Abstract
Tenofovir disoproxil fumarate (TDF) is a very effective antiviral drug that has been associated with tubular dysfunction. The aim of this study was to analyze the demographic, pharmacokinetic, and pharmacogenetic variables associated with TDF discontinuation for renal outcomes in stable HIV-positive patients using multivariable analyses. Three hundred and four patients were included (73% male, with median age and eCrCl of 45.3 years and 90.9 mL/min, respectively). After a median follow-up of 28.3 months, 27 patients discontinued TDF for renal adverse events [persistent urinary abnormalities (n = 21) or eCrCl < 60 mL/min (n = 6)] providing an incidence of 3.77 events per 100 patient-year. The probability of TDF discontinuation was higher with several features (male gender, older age, not Caucasians ancestry, absence of intravenous drug abuse, protease inhibitors, previous indinavir, HCV-positivity, lower CD4 cell count, detectable HIV-RNA, lower eCrCl, spot-urine proteinuria) and higher tenofovir concentrations but not genetic variants. Tenofovir plasma concentrations were prognostic of TDF discontinuation for renal adverse events suggesting that dose-adjustment may be warranted for long-term safety.
Similar content being viewed by others
Background
Tenofovir, administered as tenofovir disoproxil fumarate (TDF), is a highly effective nucleoside reverse-transcriptase inhibitor. Since its release in 2001 the medication has been largely used worldwide and it is recommended as the preferential backbone for antiretroviral-naïve patients by international guidelines [1]. While many years of TDF use have confirmed its efficacy and safety profile, some patients may develop kidney and/or bone toxicity that leads to TDF discontinuation [2]. On this basis tenofovir alafenamide (TAF), the novel pro-drug associated with lower plasma concentrations and better toxicity features, has been developed and has just been released into the market, while TDF lost its patent by the end of 2017 and it now available as a generic drug [3]. It is likely that the drug will be part of some cost-saving strategies and it is still included in first-line regimens in limited resource countries; as a consequence, a better understanding of the factors associated with TDF toxicity will remain of clinical interest [4].
TDF-associated renal adverse effects originate from its elimination path, as TDF clearance results from both glomerular filtration and active tubular secretion. Recipients of TDF-containing regimens show an increased risk of glomerular filtration rate (GFR) reduction and chronic kidney disease (CKD) occurrence when compared to other highly active antiretroviral treatment combinations [5]. However it has been repeatedly demonstrated that proximal tubular cells are the real key target of tenofovir toxicity [6, 7]. Such tubular impairment can very rarely manifest as complete Fanconi syndrome and acute renal failure, but more frequently as a subclinical tubular dysfunction which involves urinary waste of glucose, proteins, and ions (such as phosphate) without actual GFR contraction [8]. Phosphate urinary loss is the most plausible candidate mechanism linking tubular dysfunction to reduced bone mineral density (BMD), an event which was found to be repeatedly associated to TDF intake in all TDF registration trials.
Altered clearance of retinol-binding protein (RBP, a low molecular weight protein completely reabsorbed by the normal proximal tubule) is a marker of tubular dysfunction which has been associated to reduction of TDF clearance; in a previous study we identified a tight association between low urinary TDF concentration and higher urinary RBP levels [9, 10]. The pathogenic mechanism proposed so far is entrapment and accumulation of the drug into the proximal tubular cells resulting in some degree of mitochondrial dysfunction and decreased energy available for efflux pumps [11]. An increase in plasma concentration and/or inhibition of tubular secretion can determine increment of intracellular TDF concentration in a sort of vicious circle, to which protease inhibitors (PIs) as companion drugs may contribute by affecting both processes [12, 13]. A synergistic action on TDF-associated tubular toxicity may also result from genetic polymorphisms of transporters involved in TDF uptake and extrusion by proximal tubular cells: organic anion transporters, multidrug resistance proteins (MRP-2, MRP-4, and MRP-7), and P-glycoprotein seem to be directly or indirectly involved in TDF elimination from tubular cells into urine [14,15,16],
In the present prospective study we investigated the relationship of demographic, clinical, pharmacokinetic, and pharmacogenetic variables with treatment discontinuation due to renal toxicity in otherwise TDF-tolerant patients.
Materials and methods
Patients and study design
Patients were enrolled in a cross-sectional study whose methods [pharmacokinetics, pharmacogenetics, urinary retinol-binding protein corrected per creatininuria (i.e. tubular proteinuria)] results have been reported elsewhere [10]. They were recruited between May 2012 and June 2014 at the Unit of Infectious Diseases, Amedeo di Savoia Hospital (Torino, Italy). Inclusion criteria were adult age, confirmed HIV positivity, estimated creatinine clearance (eCrCl, evaluated through the Cockcroft-Gault equation) above 60 mL/min, being on tenofovir-containing combination antiretroviral treatment since at least 6 months. In order to exclude major confounding conditions subjects with diabetes (fasting glucose above 126 mg/dL or anti-diabetic drug use) or with kidney and urinary tract malformations were excluded. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) CKD risk score was calculated according to the manuscript from Mocroft et al. [17]. All participants signed a written informed consent before being enrolled; the protocol was approved by Comitato Etico Interaziendale di Orbassano.
Patients have been followed prospectively until March 2016 according to clinical practice; 24-h urine were collected after physicians’ assessment (and usually following spot-urine analysis). The causes of TDF discontinuation were categorized as follows: persistent 24-h urine abnormalities (two following exams with at least one among proteinuria, glycosuria with normal blood glucose, increased phosphate, calcium, or uric acid waste), renal toxicity (eCrCl decrease below 60 mL/min), treatment failure, and other causes. The outcome of interest was defined as occurrence of TDF discontinuation due to either urine abnormalities or renal toxicity, while TDF discontinuation for other causes and death were treated as competing events. Follow-up was calculated from the date of entry into the study until date of TDF discontinuation or death, whichever occurred first. Patients who did not interrupt the treatment and were still alive in March 2016 were censored at date of last follow-up in living. Sample size was calculated for the baseline cross-sectional study as reported elsewhere [10].
Statistical analysis
Patients’ characteristics are described using median values and interquartile ranges (IQR) for continuous variables and percentages for categorical variables. Multiple imputation by chained equations (MICE) approach was performed assuming the data were missing at random (MAR) [18]. Cumulative incidence functions, i.e., the probability of TDF discontinuation over time, stratified by each covariate, were calculated and potential differences were evaluated by Gray’s test. Multivariable analyses were performed using the competing risk regression (Fine and Gray) model which allows to estimate the effect of each covariate on the cumulative incidence function in terms of sub-hazard ratio (sub-HR) [19]. Proportionality assumption on the sub-hazard distribution scale was checked by including in the model the interaction between time and each covariate. The covariates sex, age, ethnicity, route of infection, body mass index, chronic HCV and HBV infection, nadir CD4 cell count, viral load, treatment, previous exposure to indinavir, duration of TDF at entry, eCrCl, retinol-binding protein, proteinuria at entry, urine, and plasma concentration were considered as potential prognostic factors. Ethnicity, route of infection, viral load, and treatment were represented by a two-level covariates (Caucasian vs. other, intravenous drug users (IVDU) vs. other, <20 vs. ≥20 copies/mL, PIs vs. other, respectively). Variables selection was carried out by backward stepwise regression procedure based on BICcr as selection criteria for the Fine and Gray model [20]. Continuous variables were modeled both assuming a linear relationship and using restricted cubic splines with three knots fixed at tertiles of their distribution. Interactions for individual combinations of predictors were tested by Wald’s test. Continuous variables’ shape and presence of interactions in the predictive model were defined according to both model’s goodness and predictive performance. Internal cross-validation was used by bootstrap resampling. The prediction models were validated on 100 bootstrap samples drawn with replacement of the same size as the original data. Discriminative ability over time was assessed by time-dependent concordance index (C-index), which quantifies the ability of the model to correctly rank events of interest up to several time-points and to discriminate them from competing events [21]. The inverse probability of censoring weighted estimator of the concordance index was used to take into account for right censoring. To assess calibration, the cumulative incidence estimated non-parametrically within quartiles of the predicted risk was plotted against the average predicted risk within the same quartiles.
The analysis described above was repeated adding each single-nucleotide polymorphism to the prognostic model and by comparing the estimated predictive ability with that obtained without taking into account the genetic data.
Results
Three hundred and four patients were included; baseline demographic and therapeutic characteristics are shown in Table 1. The most used PIs was atazanavir with low-dose ritonavir (58, 19.1%), darunavir/ritonavir (31, 10.2%), atazanavir (19, 6.3%), and lopinavir/ritonavir (19, 6.2%); among non-nucleoside reverse-transcriptase inhibitors (NNRTI) efavirenz was the most commonly used (128, 42.1%) followed by nevirapine (29, 9.5%). Thirteen subjects (4.3%) were receiving raltegravir without concomitant PIs or NNRTIs. Median tenofovir plasma and urinary C12 were 66 ng/mL (IQR: 49–93) and 25.3 μg/mL (IQR: 14.6–38.8). The prevalence of single-nucleotide polymorphisms is reported in Supplementary Table 1; all polymorphisms excluding SLC28A2 were in Hardy–Weinberg equilibrium (p > 0.05). RBP/cr was 63 μg/g (IQR: 1.5–185.8) with 98 patients (32.2%) showing abnormal age-adjusted levels. Abnormal proteins on spot-urine samples (>30 mg/dL) were observed in seven subjects (2.3%).
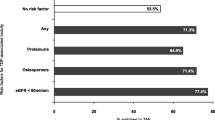
The median follow-up time was 28.3 months (23.3–33.6) accounting for 716 person-year of follow-up, 39 patients discontinued the drug, and 3 patients died. Reasons for discontinuation included persistent 24-h urine abnormalities (21, 53.8%), eCrCl decrease below 60 mL/min (6, 15.4%), osteopenia/osteoporosis at DEXA scan (5, 12.8%), other causes (3, 7.7%), or virological failure (4, 10.2%). Therefore 27 events of interest were observed (3.77 events per 100 patient-year).
Crude cumulative incidence curves of clinically relevant variables are shown in Fig. 1. There was no evidence of time-dependent effects for the variables considered, hence no interaction with time was included in the regression model. The selection procedure identified as prognostic factors are gender, age, route of infection, third drugs, chronic HCV infection, eCrCl, duration of TDF at entry, spot-urine proteinuria at entry, and TFV plasma concentrations. Continuous variables were modeled as linear predictors and no interaction was included in the model. The parameter estimates for the full and the selected models are shown in Table 2. The full model suggested that the probability of interrupting TDF for renal toxicity was higher among males, older patients, not Caucasians, PIs users, those not infected by drug injection, those with chronic HCV, those with previous exposure to indinavir, those with lower CD4 cell count, detectable plasma HIV-RNA, lower eCrCl, and those with higher proteinuria and TFV plasma concentration. The direction and the magnitude of the effects were confirmed in the selected model. C-index decreased from 0.93 at 1 year to 0.88 at 2 years and to 0.85 at 3 years after entry into the study in the study set while it ranged from 0.85 at 1 year to 0.77 at 3 years after entry in the internal cross-validation (Supplementary Figure 1). The calibration plot at 1 year indicates a clinically good calibration although high predicted risks will be slightly too high (Supplementary Figure 2). When comparing the predictive ability of the models with and without plasma concentration, C-index decreased to 0.90 at 1 year, to 0.86 at 2 years and 0.83 at 3 years after entry into the study. Estimated probabilities of interrupting therapy for renal toxicity at 3 years after entry into the study for four hypothetical patients with specific combinations of predictors and with focus on plasma concentration are shown in Fig. 2.
Estimated probabilities of interrupting therapy for renal toxicity with 95% confidence interval for: (i) a man not infected by drug injection, 45 years old, protease inhibitors user and treated with TDF for 5 years at the entry into the study, without chronic HCV infection, with eCrCl equal to 80 mL/min, with proteinuria lower than 30, and with plasma concentration equal to 60 (gray solid line, 95% CI dash line) and 80 (black solid line, 95% CI dash line); (ii) a man not infected by drug injection, 35 years old, no protease inhibitors user and treated with TDF for 5 years at the entry into the study, without chronic HCV infection, with eCrCl equal to 90 mL/min, with proteinuria lower than 30, and with plasma concentration equal to 60 (gray solid line, 95% CI dash line) and 80 (black solid line, 95% CI dash line)
Multivariable sub-hazard ratios of each single-nucleotide polymorphism estimated by Fine and Gray model are reported in Supplementary Table 2. The discrimination ability did not improve when adding the genetic information in the model.
Discussion
In a cohort of stable HIV-positive patients on TDF-based highly active antiretroviral therapy treatment interruptions due to renal toxicity were not uncommon in spite of such cohort being biased in favor to TDF tolerability, as the median time of TDF exposure at baseline exceeded 4 years. Among the different factors found to be associated to such event, the choice of the third drug was confirmed to have a significant effect on treatment discontinuations over time. As seen in previous studies, PIs were more likely to be associated to renal abnormalities and related treatment interruptions. It is worth noting that in the ARDENT study (three arms with DRV/r, ATV/r, and RAL, all with a common N/NtRTI backbone consisting of TDF/FTC) a significant role of the third drug was however seen in terms of BMD reduction, as RAL was found to possibly play a protective role as compared to PI/r. In spite of a relatively low number of patients taking RAL as a third drug, in the present study we found that no patients on RAL had to interrupt their treatment for renal function abnormalities. These results fit with the association we found here between TFV pharmacokinetic exposure and the risk of treatment interruption for renal function abnormalities. Alike in this study, in a prior cross-sectional survey on TDF-tolerant patients we found that TFV plasma concentration in RAL recipients was among the lowest recorded among patients taking TDF with a variety of third drugs [13]. In the perspective of reducing drug expenditure, this might have relevant clinical implications in the management of TDF-treated subjects with the ongoing introduction of TAF and the forthcoming availability of generic TDF [22].
Our cohort included HIV-positive stable patients with high CD4 cell count and with suppressed or low-level plasma HIV-RNA under treatment. Despite a very long exposure to TDF (almost 4.5 years) their renal function was normal with median estimated creatinine clearance of 90 mL/min (and above 60 mL/min in all). Proteinuria on spot urines was observed in seven subjects (2.3%) while tubular proteinuria in 32.2%. This prevalence is lower than the one we observed in a restricted sample and also in comparison to other cohorts that reported values as high as 62.7% [10, 23] This large sample may be representative of a cohort of HIV-positive patients in Western Europe with excellent immunovirological results and high rate of HCV-co-infection. However a significant survival bias may have occurred: patients with early tubular and glomerular toxicities were not included as they had probably prematurely discontinued TDF. This may also explain the lack of association with genetic variants reported in other studies: patients with risk genotype were probably dropped from clinical care before entering the study.
The model we developed and validated included several factors that have previously been described for TDF-attributed renal toxicity, including male gender, older age, PI use, and TFV plasma exposure. The observation that HIV acquisition through risks other than intravenous drug abuse was a predictive factor is novel and unexpected and might be at least partly explained by selection bias. It could be hypothesized that IVDU are at higher risk of renal impairment, with a reduced tolerance to TDF-containing regimens and therefore higher drop rates before study inclusion. While tubular proteinuria did not predict TDF discontinuation, proteinuria on spot urines was associated to such event in the final model. Urine analysis is already suggested in several guidelines as it may represent a simple and cheap screening test. The D:A:D CKD risk score, although associated with the outcome of interest, worsened the model predictive ability.
The inclusion of genetic variants in several transporters encoding genes did not increased the model prediction despite a significant effect of ABCB1 single-nucleotide polymorphisms at survival analysis. Previous studies highlighted several genetic associations with tubular dysfunction while only single-nucleotide polymorphisms in ABCB1 were deemed relevant in predicting TDF discontinuation in the Swiss HIV cohort [24]. Our final model did not include pharmacogenetic markers; this may be explained by different prevalence of allelic variants or by the inclusion of TFV plasma concentrations, indirectly associated with genetic variants in transporter encoding genes. Another possible explanation may be a significant survival bias: patients with unfavorable genetic variants could have stopped TDF before entering our study thus masking a potential association [25].
Tenofovir plasma exposure was, in our cohort, independently associated with TDF discontinuation even when accounting for PI co-administration; demographic and genetic factors have been associated with the drug’s exposure [26]. A longitudinal study in 105 HIV-positive women found that TFV plasma concentrations (measured as 24 h exposure) was associated with decline in estimated glomerular function [27]. Several data now confirm this association and suggest that a reduced dose may be suitable for concomitant boosted regimens or selected clinical features (low body weight, lower estimated creatinine clearance, etc.) [28]. However it should be highlighted that long-term follow-up and data on the benefits of switching TDF/INSTI-receiving patients to TAF/INSTI are still lacking. The use of therapeutic drug monitoring may be suggested for optimizing TDF dose or concomitant medications as recently proposed in an interesting editorial in AIDS; those subjects with TFV plasma concentration above 70 ng/mL had a significant higher risk of discontinuing for renal toxicity [29]. The need for an individualized tenofovir exposure is highlighted by the two available doses of TAF: 25 and 10 mg (when co-administered with ritonavir or cobicistat boosted antiretrovirals) [30].
Some limitations should be acknowledged such as the heterogeneous follow-up, the composite endpoint (although representing clinically relevant outcomes), the limited sample size (insufficient for identifying the effect or rarer phenotypes), and the absence of regimens including newer drugs (such as rilpivirine, dolutegravir, and elvitegravir). Nevertheless it should be highlighted that tenofovir exposure with concomitant elvitegravir/cobicistat and with rilpivirine was reported to be higher than with co-administered raltegravir [31]. Conversely some interesting issues peculiar to this study may be highlighted: the longitudinal design, the accurate statistical methods as well as the contemporary inclusion of pharmacokinetic and pharmacogenetic markers.
In conclusion TDF-tolerant and stable HIV-positive patients are at greater risk of discontinuing their regimens for renal events if male, older, having acquired HIV through risks other than intravenous drug use, presenting proteinuria, receiving PIs and showing higher TFV plasma concentrations. These data suggest that TDF may be safe in selected patients according to the choice of concomitant drugs and degree of tenofovir plasma exposure.
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [Internet] [cited 20 Apr 2017]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
Ustianowski A, Arends JE. Tenofovir: what we have learnt after 7.5 million person-years of use. Infect Dis Ther. 2015;4:145–57.
Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine (Baltimore). 2016;95:e5146.
World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations 2016 update [Internet]. 2016 [cited 2017 Apr 20]. http://apps.who.int/iris/bitstream/10665/246200/1/9789241511124-eng.pdf?ua=1
Monteagudo-Chu MO, Chang MH, Fung HB, Bräu N. Renal toxicity of long-term therapy with tenofovir in HIV-infected patients. J Pharm Pract. 2012;25:552–9.
Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS Lond Engl. 2012;26:867–75.
Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella F, et al. Brief report: cumulative tenofovir disoproxil fumarate exposure is associated with biomarkers of tubular injury and fibrosis in HIV-infected men. J Acquir Immune Defic Syndr. 1999;73:177–81.
Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013;24:1519–27.
Hall AM, Edwards SG, Lapsley M, Connolly JO, Chetty K, O’Farrell S, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. Am J Kidney Dis. 2009;54:1034–42.
Calcagno A, Cusato J, Marinaro L, Simiele M, Lucchiari M, Alcantarini C, et al. Tenofovir clearance is reduced in HIV-positive patients with subclinical tubular impairment. AIDS Lond Engl. 2016;30:915–20.
Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–7.
Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–8.
Calcagno A, Gonzalez de Requena D, Simiele M, D’Avolio A, Tettoni MC, Salassa B, et al. Tenofovir plasma concentrations according to companion drugs: a cross-sectional study of HIV-positive patients with normal renal function. Antimicrob Agents Chemother. 2013;57:1840–3.
Moss DM, Neary M, Owen A. The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol. 2014;5:248.
Pushpakom SP, Liptrott NJ, Rodríguez-Nóvoa S, Labarga P, Soriano V, Albalater M, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011;204:145–53.
Calcagno A, Cusato J, Marinaro L, Trentini L, Alcantarini C, Mussa M, et al. Clinical pharmacology of tenofovir clearance: a pharmacokinetic/pharmacogenetic study on plasma and urines. Pharmacogenomics J. 2015;16:514–518.
Mocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12:e1001809.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Kuk D, Varadhan R. Model selection in competing risks regression. Stat Med. 2013;32:3077–88.
Wolbers M, Blanche P, Koller MT, Witteman JCM, Gerds TA. Concordance for prognostic models with competing risks. Biostat Oxf Engl. 2014;15:526–39.
Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet Hiv. 2016;3:e23–32.
Ezinga M, Wetzels JFM, Bosch MEW, van der Ven AJAM, Burger DM. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther. 2014;19:765–71.
Lubomirov R, Colombo S, di Iulio J, Ledergerber B, Martinez R, Cavassini M, et al. Association of pharmacogenetic markers with premature discontinuation of first-line anti-HIV therapy: an observational cohort study. J Infect Dis. 2011;203:246–57.
Rungtivasuwan K, Avihingsanon A, Thammajaruk N, Mitruk S, Burger DM, Ruxrungtham K, et al. Influence of ABCC2 and ABCC4 polymorphisms on tenofovir plasma concentrations in Thai HIV-infected patients. Antimicrob Agents Chemother. 2015;59:3240–5.
Baxi SM, Greenblatt RM, Bacchetti P, Scherzer R, Minkoff H, Huang Y, et al. Common clinical conditions—age, low BMI, ritonavir use, mild renal impairment —affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS Lond Engl. 2014;28:59–66.
Baxi SM, Scherzer R, Greenblatt RM, Minkoff H, Sharma A, Cohen M, et al. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS Lond Engl. 2016;30:609–18.
Costarelli S, Cozzi-Lepri A, Lapadula G, Bonora S, Madeddu G, Maggiolo F, et al. Long-term durability of tenofovir-based antiretroviral therapy in relation to the co-Administration of other drug classes in routine clinical practice. PLoS ONE. 2016;11:e0160761.
Gagneux-Brunon A, Botelho-Nevers E, Frésard A, Lucht F. Preventing long-term tenofovir renal toxicity by pharmacokinetic assessment. AIDS Lond Engl. 2016;30:665–6.
Gervasoni C, Minisci D, Baldelli S, Mazzali C, Giacomelli A, Milazzo L, et al. Effect of cobicistat on tenofovir plasma concentration: a cross-sectional study. In: Abstract of the conference on retroviruses and opportunistic infections. Seattle, WA, USA, 2017.
Cattaneo D, Minisci D, Baldelli S, Mazzali C, Giacomelli A, Milazzo L, et al. Effect of cobicistat on tenofovir disoproxil fumarate (TDF): what is true for TAF may also be true for TDF. J Acquir Immune Defic Syndr. 1999;77:86–92.
Funding
The study was supported by internal funding. The previous study (the cross-sectional study) that originated this cohort was partially supported by a Gilead fellowship grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AC has received honoraria from Abbvie, BMS, Gilead, Janssen-Cilag, MSD, Viiv and he is currently receiving research grants from BMS, Gilead, and Viiv. GDP and SB have received honoraria from Abbvie, BMS, Gilead, Janssen-Cilag, MSD, and Viiv. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Calcagno, A., Fiumanò, M., Zugna, D. et al. Tenofovir disoproxil fumarate discontinuation for renal outcomes: any room for treatment personalization?. Pharmacogenomics J 19, 65–71 (2019). https://doi.org/10.1038/s41397-018-0064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-018-0064-y
- Springer Nature Limited
This article is cited by
-
Role of plasmatic and urinary concentration of tenofovir disoproxil fumarate in a cohort of patients affected by chronic hepatitis B
Archives of Virology (2022)
-
ABCC4 single-nucleotide polymorphisms as markers of tenofovir disoproxil fumarate-induced kidney impairment
The Pharmacogenomics Journal (2021)