiated MD2KO mice. Mice were then administered 30 mg·kg−1·d−1 ISO for 2 weeks. a Schematic illustrating this modelling process. b Representative H&E staining (longitudinal) of cardiac tissues (scale bar = 100 μm). c Representative H&E staining (transverse) of cardiac tissues (scale bar = 100 μm). d–g Representative staining images and quantification of mouse heart sections stained with Masson’s Trichrome (d, e) and Sirius Red (f, g) (scale bar = 100 μm). h, i Representative staining images and quantification of heart tissues with FITC-WGA (scale bar = 50 μm). j ANP level in the serum based on ELISA. k The mRNA levels of Myh7, Nppa, Col1a1, and Tgfb1 in the heart tissues based on real-time PCR. Actb mRNA was used as loading control. l Representative immunoblots showing cardiac tissue β-MyHC, ANP, COL-1, and TGF-β1. GAPDH was used as a loading control. m IL-6 and TNF-α levels in the serum based on ELISA. n The mRNA levels of Il6 and Tnf in the heart based on real-time PCR. Actb mRNA was used as loading control. o Activation of the NF-κB pathways was assessed by probing for the degradation of IκBα and phosphorylated NF-κB P65. Total P65 and GAPDH were used as controls. Mean ± SEM, for (b–i), n = 6, **P < 0.01; for (j–o), n = 7, *P < 0.05, **P < 0.01.
MD2 silencing alleviated ISO-induced cardiomyocyte inflammatory response and remodelling in vitro
We determined whether MD2 mediated ISO-induced inflammatory response and injuries in cultured H9c2 cardiomyocyte-like cell line and primary neonatal rat cardiomyocytes. MD2 expression was knocked down by transfection of siRNA targeting MD2 (siMD2) (Fig. 4a). We showed that MD2 silencing reversed the NF-κB p65 phosphorylation, IκBα degradation, p65 nuclear translocation, and the inflammatory gene Il6 and Tnf expression in ISO-challenged cardiomyocytes (Fig. 4b–d, Supplementary Fig. S5a). Pre-treatment of H9C2 cells with pharmacological MD2 inhibitor L6H21 mimics MD2 knockdown in preventing ISO-induced inflammatory responses (Fig. 4e, Supplementary Fig. S5b). We further confirmed these findings in isolated NRCMs (Fig. 4f, Supplementary Fig. S5c, d). Subsequently, the ISO-increased protein levels of β-MyHC, ANP, COL-I, and TGF-β1 were also significantly reduced when MD2 was knocked down in H9c2 cells (Fig. 4g, Supplementary Fig. S6a). Rhodamine phalloidin staining revealed MD2 silencing decreased hypertrophy of cardiomyocytes under ISO stimulation (Fig. 4h). Either pharmacological inhibition of MD2 in H9c2 cells or silencing MD2 in neonatal rat cardiomyocytes also prevented cellular hypertrophy and fibrotic protein induction (Fig. 4i, j, Supplementary Fig. S6b, c). Together, these data indicated that MD2 mediated ISO-induced inflammatory responses and subsequent injuries in cardiomyocytes.
H9c2 cells and neonatal rat primary cardiomyocytes were used to model ISO effects in culture. a H9c2 cells were transfected with control siRNA (NC) or MD2 siRNA (siMD2); Lysates were probed for the protein levels of MD2. GAPDH was used as a control. Densitometric quantification is shown below. b, c H9c2 cells were transfected with control siNC or siMD2 and exposed to ISO (20 μM) for 1 h. Representative Western blotting for p-P65 and IκBα in cells, total protein P65 and GAPDH were used as controls (b). Immunofluorescence staining for the NF-κB P65 subunit (red) in H9c2 cells. Cells were counterstained with DAPI (blue) (scale bar = 50 μm). Quantification is shown on the right (c). d H9c2 cells were transfected with control si NC or si MD2 and exposed to ISO (20 μM) for 24 h. The mRNA levels of inflammatory genes, Il6 and Tnf. Actb mRNA was used as a loading control. e H9c2 cells were pretreated with L6H21 (10 μM) for 1 h and exposed to ISO (20 μM) for 1 h. Representative Western blotting for p-P65 and IκBα in cells, total protein P65 and GAPDH were used as controls. f NRCMs were transfected with control siNC or siMD2 and exposed to ISO (20 μM) for 1 h. Representative Western blotting for p-P65 and IκBα in cells, total protein P65 and GAPDH were used as controls. g, h H9c2 cells were transfected with control siNC or siMD2 and exposed to ISO (20 μM) for 24 h. Representative Western blotting for β-MyHC, ANP, COL-1, and TGF-β1 in cells, GAPDH was used as controls (g). H9c2 cells were stained with TRITC Phalloidin to assess hypertrophic responses (scale bar = 50 μm). Quantification is shown on the right (h). i H9c2 cells were pretreated with L6H21 (10 μM) for 1 h and exposed to ISO (20 μM) for 24 h. Representative Western blotting for β-MyHC, ANP, COL-1, and TGF-β1 in cells, GAPDH was used as controls. j NRCMs were transfected with control siNC or siMD2 and exposed to ISO (20 μM) for 24 h. Representative Western blotting for β-MyHC, ANP, COL-1, and TGF-β1 in cells, GAPDH was used as controls. Mean ± SEM, n = 3, *P < 0.05, **P < 0.01.
Blockade of MD2 regulated ISO-induced inflammatory response in macrophages
We isolated MPMs from WT and MD2KO mice and exposed these cells to ISO. ISO significantly activated NF-κB in MPMs isolated from WT mice but not from MD2KO mice (Fig. 5a, b, Supplementary Fig. S7a). MD2 knockdown decreased cytokine IL6 and TNF-α production and mRNA expression in MPMs induced by ISO stimulation (Fig. 5c, d). Treatment with L6H21 also suppressed ISO-induced NF-κB activation MPMs (Fig. 5e, Supplementary Fig. S7b). These data indicated that MD2 mediated ISO-induced inflammatory response in macrophages. The inflammatory environment induced by macrophages promoted the development of cardiac hypertrophy and fibrosis. We next determined whether MD2-mediated macrophage cytokines affect cardiomyocytes and fibroblasts. To do this, we exposed MPMs from WT and MD2KO mice to ISO, and then collected the condition media (CM) of macrophages to treat cardiomyocytes and fibroblasts (Fig. 5f). As shown in our results, CM from ISO-challenged macrophages induced hypertrophy and fibrotic injury in cardiomyocytes and fibroblasts, while CM from ISO-challenged MD2KO macrophages failed to induce these changes (Fig. 5g–i and Supplementary Fig. S8). These data suggested that macrophage MD2 increased ISO-induced inflammatory mediators and subsequent intercellular cross-talk, resulting in injurious remodelling in cardiomyocytes and fibroblasts.
a, b Primary macrophages isolated from MD2KO and WT mice were challenged with ISO (20 μM) for 1 h. Representative Western blotting for p-P65 and IκBα in cells, total protein P65 and GAPDH were used as controls (a). Immunofluorescence staining for the NF-κB P65 subunit (red) in primary macrophages. Cells were counterstained with DAPI (blue) (scale bar = 25 μm). Quantification is shown on the right (b). c, d MPMs were isolated from MD2KO and WT mice and exposed to ISO (20 μM) for 24 h. The levels of IL-6 and TNF-α cytokines in culture media were measured by ELISA (c). The mRNA levels of inflammatory genes, Il6 and Tnf. Actb mRNA was used as a loading control (d). e Primary macrophages were pretreated with L6H21 (10 μM) for 1 h and exposed to ISO (20 μM) for 1 h. Representative Western blotting for p-P65 and IκBα in cells, total protein p65 and GAPDH were used as controls. f Schematic illustration of the experimental model employed to determine the role of ISO-induced paracrine factors. Primary macrophages isolated from MD2KO and WT mice were challenged with ISO (20 μM) for 24 h. Conditioned medium was collected from the cells. The primary cardiomyocytes and primary cardiac fibroblasts were then challenged with media for 24 h. g Western blot revealing the protein levels of β-MyHC, ANP, COL-I, and TGF-β1 in whole cell lysates of primary cardiomyocytes. GAPDH was used as a control. h Primary cardiomyocytes were stained with TRITC Phalloidin to assess hypertrophic responses (scale bar = 50 μm). Quantification is shown on the right. i Western blot revealing the protein levels of COL-I and TGF-β1 in whole cell lysates of primary cardiac fibroblasts. GAPDH was used as a control. Mean ± SEM, n = 3; *P < 0.05, **P < 0.01.
ISO activated MD2 via β1-AR-cAMP-PKA-ROS signalling axis in cardiomyocytes
We then explored how ISO activates MD2 in cardiomyocytes. Co-immunoprecipitation (Co-IP) assay results showed that ISO challenge significantly promoted MD2-TLR4 interaction in a short time in H9c2 cells (Fig. 6a). Similar result was observed in cultured primary cardiomyocytes (Supplementary Fig. S9). As expected, ISO stimulation induced the formation of the TLR4-MyD88 complex in cardiomyocytes (Supplementary Fig. S10). However, our SPR analysis showed no direct interaction between rhMD2 protein and ISO (Supplementary Fig. S11), indicating ISO activates MD2 possibly via its receptor β-AR. Indeed, pre-treatment of H9c2 cells with propranolol (a non-selective β-AR blocker) or bisoprolol (a selective β1-AR blocker) significantly blocked ISO-induced NF-κB activation and MD2-TLR4 interaction in cardiomyocytes (Fig. 6b, c). Since β1-AR is the main type of β-AR in cardiomyocytes, the selective β2-AR blocker, ICI-118551, failed to block ISO-induced these changes (Fig. 6b, c). These data indicated that β1-AR in cardiomyocytes mediated ISO-induced MD2 activation. The cAMP-PKA-ROS signalling pathway is canonical downstream of β1-AR, and we then examine whether it is involved in MD2 activation in cardiomyocytes. We then used forskolin to increase cAMP intracellular levels, which activated adenylcyclase. Our results showed that exposure of cardiomyocytes to forskolin increased the association of MD2 with TLR4 (Fig. 6d), and the ISO-increased MD2-TLR4 interaction was inhibited by a PKA inhibitor H89 (Fig. 6e). Notably, ISO stimulation in cardiomyocytes rapidly increased ROS production (Fig. 6f), which has been reported to induce MD2-TLR4 activation [22]. Both propranolol and bisoprolol, but not ICI-118551, could inhibit ROS generation induced by ISO (Fig. 6f). Our data in Fig. 6g also validated the activation of MD2-TLR4 by ROS in H9c2 cells. In addition, NAC, a ROS scavenger, significantly blocked ISO-induced the formation of the MD2-TLR4 complex in H9c2 cells (Fig. 6h, Supplementary Fig. S12). Collectively, these data revealed that the β1-AR-cAMP-PKA-ROS pathway mediated ISO-induced MD2 activation and inflammatory response in cardiomyocytes (Fig. 6i).
a H9c2 cells were incubated with ISO (20 μM) for the indicated time periods. Representative immunoblots showed the co-IP of MD2 and TLR4 in cells. Endogenous MD2 was immunoprecipitated by anti-MD2 antibody. IgG, immunoglobulin G. b H9c2 cells were pretreated with propranolol (Prop, 0.1 μM), β1-AR blocker bisoprolol (Biso, 1 μM) and β2-AR blocker ICI 118,551 (ICI, 0.1 μM) for 1 h and then treated with ISO (20 μM) for 1 h. Western blot analysis for p-P65 and IκBα in cells, total proteins and GAPDH were used as controls. c H9c2 cells were pretreated with Prop (0.1 μM), Biso (1 μM) and ICI (0.1 μM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 was IP and TLR4 was detected by IB. d H9c2 cells were exposed to forskolin at different concentrations for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. e H9c2 cells were pretreated with H89 (10 μM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. f H9c2 cells were pretreated with Prop (0.1 μM), Biso (1 μM) and ICI (0.1 μM) for 1 h and then treated with ISO (20 μM) for 30 min. DHE staining in H9c2 cells (scale bar = 25 μm). g H9c2 cells were exposed to tert-butyl hydroperoxide (TBHP) at different concentrations for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. h H9c2 cells were pretreated with ROS scavenger NAC (5 mM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. i Schematic illustrating the potential signalling. Mean ± SEM, n = 3; **P < 0.01, ns not significant.
ISO activated MD2 via β2-AR-cAMP-PKA-ROS signalling axis in macrophages
Co-IP assay showed that ISO also stimulated the formation of MD2-TLR4 complex (Fig. 7a) and the recruitment of MyD88 (Supplementary Fig. S13) in MPMs. Since macrophages mainly express β2-AR, not β1-AR, we then explored whether ISO activates MD2 via β2-AR in macrophages. As expected, pre-treatment of MPMs with propranolol and ICI-118551, but not β1-AR blocker bisoprolol, remarkedly attenuated ISO-induced NF-κB activation and MD2-TLR4 interaction in MPMs (Fig. 7b, c). As in cardiomyocytes, forskolin incubation induced MD2-TLR4 interaction and the PKA inhibitor H89 reversed ISO-induced MD2-TLR4 complex formation (Fig. 7d, e), implicating that cAMP-PKA signalling is also required for ISO induction in macrophages. Further, as those in cardiomyocytes, propranolol and ICI-118551 also blocked ISO-induced oxidative stress (Fig. 7f), increased ROS could cause the interaction between MD2 and TLR4 (Fig. 7g), and pre-treatment of cells with NAC blocked ISO-induced MD2 activation in MPMs (Fig. 7h, Supplementary Fig. S14). These findings clearly demonstrated that β2-AR-cAMP-PKA-ROS signalling mediated ISO-induced MD2 activation and the downstream inflammatory cascade in macrophages (Fig. 7i).
a Primary macrophages were incubated with ISO (20 μM) for 1 h. MD2 IP was performed and levels of TLR4 were detected by IB. IgG, immunoglobulin G. b Primary macrophages were pretreated with Prop (0.1 μM), Biso (1 μM) and ICI (0.1 μM) for 1 h and then treated with ISO (20 μM) for 1 h. Western blot analysis for p-P65 and IκBα in cells, total proteins and GAPDH were used as controls. c Primary macrophages were pretreated with Prop (0.1 μM), Biso (1 μM) and ICI (0.1 μM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 was IP and TLR4 was detected by IB. d Primary macrophages were exposed to forskolin (100 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. e Primary macrophages were pretreated with H89 (10 μM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. f Primary macrophages were pretreated with Prop (0.1 μM), Biso (1 μM) and ICI (0.1 μM) for 1 h and then treated with ISO (20 μM) for 30 min. DHE staining in cells (scale bar = 25 μm). g Primary macrophages were exposed to TBHP (100 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. h Primary macrophages were pretreated with ROS scavenger NAC (5 mM) for 1 h and then treated with ISO (20 μM) for 30 min. MD2 IP was performed and levels of TLR4 were detected by IB. i Schematic illustrating the potential signalling. Mean ± SEM, n = 3; **P < 0.01, ns not significant.
Discussion
MD2 is a key protein in endotoxin-related innate immunity. This study set out to investigate the role of MD2 in adrenergic stimulation related pathological cardiac remodelling and reveal its potential mechanism. Our results showed the increased expression and activation of MD2 in the myocardium of ISO-challenged mice. MD2 gene knockout or pharmacological inhibition attenuated ISO-induced cardiac inflammation, hypertrophy, fibrosis, and dysfunction. Using marrow depletion and reconstitution, we showed that MD2 in either cardiomyocytes or bone marrow-derived cells mediated ISO-induced cardiac inflammation and remodelling. In addition to that MD2 mediated ISO-induced inflammation in both cardiomyocytes and macrophages, MD2-mediated macrophage cytokines further promote hypertrophy and fibrosis in cardiomyocytes and fibroblasts. Furthermore, we demonstrate that ISO induces the activation of MD2 and subsequent TLR4-MyD88-NF-κB inflammatory cascade via β1-AR-cAMP-PKA-ROS signalling axis in cardiomyocytes, or β2-AR-cAMP-PKA-ROS axis in macrophages. Taken together, our findings identify MD2 as a key mediator and a promising therapeutic target for ISO-induced cardiac inflammation and heart failure (Fig. 8).
ISO induced MD2 activation and inflammatory response via β1-AR-cAMP-PKA-ROS signalling axis in cardiomyocytes, or β2-AR-cAMP-PKA-ROS axis in macrophages, respectively. Both cardiomyocyte MD2 and macrophage MD2 mediated ISO-induced inflammatory responses and subsequent viciously intercellular cross-talk between cardiomyocytes and macrophages, resulting in increased pathological cardiac remodelling.
We found that ISO could activate MD2 to induce inflammatory response via β-ARs. The relation between β-ARs and inflammation in HF has been reported. Previous study has shown the involvement of β-ARs in the inflammatory response in both cardiomyocytes and immune cells [23]. The sympathetic nervous system has been considered as an important regulator of inflammatory responses [24]. Toyoda et al showed that selective β1-AR blocker bisoprolol has anti-inflammatory effects in HF patients [7]. Xiao et al showed that β1-AR mediates ISO-induced inflammasome activation and inflammatory response in cardiomyocytes [25]. Another study found that mice lacking immune cell β2-AR showed decreased immune cell infiltration to hearts and improved cardiac function [26]. We firstly found that ISO induced MD2 activation and inflammatory cascade through β1-AR and β2-AR in cardiomyocytes and macrophages, respectively. These data provide a mechanistic explain for ISO and β-ARs regulating inflammatory response.
After β-ARs activation, the level of cAMP was enhanced in the myocardium [27]. cAMP is known as a mediator of anti-inflammatory responses, and cAMP-dependent signalling has been pharmacologically exploited for the treatment of inflammatory diseases. cAMP can act as a positive regulator of a number of inflammatory genes [28]. A new study has shown that glibenclamide inhibited the cAMP-PKA signalling pathway in mouse hearts and cardiomyocytes to alleviate β-AR overactivation-induced cardiac inflammation [29]. Cardiac overexpression of phosphodiesterase 4B, a cAMP-hydrolyzing protein, blunted β-AR response and maladaptive remodelling in ISO-induced HF [30], which suggested that cAMP plays a pathogenic role in sympathetic excitation-related HF. The cAMP-PKA activation increased cellular ROS production. Xiao et al found that ISO induced inflammasome activation is mediated by the β1-AR-ROS pathway [25]. Transgenic activation of β2-AR leads to ROS production and p38 MAPK phosphorylation, associated with cardiac inflammation and dysfunction, while antioxidant treatment protected hearts against these abnormalities, indicating ROS production to be central to link the detrimental signalling of β2-AR and inflammation [31]. What’s more, it has been reported that both mitochondrial-derived and NADPH oxidase-derived ROS are generated in cardiomyocytes during chronic β-AR activation [32, 33]. How PKA mediated oxidative stress is not our focus here, which is worth further study. Our previous study found that TBHP induced the formation of MD2-TLR4 complex on a short time and pro-inflammatory response [34]. Here, our results found that ROS induced MD2-TLR4 complex formation and NAC inhibited ISO-induced MD2-TLR4 interaction in both cardiomyocytes and macrophages. To our best knowledge, this is the first time to identify that β-AR-cAMP-PKA-ROS signalling mediated ISO-induced inflammatory response and cardiac inflammatory injuries.
Enhanced ROS level induced cellular injuries and produced DAMPs in cells [35]. Emerging evidence suggested that TLRs can be activated by endogenous DAMPs, which are produced by damaged and dying cells and promote sterile inflammation [36]. The interaction between TLR4 and DAMPs, such as Galectin-3 and High mobility group box 1 (HMGB1), have been found that contribute to the pathogenesis of cardiac injury induced by ISO [37, 38]. Disulfide HMGB1 activated the TLR4 complex by binding to MD2 [39]. Another study performed a computational approach to determine a relationship between TLR4-MD2 and heat-shock protein 70 families (HSP70) in human LV and aorta [40]. As shown in Supplementary Fig. S15, the expression of these DAMPs was up-regulated in heart tissues under ISO perfusion, indicating that ROS-mediated endogenous DAMPs production promoted the inflammatory response and the progress of HF. Thus, we may guess that increased ROS induces the production of cellular DAMPs, which activates MD2-TLR4 signalling to further drive inflammation in cardiomyocytes and macrophages. Further studies need to be performed to validate this hypothesis.
The remarkedly protective effect of blocking cardiomyocytes or macrophages MD2 on heart hypertrophy was also attributed to halting the vicious cycle of cross-talk between cardiomyocytes and macrophages. The intercellular signalling and communication between cardiomyocytes and macrophages are critical in the pathophysiology of cardiac remodelling [41]. Both cardiomyocyte and macrophage MD2 directly mediated NF-κB-dependent inflammatory response under ISO stimulation, which contributes to the pro-inflammatory extracellular milieu. The activated local macrophages and the infiltrated bone marrow-derived immune cells from the circulation subsequently increased cardiac inflammation by co-stimulation and positive feedback loops. In addition, damaged cardiomyocytes and macrophages additionally released DAMPs, which may further activate local cardiomyocyte MD2 and macrophage MD2 signalling. These mechanisms may drive the cross-talk between cardiomyocytes MD2 and macrophages MD2.
A point worth mentioning here is the concentration of ISO used in our cellular studies. The ISO concentration used in our cellular studies is super-physiological 20 μM, which is much higher than the plasma catecholamine level in heart failure patient. However, we selected this concentration of ISO according to a lot of previous studies [29, 42, 43]. Although previous studies used the concentration of 20 μM possibly for more evident cellular phenotypes, the relatively high ISO dosage may be a limitation of our research. To examine if ISO at a low dose also induce the same cellular mechanism, we treated both cardiomyocytes and macrophages with ISO at 100 nM. As we can see in the Supplementary Fig. S16 and S17, ISO at 100 nM also significantly increased ROS generation, MD2-TLR4 interaction, and inflammatory response in the cultured cardiomyocytes and macrophages. Similarly, MD2 knockdown alleviated these phenotypes induced by 100 nM ISO in both cardiomyocytes and macrophages. These results indicate that ISO at pathological concentration is able to induce MD2 activation and inflammatory response. However, the dose-dependent effects of ISO in both cell types deserved further study.
Another limitation of this study may be lacking the role of MD2 in cardiac fibroblast. Fibroblasts are an important resident cell population in the heart and partly mediate cardiac fibrosis and injuries [44]. Cardiac fibroblasts, with high β2-AR expression, respond to direct β‐AR activation and paracrine signals from cardiomyocytes, and then proliferate and increase contractile forces and express high levels of collagens [45,46,47,48]. β2-AR activation in cardiac fibroblasts has been also shown to increase the release of pro-inflammatory cytokines [47]. Although our data showed fibroblasts has a less MD2 expression compared to cardiomyocytes and macrophages, we could not completely exclude the contribution of β2-AR-cAMP-PKA-ROS-MD2 signalling pathway in fibroblasts in ISO-induced cardiac inflammation, fibrosis, and remodelling. Further studies are needed to determine the potential regulatory roles of MD2 in β2-AR-mediated signalling and function in cardiac fibroblasts. The utility of cardiomyocyte-specific and macrophage-specific MD2 knockout mice in the future study may make our conclusion more convincing. Another interesting question is how ISO upregulates MD2 expression? The inflammatory transcriptional factor NF-κB has been reported to up-regulate the gene transcription of TLR4 [49]. Therefore, we may consider that the ISO-induced inflammatory response and NF-κB activation may also be able to induce MD2 gene expression in a positive feedback manner. Nevertheless, other mechanisms may be also involved and this question deserves further investigation.
Conclusion
In summary, we have shown that β-AR-cAMP-PKA-ROS activated MD2 pro-inflammatory signalling as a central driving force in ISO-induced HF. We show that β1-AR and β2-AR mediate inflammatory response via MD2 in cardiomyocytes and macrophages, respectively. These results have shed new light on the role of MD2 in ISO-related HF and support the therapeutic potential of targeting MD2 in this disease.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart association. Circulation. 2019;139:e56–e528.
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23.
El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–41.
Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–41.
Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R, Pomérance M. A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal. 2010;22:1143–52.
Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–8.
Toyoda S, Haruyama A, Inami S, Arikawa T, Saito F, Watanabe R, et al. Effects of carvedilol vs bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J Cardiol. 2020;75:140–7.
Woo AY, Xiao RP. β-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin. 2012;33:335–41.
Kossack M, Hein S, Juergensen L, Siragusa M, Benz A, Katus HA, et al. Induction of cardiac dysfunction in developing and adult zebrafish by chronic isoproterenol stimulation. J Mol Cell Cardiol. 2017;108:95–105.
Meeran MFN, Azimullah S, Adeghate E, Ojha S. Nootkatone attenuates myocardial oxidative damage, inflammation, and apoptosis in isoproterenol-induced myocardial infarction in rats. Phytomedicine. 2021;84:153405.
Park SH, Kim ND, Jung JK, Lee CK, Han SB, Kim Y. Myeloid differentiation 2 as a therapeutic target of inflammatory disorders. Pharmacol Ther. 2012;133:291–8.
Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66.
Han J, Zou C, Mei L, Zhang Y, Qian Y, You S, et al. MD2 mediates angiotensin II-induced cardiac inflammation and remodelling via directly binding to Ang II and activating TLR4/NF-κB signaling pathway. Basic Res Cardiol. 2017;112:9.
Chen T, Huang W, Qian J, Luo W, Shan P, Cai Y, et al. Macrophage-derived myeloid differentiation protein 2 plays an essential role in ox-LDL-induced inflammation and atherosclerosis. EBioMedicine. 2020;53:102706.
Riad A, Gross S, Witte J, Feldtmann R, Wagner KB, Reinke Y, et al. MD-2 is a new predictive biomarker in dilated cardiomyopathy and exerts direct effects in isolated cardiomyocytes. Int J Cardiol. 2018;270:278–86.
Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, et al. Author Correction: Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun. 2018;9:16185.
Wang Y, Luo W, Han J, Khan ZA, Fang Q, Jin Y, et al. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat Commun. 2020;11:2148.
Wang Y, Shan X, Chen G, Jiang L, Wang Z, Fang Q, et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br J Pharmacol. 2015;172:4391–405.
Li C, Zhao M, Xiao L, Wei H, Wen Z, Hu D, et al. Prognostic value of elevated levels of plasma N-Acetylneuraminic acid in patients with heart failure. Circ Heart Fail. 2021;14:e008459.
Zhao H, Yang H, Geng C, Chen Y, Pang J, Shu T, et al. Role of IgE-FcεR1 in pathological cardiac remodelling and dysfunction. Circulation. 2021;143:1014–30.
Ye S, Luo W, Khan ZA, Wu G, Xuan L, Shan P, et al. Celastrol attenuates angiotensin II-induced cardiac remodelling by targeting STAT3. Circ Res. 2020;126:1007–23.
Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–8.
Adzika GK, Machuki JO, Shang W, Hou H, Ma T, Wu L, et al. Pathological cardiac hypertrophy: the synergy of adenylyl cyclases inhibition in cardiac and immune cells during chronic catecholamine stress. J Mol Med (Berl). 2019;97:897–907.
Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171.
Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. 2018;39:60–9.
Tanner MA, Maitz CA, Grisanti LA. Immune cell β(2)-adrenergic receptors contribute to the development of heart failure. Am J Physiol Heart Circ Physiol. 2021;321:H633–49.
Corbi G, Conti V, Russomanno G, Longobardi G, Furgi G, Filippelli A, et al. Adrenergic signaling and oxidative stress: a role for sirtuins? Front Physiol. 2013;4:324.
Hertz AL, Bender AT, Smith KC, Gilchrist M, Amieux PS, Aderem A, et al. Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA 2009;106:21978–83.
Cao N, Wang JJ, Wu JM, Xu WL, Wang R, Chen XD, et al. Glibenclamide alleviates β adrenergic receptor activation-induced cardiac inflammation. Acta Pharmacol Sin. 2022;43:1243–50.
Karam S, Margaria JP, Bourcier A, Mika D, Varin A, Bedioune I, et al. Cardiac overexpression of PDE4B blunts β-adrenergic response and maladaptive remodelling in heart failure. Circulation. 2020;142:161–74.
Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie RH, Sim K, et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol. 2011;162:1012–28.
Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–8.
Theccanat T, Philip JL, Razzaque AM, Ludmer N, Li J, Xu X, et al. Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell Signal. 2016;28:190–203.
Chen H, Song Z, Ying S, Yang X, Wu W, Tan Q, et al. Myeloid differentiation protein 2 induced retinal ischemia reperfusion injury via upregulation of ROS through a TLR4-NOX4 pathway. Toxicol Lett. 2018;282:109–20.
Koenig A, Buskiewicz-Koenig IA. Redox activation of mitochondrial DAMPs and the metabolic consequences for development of autoimmunity. Antioxid Redox Signal. 2022;36:441–61.
Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112.
Sun JH, Yang HX, Yao TT, Li Y, Ruan L, Xu GR, et al. Gentianella acuta prevents acute myocardial infarction induced by isoproterenol in rats via inhibition of galectin-3/TLR4/MyD88/NF-кB inflammatory signalling. Inflammopharmacology. 2021;29:205–19.
Bai C, Ren Y, Huang J, Zhang Y, Li L, Du G. High-mobility group Box-1 regulates acute myocardial ischemia-induced injury through the toll-like receptor 4-related pathway. Int J Clin Exp Pathol. 2017;10:8344–52.
Yang H, Wang H, Andersson U. Targeting inflammation driven by HMGB1. Front Immunol. 2020;11:484.
de Oliveira AA, Faustino J, de Lima ME, Menezes R, Nunes KP. Unveiling the interplay between the TLR4/MD2 complex and HSP70 in the human cardiovascular system: a computational approach. Int J Mol Sci. 2019;20:3121.
Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodelling. Circulation. 2015;131:1019–30.
Qian J, Liang S, Wang Q, Xu J, Huang W, Wu G, et al. Toll-like receptor-2 in cardiomyocytes and macrophages mediates isoproterenol-induced cardiac inflammation and remodelling. FASEB J. 2023;37:e22740.
Stapel B, Kohlhaas M, Ricke-Hoch M, Haghikia A, Erschow S, Knuuti J, et al. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J. 2017;38:349–61.
Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodelling. Pharmacol Ther. 2009;123:255–78.
She G, Hou MC, Zhang Y, Zhang Y, Wang Y, Wang HF, et al. Gal-3 (Galectin-3) and K(Ca)3.1 mediate heterogeneous cell coupling and myocardial fibrogenesis driven by βAR (β-adrenoceptor) activation. Hypertension. 2020;75:393–404.
Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res. 1989;65:657–70.
Tanner MA, Thomas TP, Maitz CA, Grisanti LA. β2-Adrenergic receptors increase cardiac fibroblast proliferation through the Gαs/ERK1/2-dependent secretion of interleukin-6. Int J Mol Sci. 2020;21:8507.
Aránguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, et al. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta. 2011;1812:23–31.
Wei M, Li Z, Xiao L, Yang Z. Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol Immunol. 2015;68:261–71.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82271347 to GJW) and Wenzhou City Research Project (ZY2020016 to GJW).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration among all authors. GL and GJW designed experiments. JFQ, SQL, QYW, JCX performed experiments. JFQ and WL analyzed the data collection and analysis. GL, JFQ and GJW analyzed the data and wrote the manuscript. GL and WJH revised the manuscript. All the authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qian, Jf., Liang, Sq., Wang, Qy. et al. Isoproterenol induces MD2 activation by β-AR-cAMP-PKA-ROS signalling axis in cardiomyocytes and macrophages drives inflammatory heart failure. Acta Pharmacol Sin 45, 531–544 (2024). https://doi.org/10.1038/s41401-023-01179-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01179-3
- Springer Nature Singapore Pte Ltd.