Abstract
Genetic polymorphism in YB-1 was previously shown to be associated with the prognosis of advanced prostate cancer patients treated with primary androgen-deprivation therapy. However, the significance of this polymorphism remains invalidated. In this study, we aimed to validate the prognostic significance of the YB-1 genetic polymorphism in metastatic prostate cancer. This study included 79 Japanese patients who were diagnosed as metastatic prostate cancer between 2000 and 2016. Genomic DNA was obtained from patient whole blood samples, and genotyping on YB-1 (rs12030724) was performed by PCR-based technique. The association of genotype in YB-1 with clinicopathological parameters and oncological outcome, including progression-free survival and overall survival, was examined. Homozygous wild-type (AA), heterozygous variant (AT), and homozygous variant (TT) were identified in 47 (59.5%), 26 (32.9%) and 6 patients (7.6%), respectively. Heterozygous/homozygous variant (AT/TT) in YB-1 was significantly associated with lower progression risk compared with homozygous wild-type (AA) (hazard ratio = 0.52; 95% confidence interval = 0.30–0.88, P = 0.015). Consistent with this finding, heterozygous/homozygous variant (AT/TT) in YB-1 was significantly associated with lower risk of any-cause mortality compared with homozygous wild-type (AA) (hazard ratio = 0.46; 95% confidence interval = 0.21–0.93, P = 0.031). Gene polymorphism in YB-1 rs12030724 was validated to be a promising predictive biomarker of androgen-deprivation therapy in metastatic prostate cancer to identify patients requiring more intensive therapeutics.
Similar content being viewed by others
Introduction
Androgen-deprivation therapy (ADT) is the backbone therapy for recurrent or advanced prostate cancer. However, most cases relapse in a castration-resistant fashion during ADT and become lethal [1]. Several genetic polymorphisms have been found to be associated with the efficacy of ADT and prognosis of ADT-treated prostate cancer patients [2].
The Y-box binding protein-1 (YB-1) plays pleiotropic roles such as in RNA splicing to modulate the expression of its target genes [3, 4]. We recently reported that YB-1 is a critical regulator of androgen receptor (AR) variants, which are involved in resistance to hormone therapy, including ADT, and AR-axis targeting (ARAT) agents, such as abiraterone and enzalutamide [5]. Consistent with these findings, YB-1 has also been shown to be a critical factor affecting the therapeutic efficacy of hormone therapy for prostate cancer [6,7,8,9]. In addition, a single nucleotide polymorphism (SNP) in YB-1 (rs12030724) was associated with the prognosis of patients with advanced prostate cancer treated with primary ADT [5]. This polymorphism is in the intron of the YB-1 gene and may affect gene expression of YB-1 through intron-mediated enhancement of gene expression. Thus far, a validation study on the prognostic significance of the SNP in YB-1 in prostate cancer has not been reported. In this study, we investigated the prognostic significance of the SNP in YB-1 in a distinct cohort of metastatic prostate cancer patients.
Methods
Patients
Seventy-nine male patients with metastatic prostate cancer diagnosed at Akita University Hospital (Akita, Japan) between 2000 and 2016 were included. Prostate biopsy was performed for pathological diagnosis in all patients, and the presence of distant metastasis was evaluated by radiography, computed tomography scan, and bone scintigraphy. Patients who were diagnosed with pathological diagnosis other than adenocarcinoma were excluded. All patients underwent primary hormone therapy by surgical castration or luteinizing hormone-releasing hormone analogs with or without antiandrogens while neither up-front docetaxel nor ARAT agents was utilized. Progression was defined as an increase in prostate-specific antigen (PSA) levels of >2 ng/mL with a 25% increase over the nadir or radiographic progression, which was defined as the appearance of two new lesions or the progression of one or more known lesions (as classified by the Response Evaluation Criteria in Solid Tumors) according to the criteria by Prostate Cancer Working Group 2 [10]. In the analyses of progression-free survival (PFS) and overall survival (OS), progression and any-cause mortality were defined as an event, respectively. Patients without events were censored at the last follow-up visit.
We obtained written informed consent from all patients. This study was approved by the institutional review board, and performed in accordance with the principles in the Declaration of Helsinki and the Ethical Guidelines for Epidemiological Research enacted by the Japanese Government.
Polymorphism genotyping
We extracted genomic DNA from the whole blood of patients and stored them at −80 °C until analysis. As described previously, genotyping of rs12030724 in YB-1 was performed on a CFX Connect Real-Time System with predesigned Taqman SNP Genotyping Assays (C_30860318_10, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol [5].
Statistical analysis
JMP13 software (SAS Institute, Cary, NC, USA) was utilized for all statistical analyses. Categorical and continuous data were analyzed by Pearson’s chi square and Wilcoxon rank sum tests, respectively. Survival analyses were performed by the Kaplan–Meier method and the log-rank test. The Cox hazard proportional model was used for univariate and multivariate analyses to estimate hazard ratios (HRs). All P values are two-sided. P values < 0.05 were defined as significant.
Results
The clinical and pathological characteristics of the 79 Japanese patients with metastatic prostate cancer are shown in Table 1. Genotyping revealed that the homozygous wild-type (AA), heterozygous variant (AT), and homozygous variant (TT) were detected in 47 (59.5%), 26 (32.9%), and 6 patients (7.6%), respectively. No statistical difference was observed among patient backgrounds between patients who were homozygous wild-type (AA) or heterozygous/homozygous variant (AT/TT) (Table 1).
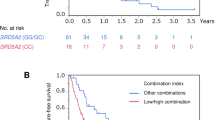
Subsequently, the prognostic impact of the genetic polymorphism in YB-1 on PFS and OS was examined. During a median follow-up of 2.5 years (interquartile range; 1.1–5.1), progression and any-cause mortality occurred in 58 (73.4%) and 35 patients (44.3%), respectively. Heterozygous variant (AT) in YB-1 was significantly associated with lower progression risk compared with homozygous wild-type (AA) (HR = 0.54; 95% confidence interval [CI] = 0.30–0.94, P = 0.028) (Table 2). In the dominant model, the heterozygous/homozygous variant (AT/TT) in YB-1 was significantly associated with lower progression risk compared with the homozygous wild-type (AA) (HR = 0.52; 95% CI = 0.30–0.88, P = 0.015) (Table 2). Better PFS among men carrying the heterozygous/homozygous variant (AT/TT) was indicated by Kaplan–Meier curve (Fig. 1a). In the recessive model, there was no significant difference between homozygous/heterozygous wild-type (AA/AT) and homozygous variant (TT) (HR = 0.54; 95% CI = 0.13–1.47, P = 0.26) (Table 2).
In terms of OS, the heterozygous variant (AT) in YB-1 was significantly associated with lower risk of any-cause mortality compared with homozygous wild-type (AA) (HR = 0.41; 95% CI = 0.18–0.87, P = 0.020) (Table 2). In the dominant model, the heterozygous/homozygous variant (AT/TT) in YB-1 was significantly associated with lower risk of any-cause mortality compared with homozygous wild-type (AA) (HR = 0.46; 95% CI = 0.21–0.93, P = 0.031) (Table 2). Better OS among men carrying the heterozygous/homozygous variant (AT/TT) was shown by Kaplan–Meier curve (Fig. 1b). In the recessive model, there was no significant difference between homozygous/heterozygous wild-type (AA/AT) and homozygous variant (TT) (HR = 1.21; 95% CI = 0.19–4.08, P = 0.81) (Table 2).
On multivariate analyses incorporating age, Eastern Cooperative Oncology Group performance status, PSA at diagnosis, and biopsy Gleason score as well as the dominant model in YB-1 genotype as parameters, the heterozygous/homozygous variant (AT/TT) in YB-1 was still significantly associated with lower progression risk (HR = 0.54; 95% CI = 0.30–0.95, P = 0.034), and lower risk of any-cause mortality (HR = 0.45; 95% CI = 0.20–0.95, P = 0.035) compared with homozygous wild-type (AA).
Discussion
As we previously reported, the SNP rs12030724 in YB-1 was associated with prognosis in advanced prostate cancer patients treated with primary ADT [5]. Here we conducted a validation study in a distinct cohort that comprises Japanese male patients with metastatic prostate cancer. Our results demonstrated that the SNP rs12030724 in YB-1 was reproducibly associated with prognosis in metastatic prostate cancer. These results indicate that the gene polymorphism YB-1 rs12030724 may be a promising prognostic factor in primary ADT for advanced prostate cancer.
In terms of molecular biology, YB-1 was shown to promote therapeutic resistance to hormonal therapy including ADT through augmenting AR signaling [5, 7]. Since higher YB-1 protein expression in prostate cancer tissues among patients with homozygous wild-type (AA) in YB-1 gene was reported, AA genotype seem to be associated with poor response to hormonal therapy via augmented YB-1/AR axis.
Up-front docetaxel as well as up-front abiraterone therapy has been established as standard therapy for metastatic hormone-sensitive prostate cancer [11]. In addition, a promise of local therapy and up-front therapy using novel ARAT agents for metastatic diseases has emerged [12]. Thus, the therapeutic landscape for metastatic hormone-sensitive prostate cancer has been changing, and multiple therapeutic options are available. Therefore, the identification of biomarkers that are useful for the selection of appropriate therapy is required, although disease volume and disease risk used in CHAARTED and LATITUDE trials would also serve as criteria for therapeutic selection [13,14,15]. Based on the obtained results, men carrying homozygous wild-type who show poor prognosis when treated with conventional primary ADT may receive benefit from treatment with more intensive treatment utilizing radiotherapy, docetaxel or novel ARAT agents. Therefore, the significance of the SNP (rs12030724) in YB-1 in the efficacy of therapy using radiotherapy, docetaxel or novel ARAT agents for advanced prostate cancer should be investigated in future studies.
This study had several limitations including relatively small sample size and its retrospective design. In addition, the patient group was limited to a Japanese population, and thus further investigations in other populations are required.
In conclusion, our study validated the gene polymorphism in YB-1 rs12030724 as a promising prognostic marker in advanced prostate cancer to identify patients requiring more intensive therapeutics for better outcome.
References
Shiota M, Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol. 2016;23:360–9.
Fujimoto N, Shiota M, Tomisaki I, Minato A. Gene polymorphism-related individual and interracial differences in the outcomes of androgen deprivation therapy for prostate cancer. Clin Genitourin Cancer. 2017;15:337–42.
Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25:691–8.
Kuwano M, Oda Y, Izumi H, Yang SJ, Uchiumi T, Iwamoto Y, et al. The role of nuclear Y-box binding protein 1 as a global marker in drug resistance. Mol Cancer Ther. 2004;3:1485–92.
Shiota M, Fujimoto N, Imada K, Yokomizo A, Itsumi M, Takeuchi A, et al. Potential role for YB-1 in castration-resistant prostate cancer and resistance to enzalutamide through the androgen receptor V7. J Natl Cancer Inst. 2016;108:djw005.
Giménez-Bonafé P, Fedoruk MN, Whitmore TG, Akbari M, Ralph JL, Ettinger S, et al. YB-1 is upregulated during prostate cancer tumor progression and increases P-glycoprotein activity. Prostate. 2004;59:337–49.
Shiota M, Takeuchi A, Song Y, Yokomizo A, Kashiwagi E, Uchiumi T, et al. Y-box binding protein-1 promotes castration-resistant prostate cancer growth via androgen receptor expression. Endocr Relat Cancer. 2011;18:505–17.
Imada K, Shiota M, Kohashi K, Kuroiwa K, Song Y, Sugimoto M, et al. Mutual regulation between Raf/MEK/ERK signaling and Y-box-binding protein-1 promotes prostate cancer progression. Clin Cancer Res. 2013;19:4638–50.
Matsumoto H, Yamamoto Y, Shiota M, Kuruma H, Beraldi E, Matsuyama H, et al. Cotargeting androgen receptor and clusterin delays castrate-resistant prostate cancer progression by inhibiting adaptive stress response and AR stability. Cancer Res. 2013;73:5206–17.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
Komura K, Sweeney CJ, Inamoto T, Ibuki N, Azuma H, Kantoff PW. Current treatment strategies for advanced prostate cancer. Int J Urol. 2018;25:220–31.
Hahn AW, Higano CS, Taplin ME, Ryan CJ, Agarwal N. Metastatic castration-sensitive prostate cancer: optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book. 2018;38:363–71.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–7.
Shiota M, Namitome R, Kobayashi T, Inokuchi J, Tatsugami K, Eto M. Prognostic significance of risk stratification in CHAARTED and LATITUDE studies among Japanese men with de novo metastatic prostate cancer. Int J Urol. 2019;26:426–8.
Acknowledgements
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this paper. This work was supported by a JSPS KAKENHI grant [17K11145].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiota, M., Narita, S., Habuchi, T. et al. Validated prognostic significance of YB-1 genetic variation in metastatic prostate cancer. Pharmacogenomics J 21, 102–105 (2021). https://doi.org/10.1038/s41397-020-00188-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-020-00188-3
- Springer Nature Limited