Abstract
We evaluated the cost-effectiveness of a genotype-guided strategy among patients with acute coronary syndromes using a decision-tree model based on the Singapore healthcare payer’s perspective over a 1-year time horizon. Three dual antiplatelet strategies were considered: universal clopidogrel, genotype-guided, and universal ticagrelor. The prevalence of loss-of-function alleles was assumed to be 61.7% and model inputs were identified from the literature. Our primary outcome of interest was incremental cost-effectiveness ratio (ICER) compared to universal clopidogrel. Both genotype-guided (72,158 SGD/QALY) and universal ticagrelor (82,269 SGD/QALY) were considered cost-effective based on a willingness-to-pay (WTP) threshold of SGD 88,991. In our secondary analysis, the ICER for universal ticagrelor was 114,998 SGD/QALY when genotype-guided was taken as a reference. Probabilistic sensitivity analysis revealed that genotype-guided was the most cost-effective strategy when the WTP threshold was between SGD 70,000 to 100,000. Until more data are available, our study suggests that funding for a once-off CYP2C19 testing merits a consideration over 1 year of universal ticagrelor.
Similar content being viewed by others
Introduction
Ischemic heart diseases, including acute coronary syndromes (ACS), is the leading cause of death worldwide and third leading cause of death in Singapore [1, 2]. Dual antiplatelet therapy (DAPT), consisting of aspirin and a P2Y12 inhibitor is considered the cornerstone of therapy for patients undergoing percutaneous coronary intervention (PCI) to prevent major adverse cardiovascular events (MACE) [3]. While the P2Y12 inhibitor, clopidogrel, had been the standard of care adjunct to aspirin, it is a pro-drug that requires a two-step conversion to its active metabolite via the cytochrome P450 2C19 (CYP2C19) enzyme [4]. This makes its efficacy susceptible to genetic polymorphisms. Unsurprisingly, patients with a loss-of-function (LOF) allele for CYP2C19 were found to have lower concentrations of the active metabolite and less platelet inhibition [5]. Retrospective analyses of clinical trial data also demonstrated higher risk of MACE among patients with LOF alleles on clopidogrel [5, 6].
Unlike clopidogrel, ticagrelor does not require metabolic activation and is able to achieve rapid and greater P2Y12 inhibition [7]. Ticagrelor also conferred fewer MACE in the PLATO study but incurred more non-coronary artery bypass grafting (CABG) major bleeding [8]. Consequently, ticagrelor has become the standard of care adjunct to aspirin for ACS, and is recommended over clopidogrel in international guidelines [3, 9]. However, the cost of ticagrelor may be prohibitive for universal adoption in many low-to-middle-income countries in Asia. Ticagrelor was also associated with more bleeding among Asians in the PHILO study, albeit not statistically significant [10]. Thus, we hypothesized that CYP2C19-guided selection of P2Y12 inhibitors may be an attractive compromise in Asian populations as it may help to minimize bleeding and overall costs while ensuring drug efficacy.
To date, several studies have evaluated the cost-effectiveness of a genotype-guided strategy [11,12,13,14]. Few were conducted from an Asian perspective and yielded conflicting results [15,16,17]. Singapore is a multi-ethnic Asian country and our prevalence of LOF alleles is estimated to be 61.7%—similar to the figures reported in other Asian populations such as the Chinese (54.4%), Japanese (67.2%), Koreans (60.6%) and Thais (55.3%) [18, 19]. Yet, we were interested to study the cost-effectiveness of a genotype-guided strategy in our local context due to Singapore’s unique approach to healthcare financing: Singapore adopts a multi-payer financing framework that comprises of subsidies and a compulsory medical savings scheme [20]. This is underpinned by the twin philosophies of affordable healthcare and individual responsibility [21]. Thus, out-of-pocket costs form the bulk of healthcare expenditures, allowing Singapore to spend just 2.1% of its GDP on healthcare [22].
Methods
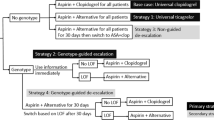
We developed a decision-tree model based on the Singapore healthcare payer’s perspective over a 1-year time horizon for ACS patients undergoing PCI (Fig. 1). Our model utilized a hypothetical cohort of Singaporean patients aged 62 years old at the time of ACS onset as this resembled the demographic of patients enrolled in clinical trials [6, 23]. All patients received 12 months of DAPT as per international guidelines and three DAPT strategies were considered: universal clopidogrel, genotype-guided, and universal ticagrelor [3, 9]. Switching between P2Y12 inhibitors was not factored in our model [3, 9]. For the genotype-guided strategy, we assumed that all patients with LOF alleles would receive ticagrelor instead of clopidogrel as per Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines [24]. Prasugrel was not considered as an alternative P2Y12 inhibitor for patients with LOF allele as it is no longer available in Singapore. We assumed that genotyping was 100% sensitive and results would be available sufficiently quickly to guide the selection of P2Y12 inhibitors during the index admission.
The prevalence of LOF alleles was assumed to be 61.7% [18]. Probabilities for MACE were obtained from Cavallari et al. while probabilities for non-CABG major bleeding were obtained from the PLATO genetic substudy [4, 6]. We assumed that event probabilities were similar for all patients on ticagrelor irrespective of LOF status and patients could not experience MACE and bleeding events concurrently [13]. Drug costs are standardized across the public healthcare sector and were available from our in-house pharmacy database. Genotyping and direct medical costs were obtained from local sources [25, 26]. The utility of each complication was identified from US adults aged 55–64 years old as locally derived values were not available [12]. Patients who died were assigned a utility score of zero. Owing to the low prevalence of intracranial hemorrhage (ICH) in the PLATO study, we decided to substitute the disutility of non-CABG major bleeding with the disutility of non-ICH bleeding [8].
In our primary analysis, our main outcome of interest was incremental cost-effectiveness ratio (ICER) compared to universal clopidogrel measured in 2019 Singapore dollars (SGD) per quality-adjusted life years (QALY). However, since ticagrelor is considered the standard of care in today’s practice, we felt that it was prudent to pitch universal ticagrelor against genotype-guided for our secondary analysis. One-way sensitivity analyses were conducted for all variables and presented as a tornado diagram to compare the relative importance of each variable. We assumed each variable followed a triangular distribution with a range ±25% of the base case unless stated otherwise (Table 1). We conducted 1000 Monte Carlo simulations to represent the combined uncertainty across all variables. Results from these simulations were presented as scatter plots over cost-effectiveness planes and cost-effectiveness acceptability curves (CEAC). We also calculated the net monetary benefit (NMB) of each strategy in order to compare the three strategies simultaneously. All analyses were performed using Excel 2011 (Microsoft, WA, USA) using methods published in the literature [27].
Results
The incremental costs, utilities, and ICERs of the three strategies are presented in Table 2. Compared to universal clopidogrel, the ICER for genotype-guided (72,158 SGD/QALY) was less than the ICER for universal ticagrelor (82,269 SGD/QALY) (Fig. 2). Both strategies were considered cost-effective compared to universal clopidogrel based on a willingness-to-pay (WTP) threshold of SGD 88,991, defined as 1× Singapore’s gross domestic product (GDP) per capita in 2019 [28]. However, when genotype-guided was taken as a reference strategy, the ICER for universal ticagrelor was 114,998 SGD/QALY and was not considered cost effective (Table 3).
We present our ten most influential variables from one-way sensitivity analyses in Fig. 3. When universal clopidogrel was taken as a reference, the ICER for universal ticagrelor was sensitive to more variables than genotype-guided. In our secondary analysis, while the ICER for universal ticagrelor versus genotype-guided was not considered cost-effective, we identified three variables that may render it cost-effective. They were probability of death among patients with no LOF alleles on clopidogrel (nonLOF-clopidogrel), probability of death among nonLOF-ticagrelor, and cost of ticagrelor.
A Genotype-guided versus universal clopidogrel, B universal ticagrelor versus universal clopidogrel and C universal ticagrelor versus genotype-guided. The bars represent the change in ICERs when the variables approach the upper (shaded) and lower (white) boundaries. The asterisk (*) denotes variables that the ICERs were sensitive to.
Compared to universal clopidogrel, probabilistic sensitivity analyses revealed that all 1000 simulations for genotype-guided and universal ticagrelor fell in the right upper quadrant of the cost-effectiveness plane (Fig. 4). While Singapore does not have an official WTP threshold for cost-effectiveness, we were cautious to adopt the World Health Organization’s definition wholeheartedly due to the low proportion of GDP spent on healthcare [29]. Thus, we constructed CEACs to determine the probability of cost-effectiveness over a range of WTP thresholds, particularly between SGD 60,000 (inferred from previous studies) and 90,000 (close to our 2019 GDP per capita) [28, 29].
At a WTP threshold of SGD 60,000, the probability of being cost-effective was 44.0% for genotype-guided versus universal clopidogrel and 24.6% for universal ticagrelor versus universal clopidogrel. When the WTP threshold was raised to SGD 90,000, the probabilities rose to 92.9% and 86.4%, respectively. Probabilistic sensitivity analyses based on NMB also revealed that genotype-guided was the most cost-effective among the three strategies when the WTP threshold was between SGD 70,000 and 100,000 (Fig. 5).
Discussion
In our study, we have demonstrated that genotype-guided and universal ticagrelor were both cost-effective strategies compared to universal clopidogrel. Probabilistic sensitivity analyses between genotype-guided and universal clopidogrel also revealed that genotype-guided was already cost-effective at a WTP threshold of SGD 70,000 and this likelihood exceeded 90% when the WTP threshold rose to SGD 90,000.
When genotype-guided was taken as a reference, the ICER for universal ticagrelor was not considered cost-effective and was most sensitive to the probability of death among nonLOF-clopidogrel (Fig. 3C). In our study, the probabilities of death among nonLOF-ticagrelor and nonLOF-clopidogrel were assumed to be 0.0173 and 0.0333, respectively (RR: 0.52). This was considered conservative compared to a meta-analysis by Wu et al. that reported a risk ratio (RR) of 0.92 when they combined the outcomes from the PLATO Asian substudy and PHILO study [30]. If we had assumed a RR of 0.92 in our analysis, universal ticagrelor would be dominated by genotype-guided (ICER: −22,8088 SGD/QALY).
International guidelines recommend ticagrelor over clopidogrel for ACS patients undergoing PCI [3, 9]. While our prescription data also echoes the preferential use of ticagrelor, high out-of-pocket cost and safety concerns were commonly cited barriers to widespread ticagrelor use [31]. We believe that CYP2C19-guided selection of P2Y12 inhibitors could be considered cost-effective in resource-strapped Asian countries by reducing bleeding and overall costs while maintaining drug efficacy [12, 13] This was demonstrated in the recent POPular Genetics trial where genotype-guided reduced minor bleeding yet it was non-inferior to universal ticagrelor in ischemic outcomes [32].
To our knowledge, several studies have evaluated the cost effectiveness of genotype-guided compared to universal ticagrelor [11,12,13,14]. However, studies from Asia have produced discordant results—two studies were consistent with our findings, while one study found universal ticagrelor to be more cost-effective than genotype-guided [15,16,17]. Thus, we were decided to pitch universal ticagrelor against genotype-guided to address this uncertainty.
A cost-effectiveness analysis from China reported that genotype-guided was dominant compared to universal prasugrel or ticagrelor [16]. However, we were cautious to interpret the results from this study as there were important differences in assumptions. In this study, patients with gain-of-function (GOF) alleles were assigned to ticagrelor as the authors explained that these patients would be at a greater risk of bleeding left on clopidogrel. Conversely, we followed CPIC’s recommendation and assigned patients with GOF alleles to clopidogrel as the association with bleeding failed to demonstrate statistical significance in several meta-analyses [33, 34]. Jiang and colleagues also assumed that the prevalence of LOF alleles was 27.8%, which was unacceptably low compared to 61.7% catered for in our study. Nevertheless, we were still able to demonstrate that genotype-guided was cost-effective compared to universal ticagrelor despite the high prevalence of LOF alleles.
A second cost-effectiveness analysis from Hong Kong also reported that genotype-guided was dominant compared to universal ticagrelor [17]. In this study, hazard ratios (HRs) for outcomes were obtained from a retrospective analysis of the PLATO study that reported more strokes in Asian patients on ticagrelor [35]. Consequently, the ICER for genotype-guided versus universal ticagrelor was the most sensitive to the HR for stroke and likely contributed to its dominance. We did not account for stroke in our analysis as there was no prospective study that reported this outcome based on drug-gene pairs.
More recently, a study from China reported that universal ticagrelor was cost-effective compared to genotype-guided at a WTP threshold of 178,980 RMB, defined as 3× China’s GDP per capita in 2017 [15]. In this study, the authors reported that the RRs for outcomes were obtained from a meta-analysis described earlier [30]. Yet, the authors did not explain why their base case RRs were different from the figures reported in the meta-analysis. As discussed earlier, we chose not to adopt the RRs in the meta-analysis as it would have favored genotype-guided as a more cost-effective strategy. Thus, we adopted more conservative estimates from Cavallari et al. [23].
There were several limitations in our study. First, probabilities for MACE were obtained from Cavallari et al. as it was the only prospective study to date that reported outcomes based on drug–gene pairs. While this study was conducted in a predominantly Caucasian population, we accounted for our local prevalence of LOF alleles when we were calculating the event probabilities of each strategy. Second, we assumed that the probabilities for MACE would be similar for patients on ticagrelor, irrespective of whether they carry a LOF allele [13]. Therefore, we decided to replace the probability of death among nonLOF-ticagrelor (p = 0.0052) with a larger probability from LOF-ticagrelor (p = 0.0173) so that the benefits of ticagrelor would not be overstated [23]. We speculate that the low probability could be attributed to the small sample of merely 193 nonLOF-ticagrelor patients [23]. While it would be most accurate to use local event probabilities based on drug-gene pairs, the data were not available to use at the time of analysis. Third, utility values from the USA may not be transferrable to our local context due to different perceptions of health across cultural groups. However, we believe that this would not affect our findings significantly as our ICERs were not sensitive to any disutility values for events. Last, we could not account for the impact of minor bleeding on QALY as we did not have local cost and probability data based on drug–gene pairs. If we had assumed that genotype-guided would result in less minor bleeding compared to universal ticagrelor, its cost effectiveness could have been even greater than what we demonstrated [32].
In conclusion, we were able to demonstrate that CYP2C19-guided antiplatelet therapy was the most cost-effective strategy despite our high prevalence of LOF alleles. Until local data are available, our study suggests that funding for a once-off CYP2C19 testing merits consideration over 1 year of universal ticagrelor.
References
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;39:119–77.
Ministry of Health Singapore. Principal causes of death. https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death. Accessed 23 March 2020.
Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017;39:213–60.
Cavallari LH. Personalizing antiplatelet prescribing using genetics for patients undergoing percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2017;15:581–9.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Eng J Med. 2009;360:354–62.
Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease. Circulation. 2009;120:2577–85.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Eng J Med. 2009;361:1045–57.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082–115.
Goto S, Huang C-H, Park S-J, Emanuelsson H, Kimura T. Ticagrelor vs clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome. Circ J. 2015;79:2452–60.
Borse MS, Dong OM, Polasek MJ, Farley JF, Stouffer GA, Lee CR. CYP2C19-guided antiplatelet therapy: a cost–effectiveness analysis of 30-day and 1-year outcomes following percutaneous coronary intervention. Pharmacogenomics. 2017;18:1155–66.
Kim K, Touchette DR, Cavallari LH, Ardati AK, DiDomenico RJ. Cost-effectiveness of strategies to personalize the selection of P2Y12 Inhibitors in patients with acute coronary syndrome. Cardiovasc Drugs Ther. 2019;33:533–46.
Limdi NA, Cavallari LH, Lee CR, Hillegass WB, Holmes AM, Skaar TC, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. 2020;20:724–35.
Sorich MJ, Horowitz JD, Sorich W, Wiese MD, Pekarsky B, Karnon JD. Cost–effectiveness of using CYP2C19 genotype to guide selection of clopidogrel or ticagrelor in Australia. Pharmacogenomics. 2013;14:2013–21.
Fu Y, Zhang X-Y, Qin S-B, Nie X-Y, Shi L-W, Shao H, et al. Cost–effectiveness of CYP2C19 LOF-guided antiplatelet therapy in Chinese patients with acute coronary syndrome. Pharmacogenomics. 2020;21:33–42.
Jiang M, You JHS. CYP2C19 LOF and GOF-guided antiplatelet therapy in patients with acute coronary syndrome: a cost-effectiveness analysis. Cardiovasc Drugs Ther. 2017;31:39–49.
Wang Y, Yan BP, Liew D, Lee VWY. Cost-effectiveness of cytochrome P450 2C19*2 genotype-guided selection of clopidogrel or ticagrelor in Chinese patients with acute coronary syndrome. Pharmacogenomics J. 2018;18:113–20.
Tan DS-Y, Aw JWX, Winther M, Goh LL, Ong HY, Wee E, et al. CYP2C19 phenotype in South-East Asian acute coronary syndrome patients and impact on major adverse cardiovascular events. J Clin Pharmacol Ther. 2020;45:52–58.
Dorji PW, Tshering G, Na-Bangchang K. CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South-East and East Asian populations: a systematic review. J Clin Pharmacol Ther. 2019;44:508–24.
Lim J. Sustainable health care financing: the Singapore experience. Glob Policy. 2017;8:103–9.
HealthHub. Costs and financing. https://www.healthhub.sg/a-z/costs-and-financing/5/costs_and_financing_overall. Accessed 21 April 2020.
Ministry of Health Singapore. Government health expenditure and healthcare financing. https://www.moh.gov.sg/resources-statistics/singapore-health-facts/government-health-expenditure-and-healthcare-financing. Accessed 24 March 2020.
Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:181–91.
Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–23.
Ministry of Health Singapore. Fee benchmarks and bill amount information. https://www.moh.gov.sg/cost-financing/fee-benchmarks-and-bill-amount-information. Accessed 26 March 2020.
Zhao YJ, Khoo AL, Lin L, Teng M, Wu TS, Chan MY, et al. Cost-effectiveness analysis of ticagrelor and prasugrel for the treatment of acute coronary syndrome. Value Health Reg Issues. 2016;9:22–27.
Craven MP, Morgan SP, Crowe JA, Lu B. Deploying a spreadsheet tool for early economic value assessment of medical device innovations with healthcare decision makers. J Manag Mark Health. 2009;2:278–92.
Department of Statistics Singapore. National accounts. https://www.singstat.gov.sg/find-data/search-by-theme/economy/national-accounts/latest-data. Accessed 28 March 2020.
Chin CTMC, Chua TS, Matchar DB. Lifetime cost-effectiveness analysis of ticagrelor in patients with acute coronary syndromes based on the PLATO trial: a Singapore healthcare perspective. Singap Med J. 2013;54:169–75.
Wu B, Lin H, Tobe RG, Zhang L, He B. Ticagrelor versus clopidogrel in East-Asian patients with acute coronary syndromes: a meta-analysis of randomized trials. J Comp Eff Res. 2018;7:281–91.
Agency for Care Effectiveness (ACE). Ticagrelor for preventing thrombotic events in adults with acute coronary syndromes. https://www.ace-hta.gov.sg/our-guidance/ticagrelor-for-preventing-thrombotic-events-in-adults-with-acute-coronary-syndromes.html. Accessed 21 April 2020.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van’t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y12 Inhibitors in primary PCI. N Eng J Med. 2019;381:1621–31.
Li Y, Tang H-L, Hu Y-F, Xie H-G. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10:199–206.
Sorich MJ, Polasek TM, Wiese MD. Systematic review and meta-analysis of the association between cytochrome P450 2C19 genotype and bleeding. Thromb Haemost. 2012;108:199–200.
Kang H-J, Clare RM, Gao R, Held C, Himmelmann A, James SK, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2015;169:899–905. e891.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.H., Tan, D.SY. & Chan, M.Y.Y. Cost-effectiveness of CYP2C19-guided antiplatelet therapy for acute coronary syndromes in Singapore. Pharmacogenomics J 21, 243–250 (2021). https://doi.org/10.1038/s41397-020-00204-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-020-00204-6
- Springer Nature Limited
This article is cited by
-
Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study
Human Genomics (2023)
-
A systematic review on the cost effectiveness of pharmacogenomics in developing countries: implementation challenges
The Pharmacogenomics Journal (2022)
-
Cost-Utility Analysis of CYP2C19 Genotype Detection for Selection of Acid-Suppressive Therapy with Lansoprazole or Vonoprazan for Patients with Reflux Esophagitis in China
Clinical Drug Investigation (2022)