Abstract
Purpose
Metformin has been suggested to reduce the risk of cancer. However, previous studies have been inconsistent regarding the relationship between metformin use and the risk of occurrence of prostate cancer (PCa). The purpose of this study was to assess the effect of metformin on clinical outcomes in patients with PCa in a meta-analysis and to explore the possible dose-response relationship.
Methods
A systematic literature search was conducted in 10 electronic databases and 4 registries. The combined relative risks (RRs) were calculated using a random-effects model with 95% confidence interval (CIs) to assess the effect of metformin on the risk of PCa. Relevant subgroup analyses and sensitivity analyses were performed.
Results
The across studies results show that metformin use associated with lower incidence of PCa (RR: 0.82, 95% CI: 0.74–0.91). Metformin use was also found to reduce PCa recurrence, but the results were not statistically significant (RR: 0.97, 95% CI: 0.81–1.15). Metformin use was not associated with PCa mortality (RR: 0.94, 95% CI: 0.81–1.09). The results of subgroup analyses indicated that the type of study was a cohort study and the population came from both Asia and Europe showed that taking metformin reduced the incidence of PCa. A linear correlation was found between the duration of metformin use and its protective effect.
Conclusions
This meta-analysis revealed an independent correlation between metformin use and reduced incidence of PCa. Metformin use was not associated with either PCa recurrence rate or mortality. Furthermore, the effect of metformin on PCa incidence was found to be related to duration.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the most frequently diagnosed cancer among men in over half of all countries worldwide and is the second leading cause of cancer deaths in men, after lung cancer [1,2,3,4,5]. PCa remains the third most prevalent cancer globally, with over 1.4 million new cases and 370,000 fatalities reported in 2020 [6]. On the other hand, apart from skin cancer, PCa is the most common cancer among men in the Western world [7]. Frequent urination, urinary weakness, urinary incontinence, blood in the urine, burning and persistent pain in the lower back, and abdominal pain are also clinical symptoms of PCa [8]. Various factors such as age, race, genetic factors, environmental factors, and family history play an important role in the progression of PCa [9, 10]. More than 670,000 PCa patients are diagnosed each year. Of these, 225,000 are in Europe and 240,000 in the United States [11]. The incidence of PCa varies between races. For example, 4–7 per 100,000 in Asian countries and 70–100 per 100,000 in European and North American countries [12, 13]. Metformin has multiple mechanisms for reducing cancer and carcinogenesis: direct action (on tumors and the microenvironment) and indirect action (on hosts that may affect tumors). Metformin is generally connected directly or indirectly through the AKT-Mtor pathway [14,15,16,17]. Mechanisms of pathway activation most commonly associated with PCa include deletion of inhibitory PTEN [18], PI3K mutations [19], or activation of growth factor receptors such as insulin [20,21,22]. The ability of metformin to reduce hyperinsulinemia may also indirectly reduce the risk of PCa [23,24,25]. In addition, laboratory evidence suggests that hyperinsulinemia regulates insulin receptors in PCa cells and promotes tumor growth [26, 27]. Reducing insulin levels in the blood stream or direct activation of AMP kinase. In this study, we used systematic evaluation and meta-analysis to investigate the relationship between metformin and PCa risk.
Methods
Study design
This study has been registered (registration number: CRD42023447013) with the PROSPERO database before July 22, 2023 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=447013). We used the Cochrane Handbook for Systematic Reviews of Interventions for the preparation and conduct of this meta-analysis [28]. We reported this meta-analysis with reference to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [29].
Search strategy
The literature search was completed before July 22, 2023 for relevant available articles from the following databases: (1) PubMed; (2) Ovid MEDLINE; (3) Scopus; (4) Embase; (5) Cochrane library; (6) Web of Science; (7) Sinomed (CBM); (8) China National Knowledge Infrastructure (CNKI); (9) Wanfang Data Knowledge Service Platform; (10) China Science and Technology Journal VIP Database. The registration search was completed by 22 July 2023 and the relevant data retrieved were from the following registration pools: (1) ClinicalTrials.gov; (2) International Clinical Trials Registry Platform (ICTRP); (3) The EU Clinical Trials Register; (4) Chinese Clinical Trial Registry. The relevant retrieval strategy was as following: (“Metformin” or “ Dimethylbiguanidine” or “ Dimethylguanylguanidine” or “ Glucophage”) and (“Prostatic Neoplasms” or “ Prostate Neoplasms” or “ Prostate Cancer” or “Cancer of Prostate” or “ Prostatic Cancers”). Relevant Chinese technical terms for the Chinese databases were used to search for published articles. Furthermore, references of all relevant articles and reviews were retrieved to search for additional eligible studies.
Inclusion and exclusion criteria
Inclusion criteria
This meta-analysis included studies based on the following criteria: (1) participants with no PCa history were selected for the incidence analysis, while those with a PCa history were chosen for recurrence and mortality analyses; (2) metformin was the exposure factor; (3) studies provided relative risks (RRs), odds ratios (ORs), or hazard ratios (HRs) along with 95% confidence intervals (CIs) or data enabling their calculation; (4) in cases of multiple publications from the same population, the study with the larger sample size or more comprehensive data was chosen; and (5) studies were assessed for quality using the Newcastle Ottawa scale (NOS), requiring a score of at least 6 stars.
Exclusion criteria
(1) Antidiabetic drugs which did not include metformin; (2) comments or letters to the editor, case reports, and abstract-only publications; (3) Preprint servers, such as medRxiv/bioRxiv, etc.
Data extraction
After removing duplicates, two reviewers (Y Liu and Q Zhang) independently screened all abstracts and titles to exclude irrelevant articles. Full texts of potentially relevant studies were then downloaded and reviewed, with those meeting the selection criteria included in this systematic review. Two independent investigators (Y Liu and Q Zhang) extracted data from the included articles. The extracted data comprised the first author’s name, year of publication, study location, study methods, sample size, metformin usage, primary outcomes, raw data of patient numbers in the trial (metformin) and control groups, and adjusted RRs/ORs/HRs with corresponding 95% CIs.
Quality assessment
Two investigators (Y Liu and Q Zhang) independently evaluated the methodological quality of the included case-control and cohort studies using the nine-star NOS [30]. The assessment considered eight items across four categories: selection of cohort studies, comparability, outcomes, or exposure for case-control studies. Studies were rated as low-, moderate-, or high-quality based on their NOS scores (0–3, 4–6, 7–9, respectively). The certainty of evidence was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [31].
Statistical analysis
Statistical analyses were conducted using Stata (version 16.0; Stata Corp, College Station, TX) and RevMan (version 5.3; Cochrane Library) software. The pooled RR with 95% CI was calculated from the extracted raw data to assess the association between metformin use and PCa risk. When multiple RRs were available, the effect value controlling for the most confounding factors was chosen. Subgroup analyses were performed to explore the relationship between metformin use and PCa incidence and mortality, considering study region, study design, dosage type, and diagnosis type. HRs were directly considered as RRs [32, 33], and ORs were converted to RRs using the formula: RR = OR/((1–P0) + (P0 × OR)), where P0 represents the incidence of the outcome in the non-exposed group [34]. The standard error (SE) of the converted RR was calculated using SElog(RR) = SElog(OR) × log(RR)/log(OR). This formula was also applied to determine the upper and lower confidence limits of the CI based on the adjusted odds ratio [35]. To generalize our study results beyond the included studies, a random-effects model was used as it is the most suitable for meta-analysis [36]. Studies reporting data on PC incidence in terms of person-years, number of cases, and metformin dose or duration were included in the dose-response analysis. Restricted cubic splines with three knots at the 10%, 50%, and 90% percentiles of the distribution were used for both linear and non-linear dose-response analyses [37]. The study-specific estimates were then combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis. Sensitivity analysis was conducted to determine if any single study significantly influenced the results [38]. Publication bias was evaluated qualitatively with funnel plots and quantitatively using Begg’s and Egger’s tests [39]. A P value of less than 0.05 was considered statistically significant in all analyses.
Results
Study selection and characteristics
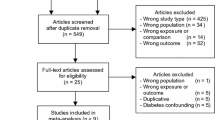
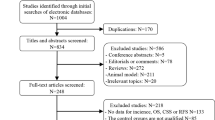
A search of databases and registries identified 3950 database records and 82 registry records, and 415 potentially eligible studies were selected after removing duplicate information and screening titles and abstracts. Of the 415 potentially eligible studies, a total of 41 studies met the inclusion criteria, including 34 studies of PCa incidence [15, 40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], 5 studies of PCa recurrence [73,74,75,76,77], and 5 studies of PCa mortality [52, 55, 75, 78, 79]. Google Scholar and Baidu Scholar were also searched, with 682 records being identified as potentially relevant to this study. However, these records were excluded as they were duplicates to the studies in the databases and the registries. The detailed process of literature screening is shown in Fig. 1.
Finally, forty-one studies with a total of 3,933,414 subjects were included in this meta-analysis. The characteristics of the included studies are shown in Tables 1 and 2.
Overall meta-analysis of metformin use on PCa risk
The results showed that metformin use was associated with a lower incidence of PCa (RR: 0.82, 95% CI: 0.74–0.91, P < 0.001, I2 = 97%, Fig. 2), and the random effects model was adopted. Meanwhile, metformin use was found to be associated with reduced recurrence (RR: 0.97, 95% CI: 0.81–1.15, P = 0.71, I2 = 0%, Fig. 3) and mortality (RR: 0.94, 95% CI: 0.81–1.09, P = 0.42, I2 = 75%, Fig. 4) in PCa with a random effects model, but the results were not statistically significant.
Subgroup analysis of metformin use on PCa risk
The results of subgroup analyses based on the study design showed that metformin administration was associated with a reduced incidence of PCa in the cohort study subgroup (RR: 0.81, 95% CI: 0.73–0.90, P < 0.001, I2 = 97%), and that metformin administration did not increase the risk of recurrence (RR: 0.93, 95% CI: 0.81–1.18, P = 0.77, I2 = 0%) and mortality (RR: 0.94, 95% CI: 0.81–1.09, P = 0.42, I2 = 75%,) from PCa. Meanwhile, the results of the case-control study suggested that metformin was not associated with either PCa incidence (RR: 0.90, 95% CI: 0.78–0.91, P = 0.16, I2 = 81%).
Subgroup analysis by study area showed that metformin was found to be associated with a reduced incidence of PCa in Asia (RR: 0.67, 95% CI: 0.56–0.79, P < 0.001, I2 = 96%) and Europe (RR: 0.89, 95% CI: 0.81–0.97, P = 0.01, I2 = 83%). In the North American study, metformin use was also found to reduce the risk of PCa, but the difference was not statistically significant (RR: 0.83, 95% CI: 0.66–1.05, P = 0.11, I2 = 97%). Meanwhile, in the North American study, metformin use was found to reduce the risk of mortality from PCa, but the difference was not statistically significant (RR: 0.91, 95% CI: 0.77–1.07, P = 0.24, I2 = 78%).
The results of all subgroup analyses are shown in Table 3.
Dose-response meta-analysis
Three studies with a total of 515,615 participants were included in a dose-response analysis of incidence [49, 66, 69]. Among these studies, metformin exposure was expressed as duration of exposure.
Linear dose-response models showed a significant negative association between duration of metformin exposure and risk of PCa (exb(b): 0.980, P < 0.001). Furthermore, nonlinear dose-response analysis showed a similar association (Coef1 = –0.299, P1 < 0.001, Coef2 = 0.325, P2 < 0.001). Each 1-year increment in metformin exposure was associated with a 22% reduction in the risk of PCa (RR: 0.78, 95% CI: 0.77–0.79, p < 0.001, Fig. 5).
Study quality assessment and risk of bias
All included observational studies were considered to be above moderate quality studies, as depicted by NOS ≥ 6. The NOS-based assessment indicated a low to moderate risk of bias, while the GRADE assessment revealed low certainty in the evidence supporting metformin’s ability to reduce PCa incidence, recurrence and mortality. This is mainly due to the retrospective nature of the studies and potential selection and publication biases (See Supplementary Information: Fig. S1). In addition, Begg’s test and Egger’s test found publication bias in some studies. In this study, for PCa incidence, P value of Begg’s test was 0.047, and P value of Egger’s test was 0.004. For PCa recurrence, P value of Begg’s test was 0.806, and P value of Egger’s test was 0.182. For PCa mortality, P value of Begg’s test was 0.825, and P value of Egger’s test was 0.573. Some of the funnel plots were shown to be unsymmetrical (Fig. S2).
Sensitivity analysis
For incidence, recurrence, and mortality, conclusions were unchanged after excluding individual papers and calculating heterogeneity and effect sizes (Fig. S3).
Discussion
In this study, we synthesized evidence from cohort and case-control studies involving 3,933,414 participants in 15 countries and regions. Our findings suggest a potential chemoprotective effect of metformin on PCa incidence, recurrence, and mortality. We reported the effect of metformin use on PCa risk in different types of studies and regions. In addition, our study explored a possible dose-response relationship between metformin use and PCa incidence based on exposure time. Finally, the main findings of this study support the mechanistic hypothesis that metformin use is negatively associated with the risk of PCa incidence, recurrence, and mortality.
The relationship between metformin use and cancer has been widely debated. Several studies have explored metformin’s potential chemopreventive effects on various tumors, including breast [80], brain [81], and melanoma [82]. This meta-analysis, with a larger participant pool than previous ones [83, 84], further substantiates metformin’s protective effect against PCa.
Several studies suggest that metformin’s antitumor effects may involve multiple mechanisms. It has been reported as an indirect activator of AMP-activated protein kinase (AMPK), inhibiting the growth of PCa cells [85] and being selectively toxic to p53-deficient tumor cells [86]. However, metformin also inhibits the proliferation of most breast cancer cells, regardless of p53 status [87]. Ben Sahra et al. [16] showed that metformin decreases the level of cell cycle protein D1, exerting antitumor effects both in vivo and ex vivo. Huang et al. [18] demonstrated that metformin inhibition might trigger a signaling pathway that effectively inhibits cellular growth.
Existing studies have demonstrated that insulin and insulin-like growth factors are crucial in regulating cellular energy and growth. These hormones and their associated signaling networks significantly contribute to tumor formation [88]. Epidemiological studies have found that insulin-like growth factor-1 (IGF-1) promotes the proliferation of various cancer cells, including breast and prostate cancers. Additionally, it has been demonstrated that insulin can regulate the activity of the IGF-1 receptor [89]. IGF-1 is present in normal cells, but in cells with malignant growth characteristics, it exerts strong mitogenic and anti-apoptotic effects. Thus, the level of IGF-1 in the human body influences tumorigenesis. Tumor cells can produce IGF-1 through autocrine or paracrine secretion, promoting their differentiation and proliferation. When IGF-1 binds to its receptor, it initiates the mitogen-activated protein kinase 2 signaling pathway and the phosphatidylinositol 3-kinase/Akt signaling pathway, which, when activated, promotes cell proliferation and inhibits apoptosis in tumor cells [90]. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase of the phosphatidylinositol 3-kinase-related enzyme family, regulated by intracellular and extracellular signals, including nutrients like glucose and amino acids, as well as growth factors such as insulin and insulin-like growth factors. These factors regulate cell growth. When metformin is used, it reduces glucose and insulin levels in the body, thereby affecting mTOR activity and inhibiting cell growth.
Additionally, confounding mechanisms might explain the link between metformin use and reduced PCa incidence. Type 2 diabetes is a known risk factor for PCa [91]. Metformin users are often obese and have type 2 diabetes, both conditions associated with a lower risk of PCa [92, 93]. Metformin, a common antidiabetic drug, helps control hyperglycemia in type 2 diabetes patients by affecting mitochondrial respiration, leading to energy deficiency and molecular changes [94, 95]. One proposed mechanism for metformin’s antitumor effects is the inhibition of mitochondrial respiratory complex I. This inhibition reduces ATP production, activating AMPK in an LKB1-dependent manner, which then inhibits mTOR, leading to anticancer effects [95]. Additionally, new diabetes treatments, such as GLP-1 inhibitors, have been found to inhibit PCa growth, reducing the risk of PCa [96]. We hypothesize that treating diabetes can further lower the incidence of PCa.
In addition to the mechanisms described above, this may be due to lower testosterone levels in diabetic men than in non-diabetic men [97].
Based on the potential antitumor mechanism of metformin, numerous reports have explored its relationship with PCa risk, but the quality and findings of these studies vary. This article reviews and analyzes 41 studies, encompassing 3,933,414 participants, and finds that metformin use reduces the risk of PCa recurrence and death. However, the results show no significant difference and exhibit high heterogeneity. This variability may stem from differences in study design, such as drug use in control groups, drug combinations, sequential drug use, dosages, follow-up periods, control of confounding factors, duration of drug use, and variations in study populations’ age, occupation, ethnicity, and geographic area. In the subgroup analyses of this study, metformin administration was found to reduce the risk of PCa in Asian and European populations. However, no significant correlation was observed between metformin use and PCa incidence, recurrence, and mortality in North American populations. This may be due to the limited number of studies conducted in North America and the focus of current research on Asia and Europe. Additionally, significant genetic differences and susceptibility loci for PCa between Asian, European, and American populations may influence metformin’s effectiveness in preventing PCa [98,99,100].
Although this meta-analysis showed a potential benefit of metformin for PCa treatment and a better risk-benefit ratio, this study has several limitations. First, there are limitations in that the inclusion of so many retrospective studies does not lead to a reasonable and unbiased conclusion and is prone to bias. Second, in studies examining the association between metformin and PCa recurrence and mortality, there was a trend toward lower risk, but it did not reach statistical significance. Further randomized controlled trials and real-world studies are needed to explore potential dose-response relationships. Third, subgroup analyses of PCa types were not performed in this study; therefore, it was not possible to examine the effect of metformin on different types of PCa. Finally, although some confounders were corrected for in the analysis, there is no guarantee that all potential confounders were considered. Other unreported and unanalyzed confounders may have been present in the original study. However, future randomized, double-blind controlled trials with adequate sample sizes and validated study protocols are still needed to assess and confirm the potential benefits of metformin for PCa prevention and to determine the optimal dose of metformin with a favorable risk-benefit ratio.
Conclusions
This meta-analysis showed that metformin use was independently associated with a reduction in PCa incidence. A duration-dependent relationship was found between metformin and PCa incidence, suggesting that prolonged metformin use is associated with a lower risk of developing PCa. Meanwhile, this study may provide guidance to clinicians to improve the prognosis of PCa patients. In the future, larger prospective cohort studies or even randomized controlled as well as longer follow-up trials are needed to confirm the relationship between metformin use and PCa.
Data availability
The data utilized in this study was sourced exclusively from published RWS, all of which are comprehensively presented within this article (including its supplementary information files).
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–48. https://doi.org/10.1001/jamaoncol.2016.5688
Simon H (ed). The Harvard Medical School Guide to Men’s Health: Lessons from the Harvard Men’s Health Studies. New York: The Free Press and colophon are registered trademarks of Simon & Schuster, Inc; 2004.
Pourmand G, Salem S, Mehrsai A, Lotfi M, Amirzargar MA, Mazdak H, et al. The risk factors of prostate cancer: a multicentric case-control study in Iran. Asian Pac J Cancer Prev. 2007;8:422–8.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660
Pinsky PF, Prorok PC, Yu K, Kramer BS, Black A, Gohagan JK, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;123:592–9. https://doi.org/10.1002/cncr.30474
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. https://doi.org/10.3322/caac.21208
Nebert DW, McKinnon RA, Puga A. Human drug-metabolizing enzyme polymorphisms: effects on risk of toxicity and cancer. DNA Cell Biol. 1996;15:273–80. https://doi.org/10.1089/dna.1996.15.273
Hsing AW, Sakoda LC, Chua S Jr. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–57. https://doi.org/10.1093/ajcn/86.3.843S. Sep
Jackson MD, Walker SP, Simpson-Smith CM, Lindsay CM, Smith G, McFarlane-Anderson N, et al. Associations of whole-blood fatty acids and dietary intakes with prostate cancer in Jamaica. Cancer Causes Control. 2012;23:23–33.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. https://doi.org/10.3322/canjclin.55.2.74
Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–413. https://doi.org/10.2741/1891
Mallick S, Blanchet P, Multigner L. Prostate cancer incidence in guadeloupe, a French Caribbean archipelago. Eur Urol. 2005;47:769–72. https://doi.org/10.1016/j.eururo.2005.02.020
Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res. 2010;3:1060–5. https://doi.org/10.1158/1940-6207.CAPR-10-0175
Margel D, Urbach D, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst. 2013;105:1123–31. https://doi.org/10.1093/jnci/djt170
Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–86. https://doi.org/10.1038/sj.onc.1211024
Prabhakaran S, Thirumal D, Gimbun J, Ranganathan B. Metformin-A panacea pharmaceutical agent through convergence revolution initiative. J Nat Rem. 2018;17:69–79. https://doi.org/10.18311/jnr/2017/17938
Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–21. https://doi.org/10.1042/BJ20080557
Niehr F, von Euw E, Attar N, Guo D, Matsunaga D, Sazegar H, et al. Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations. J Transl Med. 2011;9:76 https://doi.org/10.1186/1479-5876-9-76
Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–11. https://doi.org/10.1158/1078-0432.CCR-09-2229
Hasanvand A. Antioxidative and anti-inflammatory effects of metformin; a new look to an old drug. J Ren Endocrinol. 2018;4:e02.22.
Unterberger CJ, Maklakova VI, Lazar M, Arneson PD, Mcilwain SJ, Tsourkas PK, et al. GH Action in Prostate Cancer Cells Promotes Proliferation, Limits Apoptosis, and Regulates Cancer-related Gene Expression. Endocrinology. 2022;163:bqac031. https://doi.org/10.1210/endocr/bqac031
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–19. https://doi.org/10.1016/j.diabres.2018.05.023
Clements A, Gao B, Yeap SHO, Wong MKY, Ali SS, Gurney H. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011;22:2556–60. https://doi.org/10.1093/annonc/mdr037
Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17–29. https://doi.org/10.1146/annurev-med-062613-093128
Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The effect of nutrition therapy and exercise on cancer-related fatigue and quality of life in men with prostate cancer: a systematic review. Nutrients. 2017;9:1003 https://doi.org/10.3390/nu9091003
Venkateswaran V, Haddad AQ, Fleshner NE, Fan R, Sugar LM, Nam R, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–800. https://doi.org/10.1093/jnci/djm231.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2020;339:b2535 https://doi.org/10.1136/bmj.b2535
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 https://doi.org/10.1136/bmj.d5928.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. https://doi.org/10.1007/s10654-010-9491-z
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Stare J, Maucort-Boulch D. Odds ratio, hazard ratio and relative risk. Metodol Zv. 1998;13:59–67.
BMJ Best Practice (n.d.) A glossary of EBM terms. https://bestpractice.bmj.com/info/us/toolkit/ebm-tools/a-glossary-of-ebm-terms/. Accessed 22 July 2023.
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671 https://doi.org/10.1136/bmj.d671.
Zhang JYK. What’ s the Relative Risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–91. https://doi.org/10.1001/jama.280.19.1690
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Health. 2015;13:196–207. https://doi.org/10.1097/XEB.0000000000000065
Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202. https://doi.org/10.1093/jnci/80.15.1198
Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14:29–37.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629
Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomark Prev. 2011;20:337–44. https://doi.org/10.1158/1055-9965.EPI-10-0940
But A, Wang H, Männistö S, Pukkala E, Haukka J. Assessing the effect of treatment duration on the association between anti-diabetic medication and cancer risk. PLoS ONE. 2014;9:e113162 https://doi.org/10.1371/journal.pone.0113162
Chen CB, Eurich DT, Majumdar SR, Johnson JA. Metformin and the risk of prostate cancer across racial/ethnic groups: a population-based cohort study. Prostate Cancer Prostatic Dis. 2017;20:122–6. https://doi.org/10.1038/pcan.2016.65
Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. https://doi.org/10.1007/s00125-009-1440-6
Ferrara A, Lewis JD, Quesenberry CP Jr, Peng T, Strom BL, Van Den Eeden SK, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–9. https://doi.org/10.2337/dc10-1067
Freedman LS, Agay N, Farmer R, Murad H, Olmer L, Dankner R. Metformin treatment among men with diabetes and the risk of prostate cancer: a population-based historical cohort study. Am J Epidemiol. 2022;191:626–35. https://doi.org/10.1093/aje/kwab287
Geraldine N, Marc A, Carla T, Chantal M, Stefaan B, Welcome W, et al. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res Clin Pr. 2012;97:331–6. https://doi.org/10.1016/j.diabres.2012.02.002
Goldberg H, Mohsin FK, Berlin A, Chandrasekar T, Wallis CJD, Klaassen Z, et al. The suggested chemopreventive association of metformin with prostate cancer in diabetic patients. Urol Oncol. 2021;39:191.e17–191.e24. https://doi.org/10.1016/j.urolonc.2020.08.032
Häggström C, Van Hemelrijck M, Zethelius B, Robinson D, Grundmark B, Holmberg L, et al. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int J Cancer. 2017;140:611–7. https://doi.org/10.1002/ijc.30480
Haring A, Murtola TJ, Talala K, Taari K, Tammela TL, Auvinen A. Antidiabetic drug use and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Scand J Urol. 2017;51:5–12. https://doi.org/10.1080/21681805.2016.1271353
Jo JK, Song HK, Heo Y, Kim MJ, Kim YJ. Risk analysis of metformin use in prostate cancer: a national population-based study. Aging Male. 2023;26:2156497 https://doi.org/10.1080/13685538.2022.2156497
Kincius M, Patasius A, Linkeviciute-Ulinskiene D, Zabuliene L, Smailyte G. Reduced risk of prostate cancer in a cohort of Lithuanian diabetes mellitus patients. Aging Male. 2020;23:1333–8. https://doi.org/10.1080/13685538.2020.1766013
Koo HY, Jeong SM, Cho MH, Chun S, Shin DW, Park J. Population-wide impacts of aspirin, statins, and metformin use on prostate cancer incidence and mortality. Sci Rep. 2021;11:16171 https://doi.org/10.1038/s41598-021-95764-3
Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf. 2015;24:865–74. https://doi.org/10.1002/pds.3823
Kuo YJ, Sung FC, Hsieh PF, Chang HP, Wu KL, Wu HC. Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: a nationwide population-based cohort study. Cancer Med. 2019;8:2514–23. https://doi.org/10.1002/cam4.2025
Lopez DS, Malagaris I, Polychronopoulou E, Tsilidis KK, Milani SA, Kristen Peek M, et al. Metformin and testosterone replacement therapy inversely associated with hormone-associated cancers (prostate, colorectal and male breast cancers) among older White and Black men. Clin Endocrinol. 2022;97:792–803. https://doi.org/10.1111/cen.14803
Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care. 2012;35:1002–7. https://doi.org/10.2337/dc11-1829
Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. 2011;34:1965–71. https://doi.org/10.2337/dc11-0699
Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168:925–31. https://doi.org/10.1093/aje/kwn190
Nair-Shalliker V, Bang A, Egger S, Yu XQ, Chiam K, Steinberg J, et al. Family history, obesity, urological factors and diabetic medications and their associations with risk of prostate cancer diagnosis in a large prospective study. Br J Cancer. 2022;127:735–46. https://doi.org/10.1038/s41416-022-01827-1
Nordström T, Clements M, Karlsson R, Adolfsson J, Grönberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer. 2015;51:725–33. https://doi.org/10.1016/j.ejca.2015.02.003
Onitilo AA, Stankowski RV, Berg RL, Engel JM, Glurich I, Williams GM, et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur J Cancer Prev. 2014;23:134–40. https://doi.org/10.1097/CEJ.0b013e3283656394
Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, et al. Metformin use and prostate cancer risk. Eur Urol. 2014;66:1012–20. https://doi.org/10.1016/j.eururo.2014.04.027
Qiu H, Rhoads GG, Berlin JA, Marcella SW, Demissie K. Initial metformin or sulphonylurea exposure and cancer occurrence among patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:349–57. https://doi.org/10.1111/dom.12036
Raval AD, Mattes MD, Madhavan S, Pan X, Wei W, Sambamoorthi U. Association between metformin use and cancer stage at diagnosis among elderly medicare beneficiaries with preexisting type 2 diabetes mellitus and incident prostate cancer. J Diabetes Res. 2016;2016:2656814 https://doi.org/10.1155/2016/2656814
Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–24. https://doi.org/10.2337/dc11-0857
Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care. 2011;34:616–21. https://doi.org/10.2337/dc10-1640
Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer. 2014;50:2831–7. https://doi.org/10.1016/j.ejca.2014.08.007
Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37:2522–32. https://doi.org/10.2337/dc14-0584
van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. 2012;55:654–65. https://doi.org/10.1007/s00125-011-2390-3
Vicentini M, Ballotari P, Giorgi Rossi P, Venturelli F, Sacchettini C, Greci M, et al. Effect of different glucose-lowering therapies on cancer incidence in type 2 diabetes: an observational population-based study. Diabetes Res Clin Pr. 2018;143:398–408. https://doi.org/10.1016/j.diabres.2018.04.036
Wang CP, Lehman DM, Lam YF, Kuhn JG, Mahalingam D, Weitman S, et al. Metformin for reducing racial/ethnic difference in prostate cancer incidence for men with type II diabetes. Cancer Prev Res. 2016;9:779–87. https://doi.org/10.1158/1940-6207.CAPR-15-0425
Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–22. https://doi.org/10.1007/s10552-009-9407-y.
Allott EH, Abern MR, Gerber L, Keto CJ, Aronson WJ, Terris MK, et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2013;16:391–7. https://doi.org/10.1038/pcan.2013.48
Aminsharifi A, Howard LE, Amling CL, Aronson WJ, Cooperberg MR, Kane CJ, et al. Statins are associated with increased biochemical recurrence after radical prostatectomy in diabetic men but no association was seen in men also taking metformin: results from the SEARCH database. Clin Genitourin Cancer. 2019;17:e140–e149. https://doi.org/10.1016/j.clgc.2018.09.020
Kaushik D, Karnes RJ, Eisenberg MS, Rangel LJ, Carlson RE, Bergstralh EJ. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urol Oncol. 2014;32:43.e1–7. https://doi.org/10.1016/j.urolonc.2013.05.005
Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76:1240–4. https://doi.org/10.1016/j.urology.2010.03.059
Rieken M, Kluth LA, Xylinas E, Fajkovic H, Becker A, Karakiewicz PI, et al. Association of diabetes mellitus and metformin use with biochemical recurrence in patients treated with radical prostatectomy for prostate cancer. World J Urol. 2014;32:999–1005. https://doi.org/10.1007/s00345-013-1171-7
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. https://doi.org/10.2337/dc11-1313. Feb
Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31:3069–75. https://doi.org/10.1200/JCO.2012.46.7043
Bashraheel SS, Kheraldine H, Khalaf S, Moustafa AA. Metformin and HER2-positive breast cancer: mechanisms and therapeutic implications. Biomed Pharmacother. 2023;162:114676. https://doi.org/10.1016/j.biopha.2023.114676
Takhwifa F, Aninditha T, Setiawan H, Sauriasari R. The potential of metformin as an antineoplastic in brain tumors: a systematic review. Heliyon. 2021;7:e06558 https://doi.org/10.1016/j.heliyon.2021.e06558
Cerezo M, Tomic T, Ballotti R, Rocchi S. Is it time to test biguanide metformin in the treatment of melanoma? Pigment Cell Melanoma Res. 2015;28:8–20. https://doi.org/10.1111/pcmr.12267
Deng D, Yang Y, Tang X, Skrip L, Qiu J, Wang Y, et al. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: evidence from a meta-analysis. Diabetes Metab Res Rev. 2015;31:595–602. https://doi.org/10.1002/dmrr.2645
He K, Hu H, Ye S, Wang H, Cui R, Yi L. The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Sci Rep. 2019;9:2218 https://doi.org/10.1038/s41598-018-38285-w
Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res. 2008;1:369–75. https://doi.org/10.1158/1940-6207.CAPR-08-0081
Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. https://doi.org/10.1158/0008-5472.CAN-06-4447
Zhuang Y, Miskimins WK. Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. https://doi.org/10.1186/1750-2187-3-18
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. https://doi.org/10.1038/nrc2536.
Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer. 2009;16:429–41. https://doi.org/10.1677/ERC-08-0240
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. https://doi.org/10.1038/nrc1387
Liu X, Hemminki K, Försti A, Sundquist K, Sundquist J, Ji J. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int J Cancer. 2015;137:903–10. https://doi.org/10.1002/ijc.29440
Hu MB, Liu SH, Jiang HW, Bai PD, Ding Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: a dose-response meta-analysis of 29,464 patients. PLoS ONE. 2014;9:e106677 https://doi.org/10.1371/journal.pone.0106677
Zhang F, Yang Y, Skrip L, Hu D, Wang Y, Wong C, et al. Diabetes mellitus and risk of prostate cancer: an updated meta-analysis based on 12 case-control and 25 cohort studies. Acta Diabetol. 2012;49:S235–46. https://doi.org/10.1007/s00592-012-0439-5
Schäfer G. Site-specific uncoupling and inhibition of oxidative phosphorylation by biguanides. II. Biochim Biophys Acta. 1969;172:334–7. https://doi.org/10.1016/0005-2728(69)90077-2
Amengual-Cladera E, Morla-Barcelo PM, Morán-Costoya A, Sastre-Serra J, Pons DG, Valle A, et al. Metformin: from diabetes to cancer-unveiling molecular mechanisms and therapeutic strategies. Biology. 2024;13:302 https://doi.org/10.3390/biology13050302
Li XN, Bu HM, Ma XH, Lu S, Zhao S, Cui YL, et al. Glucagon-like peptide-1 analogues inhibit proliferation and increase apoptosis of human prostate cancer cells in vitro. Exp Clin Endocrinol Diabetes. 2017;125:91–97. https://doi.org/10.1055/s-0042-112368
Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34:528–40. https://doi.org/10.1111/j.1365-2605.2010.01117.x
Jang A, Lanka SM, Huang M, Casado CV, Caputo SA, Sweeney PL, et al. Comparison of circulating tumor DNA between African American and Caucasian patients with metastatic castrate-resistant prostate cancer post-abiraterone and/or enzalutamide. Prostate. 2023;83:1028–34. https://doi.org/10.1002/pros.24544
Shibata A, Whittemore AS. Genetic predisposition to prostate cancer: possible explanations for ethnic differences in risk. Prostate 1997;32:65–72.
Li J, Mercer E, Gou X, Lu YJ. Ethnical disparities of prostate cancer predisposition: genetic polymorphisms in androgen-related genes. Am J Cancer Res. 2013;3:127–51.
Acknowledgements
We appreciate support from the Nanchang University Queen Mary School and Nanchang University.
Author information
Authors and Affiliations
Contributions
Yuchen Liu and Xuan Huang designed research; Yuchen Liu, Qingfang Zhang, and Xuan Huang conducted literature search; Yuchen Liu participated and assisted in literature search and data collection; Yuchen Liu and Qingfang Zhang analyzed and interpreted data; and Yuchen Liu wrote the paper. Xuan Huang and Qingfang Zhang provided critical opinion. Yuchen Liu, Qingfang Zhang, and Xuan Huang revised the paper. Yuchen Liu had primary responsibility for final content. Xuan Huang is the corresponding authors. All authors read and approved the final manuscript. Guarantor of the article: Xuan Huang. The authors are responsible for the reported research, and have participated in the concept and design, analysis and interpretation of data, drafting or revising of the manuscript, and have approved the manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. We did not use individual data but published data. These data have been widely utilized in research and are generally available. Therefore, we confirm that any aspect of the work covered in this manuscript has been conducted with ethical approval. And this study has been registered (registration number: CRD42023447013) with the PROSPERO (International Prospective Register of Systematic Reviews) and was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement.
Consent for publication
All individuals gave written informed consent for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhang, Q. & Huang, X. Effect of metformin on incidence, recurrence, and mortality in prostate cancer patients: integrating evidence from real-world studies. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00871-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00871-7
- Springer Nature Limited