Abstract
Background
Tibetan medicine Gaoyuan’an capsule (GYAC) is widely used to prevent pulmonary edema at high altitude, but the specific mechanism has not been explored. In this study, we analyzed the mechanism of GYAC in hypoxia tolerance, and provided a new idea for the prevention and treatment of altitude disease.
Methods
The effective components and corresponding targets of GYAC were screened out by the Chinese herbal medicine network database, and the key targets of hypoxia tolerance were retrieved by Genecards, OMIM and PubMed database. Cytoscape 3.7.2 was used to construct GYAC ingredient-target-hypoxia tolerance-related target network. GO function annotation and KEGG enrichment analysis were performed to predict the pathways in which target genes may be involved, and molecular docking was used to verify the binding ability of the compound to target genes. In vitro, the above results were further verified by molecular experiment.
Results
We found that GYAC can improve hypoxia tolerance by regulating various target genes, including IL6, IFNG, etc. The main regulatory pathways were HIF-1 signaling pathway. Molecular docking showed that the affinity between luteolin and target genes (IL6, IFNG) were better. In vitro, we observed that hypoxia can inhibit cell viability and promote apoptosis of H9C2 cell. And hypoxia can promote the expression of LDH. After the addition of luteolin, the decrease of cell viability, the increase of cell apoptosis, LDH release and the decrease of mitochondrial membrane potential were inhibited. Besides, inflammatory related factors (IL-6, IL-10, IL-2, IFNG and VEGFA) expression were also inhibited hypoxic cell models.
Conclusions
The results of network pharmacology and molecular docking showed that luteolin, a monomeric component of GYAC, played a role in hypoxia tolerance through a variety of target genes, such as IL6, IFNG. What’s more, we have discovered that luteolin can reduce the inflammatory response in cardiac myocytes, thereby alleviating mitochondrial damage, and ultimately enhancing the hypoxia tolerance of H9C2 cardiomyocytes.
Similar content being viewed by others
Introduction
Continuous hypoxia under low-pressure is a defining characteristic of high-altitude environments, significantly impacting the metabolic functions of skeletal muscles and heart depending on the duration of exposure [1]. Hypoxic environments contribute to various diseases, acute alpine disease, high-altitude cerebral edema and high-altitude pulmonary edema [2]. As global development continues, an increasing number of people are visiting high-altitude areas for tourism and work, making the prevention of altitude sickness a critical concern.
Current studies have shown that traditional Chinese medicine (TCM) has certain potential in hypoxia tolerance [3, 4]. GYAC is a kind of Tibetan medicine, its main ingredients include rhodiola, astragalus, salvia miltiorrhiza and so on. It is widely used to prevent high-altitude reactions. Its primary function is to enhance hypoxia tolerance and boost immunity, but the specific mechanism remains unclear. The ingredients of TCM have attracted the attention of researchers. Astragalus extract-Astragaloside IV (AST-IV) exhibits anti-inflammatory, anti-fibrotic, antioxidant, anti-asthmatic, anti-diabetic and immunomodulatory properties [5, 6]. It has been proved that AST-IV can inhibit hypoxia-induced apoptosis of PC-12 cells by down-regulating miR-124 [7]. Hypoxia inducible Factor-1α (HIF-1α) has become a key regulator of hypoxia responses, and is also a major regulator of homeostasis in hypoxic cells and systems by activating transcription of numerous genes [8], and AST-IV can upregulate HIF-1 [9]. Rhodiola rosea is a common medicinal plant in Asian countries. It is reported that rhodiola rosea has significant anti-hypoxic, neuroprotective, anti-fatigue, and radiation-protective activities [10, 11]. Rhodiola rosea can alleviate apoptosis and mitochondrial energy metabolism within rat models of hypoxia-induced brain injury by regulating HIF-1/microRNA 210/ISCU1/2(COX10) signaling pathway [12]. Saponins, a key bioactive component of Panax quinquefolium, exhibit a range of pharmacological effects, such as blood glucose regulation, blood lipid reduction, anti-tumor, anti-oxidation, anti-arrhythmic properties and enhancement of myocardial ischemia recovery. Danhong injection (DHI) is extracted from Salvia miltiorrhiza and safflower, has been shown to protect myocardial cells from hypoxia/reoxygenation and H2O2-induced injury by inhibiting the opening of mitochondrial permeability transition pore [13].
TCM is frequently utilized in treating various diseases. Nonetheless, the intricacies of TCM’s mechanisms of action remain elusive, primarily due to the absence of robust methodologies for analyzing its complex chemical constituents [14]. The integration of network biology and multi-pharmacology is anticipated to broaden the scope for identifying drug-actionable targets, thereby establishing a solid foundation for efficacious drug discovery strategies [15]. Molecular docking, a theoretical simulation technique, predicts the binding mode and affinity of a drug based on the interactions between the drug molecule and its receptor. It can reduce the time and consumables we spend in drug development, at the same time, there is great practical value in drug design, making it a highly attractive tool [16].
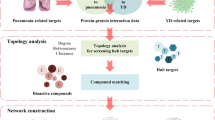
In the present study, we used network pharmacology and molecular docking to predict the potential mechanism of GYAC in improving hypoxia tolerance. In vitro experiments, we constructed a cell hypoxia model for drug administration to verify the above results. This study may reveal new therapeutic pathways or drug targets for high-altitude capsules, and provide new ideas for their application in hypoxia related diseases. The flow chart of this research is shown in Supplementary Fig. 1.
Materials and methods
Composition of GYAC and the potential targets of GYAC
In order to collect the chemical constituents of five herbs in GYAC, we used the traditional Chinese medicine system (TCMSP, http://lsp.nwu.edu.cn/TCMSP.php; HERB, http://herb.ac.cn/; ETCM, http://www.tcmip.cn/ETCM/; Pubchem, https://pubchem.ncbi.nlm.nih.gov/; swisstargetprediction, http://www.swisstargetprediction.ch/) [17], and the criteria of drug screening was: OB (oral bioavailability) ≥ 30%, DL (similarity of patent medicine) ≥ 0.18. Finally, the effective compound information and corresponding targets of GYAC were obtained. In addition, comprehensive database, such as the traditional Chinese medicine integrative database (TCMID, http://183.129.215.33/TCMID/search/) [18], PubMed (https://pubmed.ncbi.nlm.nih.gov/) and OMIM (https://www.omim.org/) were used to retrieve the compounds.
Targets associated with hypoxia tolerance
Target genes related to hypoxia were obtained from Genecards (https://www.genecards.org/), OMIM (https://omim.org/) and PubMed database. The common targets were obtained by intersecting the targets of active ingredients and hypoxia related targets. Taking “Homo sapiens” as research species, the predicted targets were selected and standardized by UniProt database.
Construction of the GYAC ingredient-target-hypoxia tolerance-related target network
Based on the target genes of active ingredients of GYAC and the target genes of hypoxia tolerance, the common targets were introduced into STRING database (https://string-db.org/cgi/input.pl) to establish PPI network [19]. We set the minimum required interaction score at 0.4. Only when the interaction score between two proteins reaches or exceeds 0.4 will they be included in our network analysis. Based on Cytoscape 3.7.2, we visualize complex networks and integrate different types of attribute data [20]. In the network, nodes represent the targets, edges represent the interactions between these nodes. According to the degree value, the top ranked compounds were selected as ligands for molecular docking. The higher the degree value, the more likely it is that the molecule will be as a key component of GYAC.
Enrichment analysis of GO function and KEGG pathway
The functional annotation of Gene Ontology (GO) was analyzed by DAVID database (https://david.ncifcrf.gov/summary.jsp) and the pathway enrichment analysis database-Kyoto Encyclopedia of genes and genomes (KEGG) was used to predict the mechanism of target genes. EHBIO (https://www.ehbio.com) was used to visualize the enrichment results of GO functional annotation analysis, including biological process (BP), molecular function (MF) and cell composition (CC) and KEGG enrichment analysis.
Molecular docking
The core targets (IL-6 and IFNG) were obtained from PPI network as protein receptors, and theirs PDB files were download through RCSB PDB database (http://www.rcsb.org/). The SDF format files of 2D structure of core active compounds were obtained in PubChem database, and the 3D molecular structure of luteolin from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). PyMOL software was used to remove water and original ligands from the original PDB file. Autodock charging software was used to add polar hydrogen to target protein receptor molecules. Molecular docking was accomplished by Autodock Vina and Python scripts. The lower the binding energy, the better the affinity between receptor and ligand. In this study, the binding energy ≤ −5.0 kcal/mol was selected as the screening basis for effective binding of luteolin to target proteins.
Cell culture
Rat cardiomyocytes H9C2 was obtained from the iCell. The cells were cultured in DMEM medium mixed with 10% fetal bovine serum and 1% penicillin-streptomycin. The cells were cultured in an incubator at 37 °C, 5% CO2.
Cell viability
Cell viability assay was performed by Cell Counting Kit-8 (MCE, HY-K0301). Firstly, H9C2 cells were inoculated into 96-well plates at 2 × 103 cells/well and treated with luteolin (Solarbio, SL8300) at a series of concentration (0 μm/mL, 1 μm/mL, 10 μm/mL, 50 μm/mL, 100 μm/mL, 150 μm/mL) for 6 h, 12 h and 24 h, respectively. At 450 nm wavelength, we detected the absorbance value of the above groups.
Cell apoptosis
Cell apoptosis and Cell cycle were performed by cell apoptosis kit (Muse, MCH100105) based on the flow cytometer instrument. After cells were collected, we added we added the staining agents-Annexin V-fluorescein isothiocyanate (FITC) and Propidium Iodide (PI) to the above groups. Besides, the cell apoptosis of each group was determined by calculating the sum of early and late apoptosis in each group.
Quantitative real‑time polymerase chain reaction (RT-qPCR)
After cells were collected, total RNA was extracted with Trizol reagent according to the instructions. Nanodrop 2000 was used to detect RNA concentration and purity. RNA integrity was detected by 0.8% denaturing gel electrophoresis. Then, reverse transcribe 500 ng RNA into cDNA with the TAKARA reverse transcription kit. Finally, we completed the PCR procedure by the fluorescence quantitative instrument (ABI 7500).
Western blotting
Western blot experiment was done with sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). Herein, polyvinylidene fluoride (PVDF) membranes were blocked with blocking solution containing 5% non-fat milk and then incubated with primary antibodies at 4 °C overnight. Clean the PVDF membrane with TBST and the membrane was incubated with a secondary antibody for 1 h at room temperature. Finally, the electrochemiluminescence (ECL) solutions were prepared in a dark chamber to visualize the target protein bands. The antibodies used in this study were as follows: BAX (proteintech, 50599-2-Ig, 1:9000), BCL2 (proteintech, 26593-1-AP, 1:3000), IgG (ZEN BIO, 511103, 1:20000).
Statistical analysis
Data was expressed as mean ± standard deviation (SD). SPSS software 20.0 and GraphPad Prism 8.3.1 software were used to complete data collation and analysis. One-way analysis of variance was used for multiple group comparisons. p < 0.05 indicates significant.
Results
Network pharmacology identified ingredients and targets of GYAC and hypoxia tolerance-related targets and Protein-protein interaction (PPI) network
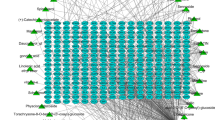
A total of 121 active compounds were retrieved in the TCMSP, HERB, ETCM, Pubchem and swisstargetprediction database, and then 707 corresponding target genes were acquired. We searched out 121 hypoxia-related targets in Genecards and OMIM database. Finally, 40 overlapping targets were obtained through the intersection of ingredient targets and disease-related targets (Fig. 1A) and the top 9 targets were used as core targets (Table 1). Subsequently, in order to further explore the mechanism of action of GYAC in hypoxia tolerance, 40 overlapping targets were imported into STRING database for PPI network analysis, and the result was visualized by Cytoscape 3.7.2, as shown in Fig. 1B–D. In PPI network diagram, there were 40 nodes, 605 edges. The top targets were CCL2, IFNG, IL1B, EGFR, CXCL8, IGF1, INS, IL6, HIF1A which indicated that these proteins have strong interactions with other proteins. The above showed that GYAC has a multi-components and multi-targets synergistic effect in improving hypoxia tolerance, especially in inflammatory response.
GO and KEGG enrichment analyses
In order to further understand the potential mechanism of 40 common targets of improving hypoxia tolerance, GO enrichment analysis was performed, as shown in Supplementary Fig. 2 and Table 2. The results showed that 40 common target genes were enriched in 536 GO terms (448 BP, 29 CC and 59 MF). The top 20 GO terms of BP mainly included positive regulation of gene expression, negative regulation of gene expression, etc. The top 20 GO terms of CC mainly included extracellular region, extracellular space, etc. The top 20 GO terms f MF mainly included identical protein binding, enzyme binding, etc. In addition, the KEGG pathway enrichment analysis showed that the common target genes were enriched in 202 pathways, as shown in Supplementary Fig. 1 and Table 3. It mainly involved HIF-1 signaling pathway, IL-17 signaling pathway, etc. Again, the above results showed that GYAC may improve hypoxic tolerance by inhibiting inflammation.
Construction of the GYAC ingredient-target-hypoxia tolerance-related target network
Based on the Cytoscape software 3.7.2, we constructed GYAC ingredient-target-hypoxia tolerance related target network, as shown in Fig. 1E. The results displayed that salidroside (HBIN042854), kaempferol (HBIN031741), luteolin (MOL000006) and ginsenoside Rb1 (C0810) were considered to be the most important effective components of GYAC against hypoxia tolerance. After reviewing the relevant studies on hypoxia tolerance and traditional Chinese medicine, we chose quercetin for further study.
Molecular docking
To verify the network pharmacology results, we performed molecular docking experiments between luteolin and common targets, as shown in Fig. 2A, B. We observed that luteolin was observed to have a strong interaction with the target gene-IL-6 and IFNG, the binding energy was −8.2 kcal/mol and −9.7 kcal/mol. It is generally believed that the lower the binding energy between ligand and receptor, the more stable the binding conformation, In Fig. 2A, the results showed that luteolin formed three hydrogen bonds (ARG-756, ASN-543, GLU-646), one Pi-Sigma (ILE-735) and one Pi-Alkyl (PRO-750) with IL-6. Luteolin and IFNG were mainly bound by four hydrogen bonds (TYR-521, LYS-185, GTP-706 and VAL-117), two bump (LYS-116 and MG-704), one donor-donor (DZ-4701), one Pi-Sigma (ILE-735) and one Pi-Alkyl (MET-115), as shown in Fig. 2B.
Construction of hypoxic tolerance model of H9C2 cardiomyocytes
In vitro, we constructed a hypoxic tolerance model of H9C2 cardiomyocytes. The cell viability and apoptosis of the model were observed under 12 h, 24 h and 36 h hypoxia conditions. In Fig. 3A–C, we observed that hypoxia can inhibit cell viability and promote apoptosis of H9C2 cell. In Fig. 3D–F, we found that hypoxia can promote the mRNA of pro-apoptotic factor Bax and inhibit the expression of anti-apoptotic factor Bcl2. This result was confirmed by the expression of Bax and Bcl2 proteins, which again confirmed that hypoxia can promote the apoptosis of H9C2 cells. According to the above results, we chose hypoxia for 24 h to construct the model and carried out the following experiments. Subsequently, we found that hypoxia can promote the expression of LDH, indicating that hypoxia can cause mitochondrial damage in cardiomyocytes (Fig. 3G).
Luteolin can improve hypoxia tolerance by reducing the inflammatory response and lowering mitochondrial damage of H9C2 cardiomyocytes
Combining with the results of network pharmacology, we selected the hypoxic tolerance cell model treated by luteolin. First, we examined the cell viability of luteolin at different concentrations (0 μm/mL, 1 μm/mL, 10 μm/mL, 50 μm/mL, 100 μm/mL, 150 μm/mL) under hypoxia conditions for 6 h, 24 h, and 36 h (Fig. 4A–C). Finally, we chose 6 h of hypoxia for the experiment, and the concentrations of luteolin treated models were 50 μm/mL, 100 μm/mL, 150 μm/mL for the following experiments. In Fig. 4D, we observed that luteolin can inhibit the decrease of cell viability in hypoxic cell model. Besides, luteolin can inhibit increase of cell apoptosis in hypoxic cell model (Fig. 4E–J). Besides, luteolin can inhibit LDH release and the decrease of mitochondrial membrane potential in hypoxic cell models (Fig. 4K–L). Moreover, luteolin can inhibit the expression of inflammatory related factors (IL-6, IL-10, IL-2, IFNG and VEGFA) in hypoxic cell models (Fig. 4M–Q). Taken together, we suggest that luteolin can reduce the inflammatory response of cardiomyocytes, thereby reducing mitochondrial damage, and thus increase the hypoxia tolerance of H9C2 cardiomyocytes.
A–C We chose 6 h of hypoxia for the experiment, and the concentrations of luteolin treated models were 50 μm/mL, 100 μm/mL, 150 μm/mL for the following experiments. D Luteolin can inhibit the decrease of cell viability in hypoxic cell model. E–J Luteolin can inhibit increase of cell apoptosis in hypoxic cell model. K Luteolin can inhibit LDH release in hypoxic cell models. L Luteolin can inhibit the decrease of mitochondrial membrane potential in hypoxic cell models. M–Q Luteolin can inhibit the expression of inflammatory related factors (IL-6, IL-10, IL-2, IFNG and VEGFA) in hypoxic cell models.
Discussion
Hypoxia is a fundamental consequence of high-altitude exposure and severe illness. In high altitude areas, the partial pressure of oxygen decreases, leading to reduced oxygen utilization in the body, which may cause various pathophysiological symptoms, such as ischemia, high altitude reaction, high altitude pulmonary edema and high altitude brain edema [21], mental disorders and memory loss, insomnia, dizziness, nausea, irritation, dyskinesia [22], as well as biochemical changes of blood-brain barrier and left ventricular dysfunction [23]. In the results of network pharmacology, we found that luteolin has potential capabilities. And we observed that luteolin was observed to have a strong interaction with the target gene-IL-6 and IFNG. The results indicated that Luteolin had an effect on the inflammatory response induced by hypoxia.
Luteolin is a natural compound that has significant anti-inflammatory and antioxidant properties [24]. Under hypoxia conditions, inflammation is usually activated in response to the damage caused by hypoxia [25]. However, an excessive inflammatory response can lead to tissue damage and organ dysfunction [26]. Therefore, controlling the inflammatory response is essential to improve the body’s tolerance to hypoxia. In the study, we found that hypoxia can promote the expression of LDH, indicating that hypoxia can cause mitochondrial damage in cardiomyocytes. After the addition of luteolin, the decrease of cell viability in hypoxic cell model and the increase of cell apoptosis in hypoxic cell model were inhibited. Besides, luteolin can inhibit LDH release and the decrease of mitochondrial membrane potential in hypoxic cell models. Moreover, luteolin can inhibit the expression of inflammatory related factors in hypoxic cell models. Thus, we suggest that luteolin can reduce the inflammatory response of cardiomyocytes, thereby reducing mitochondrial damage, and thus increase the hypoxia tolerance of H9C2 cardiomyocytes.
The results of KEGG signaling pathway indicate that HIF-1 signaling pathway is involved in the hypoxia tolerance of luteolin. HIF-1α is a transcription complex that, by activating the transcription of multiple genes, has become a key regulator of hypoxia responses and a major regulator of homeostasis in hypoxic cells and systems. The role of HIF-1 pathway in hypoxia tolerance involves several aspects. First, HIF-1 can induce cellular adaptation to hypoxic environments [27]. Under hypoxia conditions, HIF-1 can up-regulate the expression of a variety of enzymes that are involved in the process of glycolysis and help cells gain energy in the absence of oxygen. In addition, HIF-1 can also promote angiogenesis, providing more oxygen and nutrients to hypoxic tissues [28]. However, it is regrettable that this study was unable to complete the research on the HIF-1 pathway in the enhancement of hypoxia tolerance by luteolin. We will continue to explore it in the future, providing a theoretical basis for the mechanism of luteolin in improving hypoxia tolerance.
Conclusion
In conclusion, our study revealed that luteolin, a monomeric component of GYAC, played a role in hypoxia tolerance through a variety of target genes, such as IL6, IFNG. What’s more, we have discovered that luteolin can reduce the inflammatory response in cardiac myocytes, thereby alleviating mitochondrial damage, and ultimately enhancing the hypoxia tolerance of H9C2 cardiomyocytes.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Murray AJ. Energy metabolism and the high-altitude environment. Exp Physiol. 2016;101:23–7.
Davis C, Hackett P. Advances in the prevention and treatment of high altitude illness. Emerg Med Clin North Am. 2017;35:241–60.
Hou Y, Wang X, Zhang Y, Wang S, Meng X. Highland mate: edible and functional foods in traditional medicine for the prevention and treatment of hypoxia-related symptoms. Curr Opin Pharmacol. 2021;60:306–14.
Su M, Ren X, Du D, He H, Zhang D, Xie R, et al. Curcumol β-cyclodextrin inclusion complex enhances radiosensitivity of esophageal cancer under hypoxic and normoxic condition. Jpn J Radiol. 2023;41:1275–89.
Ren S, Zhang H, Mu Y, Sun M, Liu P. Pharmacological effects of Astragaloside IV: a literature review. J Tradit Chin Med. 2013;33:413–6.
Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31:17–36.
Yu W, Lv Z, Zhang L, Gao Z, Chen X, Yang X, et al. Astragaloside IV reduces the hypoxia-induced injury in PC-12 cells by inhibiting expression of miR-124. Biomed Pharmacother = Biomedecine Pharmacotherapie. 2018;106:419–25.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408.
Si J, Wang N, Wang H, Xie J, Yang J, Yi H, et al. HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PloS One. 2014;9:e107832.
Lin KT, Chang TC, Lai FY, Lin CS, Chao HL, Lee SY. Rhodiola crenulata attenuates γ-ray induced cellular injury via modulation of oxidative stress in human skin cells. Am J Chin Med. 2018;46:175–90.
Chang PK, Yen IC, Tsai WC, Chang TC, Lee SY. Protective effects of rhodiola crenulata extract on hypoxia-induced endothelial damage via regulation of AMPK and ERK pathways. Int J Mol Sci. 2018;19:2286.
Wang X, Hou Y, Li Q, Li X, Wang W, Ai X, et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J Ethnopharmacol. 2019;241:111801.
Duan ZZ, Li YH, Li YY, Fan GW, Chang YX, Yu B, et al. Danhong injection protects cardiomyocytes against hypoxia/reoxygenation- and H2O2-induced injury by inhibiting mitochondrial permeability transition pore opening. J Ethnopharmacol. 2015;175:617–25.
Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26:72–80.
Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90.
Saikia S, Bordoloi M. Molecular docking: challenges, advances and its use in drug discovery perspective. Curr drug targets. 2019;20:501–21.
Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13.
Xue R, Fang Z, Zhang M, Yi Z, Wen C, Shi T. TCMID: traditional chinese medicine integrative database for herb molecular mechanism analysis. Nucleic acids Res. 2013;41:D1089–95.
Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–40.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Zubieta-Calleja GR, Zubieta-DeUrioste N. High altitude pulmonary edema, high altitude cerebral edema, and acute mountain sickness: an enhanced opinion from the high Andes - La Paz, Bolivia 3,500 m. Rev Environ health. 2023;38:327–38.
Zhou Y, Yang L, Liu X, Wang H. Lactylation may be a novel posttranslational modification in inflammation in neonatal hypoxic-ischemic encephalopathy. Front Pharmacol. 2022;13:926802.
Holloway C, Cochlin L, Codreanu I, Bloch E, Fatemian M, Szmigielski C, et al. Normobaric hypoxia impairs human cardiac energetics. FASEB J. 2011;25:3130–5.
Ahmadi SM, Farhoosh R, Sharif A, Rezaie M. Structure-antioxidant activity relationships of luteolin and catechin. J Food Sci. 2020;85:298–305.
Han J, Wan Q, Seo GY, Kim K, El Baghdady S, Lee JH, et al. Hypoxia induces adrenomedullin from lung epithelia, stimulating ILC2 inflammation and immunity. J Exp Med. 2022;219:e20211985.
Lodge KM, Vassallo A, Liu B, Long M, Tong Z, Newby PR, et al. Hypoxia increases the potential for neutrophil-mediated endothelial damage in chronic obstructive pulmonary disease. Am J Respiratory Crit care Med. 2022;205:903–16.
Yfantis A, Mylonis I, Chachami G, Nikolaidis M, Amoutzias GD, Paraskeva E, et al. Transcriptional response to hypoxia: the role of HIF-1-associated co-regulators. Cells. 2023;12:798.
Chen W, Wu P, Yu F, Luo G, Qing L, Tang J. HIF-1α regulates bone homeostasis and angiogenesis, participating in the occurrence of bone metabolic diseases. Cells. 2022;11:3552.
Acknowledgements
We thank all authors for their contributions and supports.
Funding
Natural Science Foundation of Tibet Autonomous Region (XZ202201ZR0048G), Science and Technology Major Project of Tibetan Autonomous Region of China (XZ202201ZD0001G) and Key R&D Program of Xizang (Tibet) Autonomous Region (XZ202101ZY0018G).
Author information
Authors and Affiliations
Contributions
Tianbo Jin wrote the manuscript; Xiaoli Liu and Yuhe Wang analyzed the data; Yijin Qi and Xuemei Li made tables and figures; Li Wang and Xue He provided research ideas.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by Research Ethics Committee of Xizang Minzu University. Written informed consent was obtained from individual or guardian participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, T., Liu, X., Wang, Y. et al. Network pharmacology prediction, molecular docking and in vitro experiment explored the potential mechanism of Gaoyuan’an capsule in improving hypoxia tolerance. Pharmacogenomics J 24, 8 (2024). https://doi.org/10.1038/s41397-024-00327-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00327-0
- Springer Nature Limited