Abstract
Background
This study supports a value-based approach to prostate cancer (PCa) treatment by systematically reviewing economic evaluations that compare the cost and cost-effectiveness of low-dose-rate brachytherapy (LDR-BT) with that of other treatment options for localised and locally advanced PCa.
Methods
Studies published between 2008 and 2023 were searched for in MEDLINE, EMBASE and Tufts Medical Center’s Cost-Effectiveness Analysis (CEA) Registry (Prospero protocol CRD42023-442027). Two reviewers independently screened the title and abstracts based on agreed inclusion and exclusion criteria, followed by full-text screening. The Drummond checklist was used to critically appraise the quality of the included studies.
Results
After screening 453 records, 36 were sought for retrieval and 14 eligible studies included. Of them, 11 compared treatments for low- and/or favourable intermediate-risk PCa, 2 compared options for unfavourable intermediate- and/or high-risk disease and 1 analysed treatments for both risk groups. Considerable heterogeneity was seen in the populations, perspectives, time horizons, costs and outcomes data used. If the oncological outcomes of standard treatment approaches are considered equivalent, LDR-BT was the most cost-effective type of radiation therapy (RT) in 9 (75%) of 12 studies, was more cost-effective than radical prostatectomy (RP) in 6 (67%) of 9 studies and, depending on the time horizon, was more cost-effective than active surveillance (AS) in 3 (60%) of 5 studies. LDR-BT was more cost-effective than high-dose-rate brachytherapy (HDR-BT) in all 4 (100%) of the studies that made this comparison and, overall, LDR-BT was the least costly of all active treatment options in 7 (50%) of the 14 studies.
Conclusion
The available health economic evidence suggests that LDR-BT has significant cost advantages and an important role to play in the delivery of value-based PCa care. In the future these advantages could be challenged if radiotherapy favours ultrahypofractionated strategies such as stereotactic body radiation therapy (SBRT) and reduced fractionation in HDR-BT.
Similar content being viewed by others
Background
Prostate cancer (PCa) is the most diagnosed male cancer in over half (112 of 185) the countries of the world and the leading cause of cancer death in 48 of them [1]. The widespread introduction of prostate-specific antigen testing and the emergence of a plethora of treatment options has facilitated early detection, aggressive treatment and improved survival for men with this type of cancer [2] but has simultaneously placed a significant economic burden on health systems and payers [3].
The major clinical practice guidelines for the treatment of localised PCa lack consensus on which treatment option is most effective for early locoregional disease [4,5,6,7]. The oncological outcomes of the three standard approaches—watchful waiting (WW)/active surveillance (AS), radical prostatectomy (RP) and radiation therapy (RT)—have been studied extensively and found to be equivalent in terms of PCa-specific mortality [8] though each approach has its own distinct pattern of health-related quality-of-life (HRQoL) outcomes that patients should be fully aware of when choosing treatment [9].
One of the established radiotherapy approaches is low-dose-rate brachytherapy (LDR-BT), a type of internal radiotherapy in which radioactive seeds are placed close to or within a tumour [10]. This treatment strategy enables a higher radiation dose to be delivered to a PCa tumour than can be achieved by an external radiation source [11] with toxicity outcomes that compare very favourably with other treatment options [12].
Whether used as a standalone monotherapy for patients with low- or favourable-intermediate risk disease or as a local boost in combination with external beam radiation therapy (EBRT) for patients with unfavourable intermediate or high-risk disease, LDR-BT is a clinically effective and safe way of treating localised PCa [13]. Used as a local boost, LDR-BT improves biochemical progression-free survival [14]. There is also growing evidence that, because it is a minimally invasive technique that can be performed as a one-time outpatient procedure and hence shorten treatment time, it can also be the most cost-effective approach [15]. Given the significant and rising financial impact of PCa treatment on health systems it is, however, surprising that the utilisation of LDR-BT has been in decline despite its potential cost advantages [16]. To support healthcare payers and providers that want to adopt a value-based approach [17] to PCa treatment—one that preserves outcomes, improves accessibility and reduces resource utilisation—we have therefore carried out a systematic review of economic evaluations that compare the cost and cost-effectiveness of LDR-BT with that of other treatment options for localised and locally advanced PCa. To the best of our knowledge a brachytherapy-focused review of economic evaluations has not been undertaken before.
Methods
This systematic review of economic evaluations was conducted in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses Guidelines [18]. A scoping literature review was initially undertaken in November 2022 and disseminated for comment. A check on PROSPERO showed no other prospectively registered systematic reviews of economic evaluations of LDR-BT for PCa were currently taking place. Our protocol was therefore registered with number CRD42023442027 [19].
Systematic searches of the MEDLINE and Embase databases were performed by an information specialist using the Dialogue platform. Although Mathes et al. [20] recommend an additional search of at least one health economic database, the Health Economic Evaluation Database is no longer available and the National Health Service Economic Evaluation Database (NHS EED) is no longer updated. We therefore searched the Cost-Effectiveness Analysis (CEA) Registry maintained by Tufts Medical Center which incorporates the Global Health Cost-Effectiveness Analysis (GH CEA) Registry [21].
Our search strategies were devised using concepts that described the population, intervention and outcomes of interest—ie, PCa (including prostate adenoma, prostatic hyperplasia/hypertrophy and prostatic intraepithelial neoplasia), brachytherapy, and economic evaluations of any type (including cost-benefit, cost-effectiveness, cost-utility, cost-minimisation, cost-analysis and cost-comparison). We also used broader search terms such as ‘costs’ and ‘economics’ (see Supplementary material). We looked for studies in any language that were undertaken in any country and any setting during the period from 1 January 2008 to 6 June 2023. Our search results were downloaded from Dialogue as .RIS files and uploaded to the EndNote21 reference management tool where they were deduplicated.
Two reviewers independently screened the titles and abstracts based on the inclusion and exclusion criteria in Table 1, followed by full text screening. Disagreement was resolved by consensus. Since this analysis concerns the economic evidence for LDR-BT as a primary treatment for localised and locally advanced PCa, studies concerning men receiving adjuvant treatment or treatment for recurrent or metastatic disease were excluded. Reports were also excluded if they were conference abstracts for which full text was unavailable; if they had no comparators or did not include LDR-BT as a comparator; if they were a review, summary, or commentary; if they evaluated treatments for recurrent or metastatic disease; or if they focused on utilisation rather than cost.
In many countries, partial economic evaluations that lack either comparators or measurements of health effects are not a recommended analytical perspective so are excluded from systematic reviews. However, the reference case methods for economic evaluations specified by health technology assessment bodies such as the National Institute for Health and Care Excellence (NICE) permit the use of cost-comparison analyses for technologies likely to provide equivalent health benefits at similar or lower cost than comparators that are recommended in published NICE guidance for the same population [22]. Given the established equivalency between the oncological outcomes of the standard PCa treatment approaches [8] we have therefore included this type of partial economic evaluation in this review. We also screened the references of included publications for further articles of interest.
A single reviewer extracted data from the included studies using a pre-defined Microsoft Excel template. These data included both general study characteristics (eg, author, year of publication, country, population, economic perspective and evaluation type) and the study methods and outcomes (eg, treatments compared, cost data, health outcomes data and conclusions). The Drummond checklist [23] was used to critically appraise the quality of the included studies.
Results
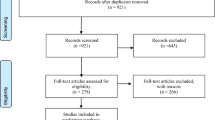
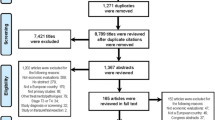
Figure 1 uses a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram to show the flow of literature. Our search identified 634 publications, from which 181 duplicates were removed. After the titles and abstracts of the 453 remaining references were screened, 36 studies were sought for retrieval and 14 were included in this review.
General characteristics
Tables 2 and 3 summarise the characteristics and key findings of the 14 economic evaluations that met the inclusion criteria—7 (50%) of which were full economic evaluations and 7 (50%) of which were partial evaluations that only considered costs. Of the full economic evaluations, 4 (29%) were cost-effectiveness analyses and 3 (21%) were cost-utility analyses. All the partial evaluations were cost-comparison analyses. Table 2 summarises the 11 (79%) studies that were focused on men with low- and/or favourable-intermediate risk PCa while Table 3 summarises the 2 (14%) studies that evaluated treatments for unfavourable intermediate- and/or high-risk disease. There was 1 (7%) study that analysed treatments for all risk groups so is included in both tables.
Countries and populations
The United States of America, where there were 9 (64%) studies, dominated the included evaluations. There were 2 (14%) studies from Canada, 2 (14%) studies from Japan and 1 (7%) from Spain.
There were 3 (21%) full evaluations that used study populations sourced from national and institutional registries, 3 (21%) that used theoretical cohorts based on systematic reviews or literature searches and 1 (7%) that used outcomes data from the ASCENDE-RT and ProtecT clinical trials. Although 3 (21%) partial evaluations also used registry or theoretical cohorts, the majority (4 = 29%) used small sample groups to estimate treatment costs by mapping real-world patient journeys.
Perspectives and time horizons
With regards to perspectives, 9 (64%) studies adopted a healthcare payer perspective, 4 (29%) a healthcare provider perspective and 1 (7%) took a societal perspective by incorporating average-wages for age-matched men into its evaluation.
There were a broad range of time horizons. A small number of cost-comparison studies used limited horizons of 6-, 12- or 18-months to capture only the costs of treatment and short-term follow-up. The median time horizon for the partial evaluations was 5 years. For full evaluations the median time horizon was 20 years, the shortest was 5 years and three studies used lifetime horizons.
Cost data
A variety of sources were used to obtain cost data. Medicare and Medicaid fee schedules were frequently used when a healthcare payer perspective was taken in a US study, whereas institutional data from hospital financial management systems were commonly used when taking a healthcare provider perspective. So-called ‘bottom-up’ micro-costing methods were used in 4 (29%) studies, including 2 cost-comparison studies (14%) that used process mapping and time-driven activity-based costing (TDABC) to generate detailed estimates of the costs of a full cycle of patient care.
Health outcomes data
The most frequently used measure of health outcomes was quality-adjusted life years (QALYs), which were reported by 5 of the 7 full economic evaluations. The evaluation that took a societal perspective used quality-adjusted life expectancies (QALEs) and one full evaluation used a trinity of clinical outcome measures—namely biochemical control, cause specific survival and overall survival—to calculate incremental cost-effectiveness ratios (ICERs) because of insufficient differences between the QALYs of the treatments being compared.
Willingness to pay
Only 4 of the 7 full evaluations clearly stated a threshold for considering an alternative as cost-effective. The 2 Canadian studies used thresholds of $50,000 per QALY gained while 2 American studies used considerably higher thresholds of $100,000 and $150,000 per QALY. All 4 of these studies carried out sensitivity analyses around these thresholds.
Quality of reporting
Both authors and reviewers have been cautioned against choosing the wrong checklist for appraising the quality of economic evaluations and against using adherence to checklist characteristics as a proxy for quality [24]. Our assessment of the methodological quality of the economic evaluations included in this study, based on Drummond’s detailed 35-item checklist and shown in Table 4, does not therefore provide an overall score or percentage but draws attention to study strengths and weaknesses. Checklist items were not applied to studies if they were not relevant—eg, the criteria on benefit measurement and validation were not applied to partial economic evaluations. The most poorly reported items on the Drummond checklist—affecting 6 (43%) of the included studies—were those relating to choice of discount rate, uncertainty due to sensitivity analyses being incomplete or missing and the failure to report incremental analysis. In 3 (21%) studies the type of brachytherapy being evaluated was not clearly described and clarification had to be sought from the authors (see Supplementary material).
Findings of included studies of treatments for low- and favourable intermediate-risk disease
LDR-BT vs AS
Of the 12 studies that compared treatments for men with low- and favourable intermediate-risk disease, 5 (42%) compared LDR-BT with AS. Two studies were unequivocal in their support for AS. Kato et al. found the costs of AS in Japan were far lower than LDR-BT ($1,074 vs $11,204) and recommended that switching patients to AS from another initial treatment could save the Japanese health system USD 13.8 million a year for 5 years [25]. Likewise, Eldefrawy et al. found that at 10-years the cumulative costs of AS ($13,116) were lower than for radical retropubic prostatectomy (RRP) ($15,084) and LDR-BT ($17,284) and much lower than for robot-assisted radical prostatectomy (RARP) ($22,762) and EBRT ($23,953) [26].
However, three studies found that, at 7-years follow-up and beyond, LDR-BT was cheaper than AS. Hayes et al., the only full evaluation to make this comparison, compared AS and LDR-BT with WW, RP and intensity-modulated radiation therapy (IMRT) over the lifetimes of men aged 65 and 75, respectively, at the time of diagnosis. Although they found WW was more effective and less costly than any comparator, and that AS provided marginally better QALE than LDR-BT (8.85 vs 8.14 for men aged 65 years and 5.98 vs 5.56 years for men aged 75), LDR-BT was less costly than AS in both age groups [27]. In their cost comparison study, Laviana et al. found that although AS was the cheapest treatment option at 5-years it reached cost-equivalency with LDR-BT at 7-years, assuming annual biopsies with MRI-fusion technology [28]. Keegan et al., who used the AS protocol and actual hospital costs at an academic medical centre in California, found that although AS with every-other-year biopsy was the cheapest treatment option at 5-years it became more expensive than LDR-BT from the ninth year of follow-up onwards [29].
LDR-BT vs RP
Of the 12 studies of men with low- and favourable intermediate-risk disease that were included in this review, 9 (75%) used at least one surgical modality as a comparator. Three of these studies were full economic evaluations. Weng et al. used real-world data registry evidence for costs, mortality and patient-derived and time-specific utility outcome differences from baseline. They found that compared with RP, LDR-BT was the lowest cost treatment for both low-risk ($39,729 vs $55,059) and intermediate-risk patients ($52,723 vs $75,064) [30]. Hayes et al. found LDR-BT to be both cheaper ($35,374 vs $38,180) and more effective (8.14 v 7.95 QALYs) than RP for men aged 65 at time of diagnosis [27]. However, Cooperberg et al. came to the opposite conclusion. They found the lifetime costs of three types of surgery—open radical prostatectomy (ORP), RARP and laporascopic-assisted radical prostatectomy (LRP)—to be statistically and clinically similar and to be consistently cheaper than RT modalities [31].
Of the 6 partial evaluations making this comparison, four studies found LDR-BT to be less expensive than surgical modalities. In some studies—such as Kato et al. [25] and Becerra Bachino et al. [32]—these differences were tangible but modest ($11,204 vs $12,689 and €5,369 vs €6,266 respectively) whereas in others—such as Laviana et al. [28]—they were substantial ($8,978 vs $16,946). Keegan et al. found LDR-BT to be less expensive than RP at both 5-years ($23,717 vs $29,862) and 10-years follow-up ($25,467 vs $31,612) [29].
Two of the partial evaluations found LDR-BT to be slightly more costly than RP. Eldefrawy et al. found LDR-BT ($17,284) to be sandwiched between the cheaper RRP ($15,084) and the more expensive RARP ($22,762) [26] while in a Japanese setting Satoh et al. found LDR-BT to be more expensive than either LRP or RRP. Indeed, whereas reimbursement for LDR-BT left the study site with just ¥199 in profit, the high fees and low costs associated with LRP yielded a profit of ¥75,672 per patient [33].
LDR-BT vs RT
All 12 (100%) studies in the low- and favourable intermediate-risk category compared LDR-BT with at least one other radiation modality. In the full economic evaluation by Weng et al., LBR-BT dominated EBRT in the low-risk group. For men with intermediate-risk disease, LDR-BT also had lower costs than either EBRT alone or EBRT + LDR-BT boost [30]. Similar results were seen by Hayes et al., who found LDR-BT offered broadly equivalent QALEs to IMRT but was considerably cheaper—both for men aged 65 years ($35,374 vs $48,699) and aged 75 years ($28,810 vs $42,286) at their time of diagnosis [27]. Notwithstanding that they had found surgical modalities to be consistently less expensive than radiation therapies, Cooperberg et al. also found that LDR-BT offered low-risk patients similar effectiveness at less cost ($25,067) than either three-dimensional conformal radiation therapy (3DCRT) ($27,626), IMRT ($37,718) or EBRT + LDR-BT boost ($40,588). For intermediate-risk patients, however, 3DCRT ($30,838) was marginally cheaper than LDR-BT ($32,533) [31].
Three of the reviewed studies focused on radiation modalities only. In patients with low- and intermediate-risk PCa Shah et al. found no significant differences in clinical outcomes but saw that LDR-BT was far less costly than high-dose-rate brachytherapy (HDR-BT) and IMRT for both providers ($2,395 vs $5,467 vs $23,665) and payers ($9,938 vs $17,514 vs $29,356) [34]. These results are corroborated by Ilg et al. who used TDABC to calculate that from a health system perspective the ‘true’ costs of LDR-BT ($6,869) were lower than those of HDR-BT ($9,538) [35].
Among the partial economic evaluations, Kato et al. found LDR-BT ($11,204) marginally cheaper than IMRT ($12,833) [25]. Laviana et al. found LDR-BT ($8,978) less expensive than HDR-BT ($11,448) and stereotactic body radiation therapy (SBRT) ($11,665) and significantly less costly than IMRT ($23,565) [28]. Eldefrawy et al. found LDR-BT ($17,284) less expensive than EBRT ($23,953) [26]. Keegan found LDR-BT cost substantially less than either image-guided radiation therapy (IGRT) alone or IGRT + LDR-BT boost at both 5-years ($23,717 vs $55,681 vs $59,381) and 10-years of follow-up ($25,467 vs $57,431 vs $61,131) [29].
Helou et al. found that, in a head-to-head comparison, the cost-effectiveness of SBRT versus LDR-BT was marginal and highly sensitive to the probability of biochemical recurrence [36]. Becerra Bachino et al. found 3DCRT to be somewhat cheaper than LDR-BT (€3,229 vs €5,369) [32] but Satoh et al. estimated it to be only about one third the cost of LBR-BT (¥470,573 vs ¥1,289,911) in a Japanese setting [33].
LDR-BT vs HDR-BT
There were 4 (33%) studies in this risk group that compared HDR-BT with LDR-BT and they all found the latter to be the most economically advantageous of the two types of brachytherapy monotherapies. Shah et al.’s full economic analysis found LDR-BT to be more cost-effective than HDR-BT [34] while all three partial evaluations to make a cost-comparison found in favour of LDR-BT. Notably, Ilg et al. used the TDABC methodology to determine the true cost of LDR-BT and HDR-BT for PCa and demonstrate opportunities for cost containment at an academic referral centre, concluding that the calculated cost to deliver HDR-BT was $2,669 greater than LDR-BT ($9,538 vs $6,869) [35]. This result was corroborated by Laviana et al. who used the same methodology at the same centre to reach a similar result ($11,448 vs $8,978) [28]. In a Japanese setting, Satoh et al. found HDR-BT to be slightly more costly than LDR-BT (¥1,416,894 vs ¥1,289,911). Moreover, due to the poor remuneration associated with HDR-BT, it was associated with a loss of ¥654,016 per patient whereas LDR-BT yielded a profit of ¥75,672 [33].
Findings of included studies of treatments for unfavourable intermediate- and high-risk disease
There were relatively few economic evaluations comparing LDR-BT with other treatment options for men whose unfavourable intermediate- and high-risk disease requires definitive treatment to begin without delay. This review identified only three such studies that met the inclusion criteria [31, 37, 38].
The most recent study is that of Kowalchuk et al., who compared three competing treatment strategies for men with high-risk disease: EBRT + LDR-BT boost, EBRT alone and RP. For patients with a life expectancy of at least 15 years, EBRT + LDR-BT boost was the treatment approach that best optimised long-term QALYs and costs, with an ICER of $20,929 per QALY gained [37]. Looking at the same risk group, Cooperberg et al. found the three surgical modalities in their study (ORP, RARP and LRP) to be less costly and marginally more effective than the four radiation modalities—of which 3DCRT ($42,397) and LDR-BT ($43,952) were significantly less expensive than EBRT + LDR-BT boost ($50,276) and IMRT ($53,539) [31].
Focusing on intermediate-risk patients, Alyamani et at compared radiation modalities, concluding that LDR-BT ($8,940) was the least costly treatment modality for the health system when including both initial and long-term outcomes. Though slightly more costly, SBRT ($10,048) provided more QALY gain (0.37) with an ICUR of $2,985 per QALY. The other four comparators in this study—conventionally fractionated (cf) IMRT, hypofractionated (hf) IMRT, HDR-BT and IMRT + HDR-BT boost—were all found to be dominated. However, a probabilistic analysis showed that the results for several comparators closely overlapped due to wide confidence intervals. The authors conceded that a more intensive surveillance of patient outcomes across each modality would improve evidence precision and clarify differences between treatments. It was also noted that the cost analysis of SBRT in this study was based on a conventional linear accelerator rather than a more expensive technique such as CyberKnife, which would be three times more costly [38].
Discussion
This systematic review identified 14 studies published between 2008 and 2023 that carried out either a full or partial economic evaluation in which LDR-BT was a comparator. The majority of these studies compared treatments for low- and/or favourable intermediate-risk disease whereas a much smaller group of studies compared treatments for men with unfavourable intermediate- and/or high-risk disease. There was significant variation between the treatments, methodologies, data sources, time horizons and settings in the compared studies. However, if the oncological outcomes of the three standard treatment approaches are considered equivalent, LDR-BT was the most cost-effective type of radiation therapy in 9 (75%) of 12 studies [25,26,27,28,29,30,31, 34, 35], was more cost-effective than RP in 6 (67%) of 9 studies [25, 27,28,29,30, 32] and, depending on the time horizon, was less costly than active surveillance (AS) in 3 (60%) of 5 studies [27,28,29] and more cost-effective in 2 (40%) studies [28, 29]. LDR-BT was more cost-effective than HDR-BT in all 4 (100%) of the studies where this comparison was made [28, 33,34,35]. Overall, LDR-BT was the cheapest of all active treatment options in 7 (50%) of the 14 studies included in this review [25, 27,28,29,30, 34, 35].
Low- and favourable intermediate-risk disease
LDR-BT vs AS
Notwithstanding the risk that left untreated it could spread, the orthodox approach to the management of low-risk PCa has traditionally been AS involving regular assessments to monitor the disease. AS is also routinely considered for men with favourable intermediate-risk cancers since there is a growing body of evidence that their outcomes are very similar to those of men with low-risk cancers [39].
The AS orthodoxy is habitually justified on the grounds of both its clinical- and cost-effectiveness. Reporting on data from the Prostate Testing for Cancer and Treatment (ProtecT) trial, for instance, Hamdy et al. found that at a median of 15-years of follow-up there were no significant differences in PCa-specific mortality between patients receiving AS, RP and RT—though men receiving AS were more likely to develop metastases [8]. Looking at the economic outcomes of the same trial at 10-years follow-up Noble et al. found that although AS yielded similar QALYs to RT and RP the average costs of AS, at £5,913, were lower than for RT (£7,361) or RP (£7,519) [40]. Randomised trial outcomes such as these have led to the widespread acceptance of AS in Europe as an initial management strategy for low- and favourable intermediate-risk disease. AS has also found favour in North America due to excellent cancer-specific outcomes reported by long-standing cohorts of AS patients at the University of Toronto and Johns Hopkins University [39].
It should be borne in mind, however, that studies that support AS sometimes fail to account for the fact that it is likely to be executed less carefully in the real-world than when done under the auspices of a clinical trial and that a high number of patients managed in this way will eventually receive radical treatment and/or develop metastatic disease. Degeling et al., for instance, have recently demonstrated that when these clinical pathways and their associated costs are included in a model-based analysis, the discounted total lifetime costs for Australian men with favourable-risk localised PCa managed with AS were greater, at AUD 17,912, than those for RP (AUD 15,609) or RT (AUD 15,118). In that study RT was the dominant strategy yielding higher QALYs at lower cost [41].
An unexpected outcome of this review has been that three out of five studies found that, at a point in time that could begin as early as the seventh year of follow-up, treatment with LDR-BT monotherapy can become cheaper than AS [27,28,29]. If surveillance protocols evolve to employ more costly strategies—for instance, the replacement of transrectal biopsy with a transperineal approach that requires sedation or general anaesthetic [42]—then LDR-BT may become less expensive than AS after only a few years of follow-up. If, on the other hand, greater trust is placed in magnetic resonance imaging (MRI) and the frequency of recurrent biopsy diminishes [43], AS may yet reassert itself as the most cost-effective treatment option for men in this risk group.
LDR-BT vs RP
The SPCG-4 and PIVOT studies both made a clear case for offering RP to patients with intermediate-risk disease and a life expectancy of >10 years [44]. Moreover, although AS is recommended as the default management strategy in patients with this life expectancy and low-risk disease, it is reasonable to consider RP as an alternative to AS in suitable patients who accept the inevitable trade-off between toxicity and preventing disease progression [5].
There were 9 studies in our review that compared LDR-BT with at least one surgical modality, of which 6 (67%) found LDR-BT to be less costly or more cost-effective [25, 27,28,29,30, 32]. In some studies the cost differences were marginal [25, 32] whereas in others they were substantial [28]. These findings demonstrate that LDR-BT is, more often than not, an economical alternative to RP associated with similar PCa-specific mortality but lower risk of sexual dysfunction and urinary incontinence [45]. They should also reassure healthcare providers in low- and middle-income countries that investing in LDR-BT as an alternative to expensive surgical robots will by no means disadvantage patients [46].
LDR-BT vs RT
Radiation therapy, like RP, is a reasonable alternative to AS in suitable patients wishing to prevent disease progression who can accept treatment-associated toxicity [5]. For patients with low- and favourable intermediate-risk disease and good urinary function LDR-BT is a convenient, effective and well-tolerated alternative to therapies that use an external radiation source [7].
All 12 of the reviewed economic studies of treatments for this risk group included at least one alternative RT modality as a comparator, with LDR-BT emerging as the least costly or most cost-effective treatment in 9 (75%) of those studies [25,26,27,28,29,30,31, 34, 35]. This outcome is not unexpected given that LDR-BT is a one-time intervention whereas conventionally fractionated RT is delivered through daily sessions over a period of 3–4 weeks. However, the economic advantage of LDR-BT is likely to become more marginal as contemporary radiotherapy adopts ultrahypofractionated strategies such as SBRT that are capable of hitting PCa tumours “harder, faster, and smarter and all for less cost and greater convenience for patients” [47]. Although the reviewed study by Helou et al. found that, in a head-to-head comparison, the cost-effectiveness of SBRT versus LDR-BT is marginal and highly sensitive to the probability of biochemical recurrence [36] we can now revisit this conclusion in the light of the HYPO-RT-PC trial’s demonstration of the noninferiority of this type of ultrahypofractionation [48]. By way of contrast, Satoh et al.’s finding that 3DCRT was about one third the cost of LBR-BT in Japan [33] is unlikely to lead to a renaissance for a modality that is being progressively replaced by modulated techniques, such as IMRT, that deliver lower toxicity and improved biochemical relapse-free survival [49].
LDR-BT vs. HDR-BT
Given that the two treatment approaches sit at opposite ends of the fractionation spectrum, it is not surprising that all 4 of the studies that compared temporary HDR-BT and permanent LDR-BT monotherapies found the latter to be the most economically advantageous technique. Outcome studies have shown they have similar biochemical recurrence rates, yet despite the flexible dosimetry of HDR-BT being associated with fewer side effects—including a statistically significant reduction in rates of dysuria, urinary frequency and rectal pain [50]—it is the less dosimetrically controllable LDR-BT that has found favour due to its practicality as a one-time procedure [51]. Its cost advantages over HDR-BT may, however, be less emphatic than the economic evidence appears to suggest. Shah et al.’s 2012 comparison was of LDR-BT and four fractions of HDR-BT [34] but this fractionation is no longer endorsed in the 2023 NCCN guidelines, which favours two fraction implants, so would not be the comparator today [52]. Moreover, while Ilg et al. found HDR-BT delivered via two separate implants over two treatment days to be $2,669 more costly than LDR-BT delivered in one treatment day ($9,538 vs $6,869) they conceded that a hypothetical single-fraction HDR-BT treatment would (at just $5,582) be markedly cheaper than multifraction therapy and, crucially, less costly than LDR-BT [35].
Unfavourable intermediate- and high-risk disease
Only 3 of the 14 evaluations included in this review studied the economic impact of LDR-BT as a local boost [31, 37, 38] for men with unfavourable intermediate- and high-risk disease. In these studies fractionation was the main cost driver, as illustrated by Kowalchuk et al.’s [37] comparison of the cost-effectiveness of RP with 20 fraction EBRT and 23 fraction EBRT + LDR-BT boost that used a Markov model powered by treatment outcomes and toxicity data from the ASCENDE-RT [14] and ProtecT trials [8]. That EBRT + LDR-BT boost was, despite its higher costs, robustly demonstrated to be the treatment approach best optimising long-term QALYs and costs is a testament to the impact LDR-BT boost is having on biochemical recurrence rates in men in this risk group [37]. HDR-BT boost was not included in this study, despite its frequent adoption as an alternative to LDR-BT due to improved impact on HRQoL—particularly its advantages for long-term bowel function [53].
Study limitations
This review has a several limitations. It contains studies that use theoretical-, registry- and real-world cohorts and the perspectives of payers, providers and society. The reporting of cost estimates in some evaluations was opaque, as was the reporting of health outcome measurements. Additional methodological heterogeneity was seen in assumptions, variables and nomenclature which limited the ability to directly compare studies.
The type of brachytherapy being evaluated was not clearly stated in the title or abstract of 4 (29%) studies [27, 29, 30, 32] and could only be ascertained by careful reading of the narrative. Furthermore, in 3 (21%) studies [25, 26, 31] there was no reference whatsoever to the type of brachytherapy being evaluated so clarification was sought from their authors (see Supplementary material).
The review was dominated by studies undertaken in the USA, a setting whose healthcare system and population characteristics—including prices, costs, productivity, clinical practices, PCa incidence, case mix and life expectancy—may not be transferrable to other countries or regions. This difference in characteristics may compromise the transferability of an American study’s ICERs. Given these limitations in the existing literature there is a strong need for further full evaluations that build the economic case for LDR-BT in those countries where it is declining in popularity or remains underutilised as a primary treatment for localised PCa—particularly European countries where public healthcare systems currently face significant cost pressures.
With regard to the comparisons of LDR-BT with other treatment strategies for men with low- and favourable intermediate-risk disease, we note that the average age at the time of PCa diagnosis is currently 66 years old [1]. It was therefore surprising to see one study [25] support AS as a cost-effective treatment based on a 5-year time horizon—an arguably inappropriate life expectancy to use when triaging between aggressive and conservative treatment options. While it is true that diminishing life expectancy decreases the oncologic benefit of PCa treatment and increases the risk of patient harm and overtreatment, increased male life expectancy requires that credible studies use appropriate follow-up durations.
The outputs of cost-effectiveness studies can only ever be as accurate as their inputs, yet a number of studies [29, 30, 37] provided sparse, limited or oversimplified cost data making it impossible to understand how procedure costs were calculated. This reduced study credibility and imported potential bias into the results of this review.
Conclusions
The available health economic evidence suggests that LDR-BT has significant cost advantages and an important role to play in the delivery of value-based PCa care. If the oncological outcomes of the three standard treatment approaches are equivalent for men with low-risk and/or favourable-intermediate risk PCa then LDR-BT can, depending on the time horizon, be less costly and more cost-effective than AS—challenging the orthodoxy that AS is always the most economically advantageous treatment strategy for men in these risk groups. LDR-BT is more cost-effective than RP in many settings. It is consistently cost-effective in comparison to most other types of RT and is always more cost-effective than HDR-BT. In the future, these economic advantages could be challenged if radiotherapy favours ultrahypofractioned strategies such as SBRT and reduced fractionation in HDR-BT.
With regards to unfavourable intermediate- and/or high-risk PCa there is currently insufficient economic evidence to draw any firm conclusions regarding the comparative costs and cost-effectiveness of the recommended treatment strategies, though EBRT + LDR-BT boost has been shown by at least one study to optimise long-term QALYs and costs [37].
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomatarum I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Sharma R. The burden of prostate cancer is associated with human development index: evidence from 87 countries, 1990-2016. EPMA J. 2019;10:137–52.
Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9:465–72.
Eastham J, Auffenberg G, Barocas D, Chou R, Crispino T, Davis J, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol. 2022;208:10–18.
Mottet N, Cornford P, van den Berg R, Briers E, De Santis M, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
National Institute for Health and Care Excellence (2022). Prostate cancer: diagnosis and management (NG131). Last updated 15 December 2021.
King M, Keyes M, Frank S, Crook J, Butler W, Rossi P, et al. Low dose rate brachytherapy for primary treatment of localized prostate cancer: asystemic review and executive summary of an evidence-based consensus statement. Brachytherapy. 2021;20:1114–29.
Hamdy F, Donovan J, Lane J, Metcalfe C, David M, Turner E, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2023;388:1547–58.
Donovan J, Hamdy F, Athene Lane J, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2016;375:1425–37.
Skowronek J. Current status of brachytherapy in cancer treatment—a short overview. J Contemp Brachytherapy. 2017;9:581–9.
Ragde H, Grado G, Nadir B, Elgamal AA. Modern prostate brachytherapy. CA Cancer J Clin. 2000;50:380–93.
Schlussel Markovic E, Buckstein M, Stone N, Stock R. Outcomes and toxicities in patients with intermediate risk prostate cancer treated with brachytherapy alone or brachytherapy and supplemental external beam radiation therapy. BJU Int. 2018;121:774–80.
Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guérin F, et al. Brachytherapy: an overview for clinicians. CA Cancer J Clin. 2019;69:386–401.
Oh J, Tyldesley S, Pai H, McKenzie M, Halperin R, Duncan G, et al. An updated analysis of the survival endpoints of ASCENDE-RT. Int J Radiat Oncol Biol Phys 2023;115:1061–70.
Vu C, Jawad M, Krauss D. The cost-effectiveness and value proposition of brachytherapy. Semin Radiat Oncol. 2020;30:87–93.
Williams V, Kahn J, Thaker N, Beriwal S, Nguyen P, Arthur D, et al. The case for brachytherapy: why it deserves a renaissance. Adv Radiat Oncol. 2021;6:100605.
Teisberg E, Wallace S, O’Hara S. Defining and implementing value-based health care: a strategic framework. Acad Med. 2020;95:682–5.
Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Prospero. Low-dose-rate brachytherapy as a primary treatment for localised and locally advanced prostate cancer: a systematic review of economic evaluations. National Institute for Health Research 2023. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023442027.
Mathes T, Walgenbach M, Antione S-L, Pieper D, Eikermann M. Methods for systematic reviews of health economic evaluations: a systematic review, comparison, and synthesis of method literature. Med Decis Mak. 2014;34:826–40.
Tufts Medical Center—Center for the Evaluation of Value and Risk in Health. https://cevr.tuftsmedicalcenter.org/databases/cea-registry.
National Institute for Health and Care Excellence (2022). NICE health technology evaluations: the manual (PMG36). Last updated 31 October 2023.
Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–83.
Frederix G. Check your checklist: the dangers of over- and underestimating the quality of economic evaluations. Pharmacoecon Open. 2019;3:433–5.
Kato T, Yokomizo A, Matsumoto R, Tohi Y, Miyakawa J, Mitsuzuka K, et al. Comparison on the medical costs between active surveillance and other treatment for early prostate cancer in Japan: from the data of PRAIS-JAPAN study. Int J Urol. 2022;29:1271–8.
Eldefrawy A, Katkoori D, Abramowitz M, Soloway M, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013;31:576–80.
Hayes J, Ollendorf D, Pearson S, Barry M, Kantoff P, Lee P, et al. Observation versus initial treatment for men with localised, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158:853–60.
Laviana A, Ilg A, Veruttipong D, Tan H-J, Burke M, Niedzwiecki D, et al. Utilising time-driven activity-based costing to understand the short- and long-term costs of treating localised, low-risk prostate cancer. Cancer. 2016;122:447–55.
Keegan K, Dall’Era M, Durbin-Johnson B, Evans C. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer. 2012;118:3512–8.
Weng X, Zhong L, Xiang P, Li Y, Paciorek A, Dong L, et al. Cost-effectiveness analysis of primary treatments for localised prostate cancer: a population-based Markov analysis using real-world evidence. Eur J Cancer Care. 2022;31:e13740.
Cooperberg M, Ramakrishna N, Duff S, Hughes K, Sadownik S, Smith J, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–50.
Becerra Bachino V, Cots F, Guedea F, Pera J, Boladeras A, Aguiló F, et al. Cost comparison of three treatments for localised prostate cancer in Spain: radical prostatectomy, prostate brachytherapy and external 3D conformal radiotherapy. Gac Sanit. 2011;25:35–43.
Satoh T, Ishiyama H, Matsumoto K, Tabata K-I, Kitano M, Iwamura M, et al. Cost comparison of curative therapies for localised prostate cancer in Japan: a single-institution experience. Jpn J Radio. 2009;27:348–54.
Shah C, Lanni T, Ghilezan M, Gustafson G, Marvin K, Ye H, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. 2012;11:441–5.
Ilg A, Laviana A, Kamrava M, Veruttipong D, Steinberg M, Par S-J, et al. Time-driven activity-based costing of low-dose-rate and high-dose-rate brachytherapy for low-risk prostate cancer. Brachytherapy. 2016;15:760–7.
Helou J, Torres S, Musunuru H, Raphael J, Cheung P, Vesprini D, et al. Stereotactic body radiotherapy versus low dose rate brachytherapy for localised prostate cancer: a cost-utility analysis. Clin Oncol (R Coll Radio). 2017;29:718–31.
Kowalchuk R, Kim H, Harmsen W, Jeans E, Morris L, Mullikin T, et al. Cost effectiveness of treatment strategies for high risk prostate cancer. Cancer. 2022;128:3815–23.
Alyamani N, Song J, van Katwyk S, Thavorn K, Renaud J, Haddad A, et al. Cost-utility analysis of radiation treatment modalities for intermediate-risk prostate cancer. Curr Oncol. 2021;28:2385–98.
Filson C. Quality of care and economic considerations of active surveillance of men with prostate cancer. Transl Androl Urol. 2018;7:203–13.
Noble S, Garfield K, Athene Lane J, Metcalfe C, Davis M, Walsh E, et al. The ProtecT randomised trial cost-effectiveness analysis comparing active monitoring, surgery, or radiotherapy for prostate cancer. Br J Cancer. 2020;123:1063–70.
Degeling K, Corcoran N, Pereira-Salgado A, Hamid A, Siva S, IJzerman M. Lifetime health and economic outcomes of active surveillance, radical prostatectomy, and radiotherapy for favourable-risk localised prostate cancer. Value Health. 2021;24:1737–45.
Grummet J, Gorin M, Popert R, O’Brien T, Lamb A, Hadaschik B, et al. “TREXIT 2020”: why the time to abandon transrectal prostate biopsy starts now. Prostate Cancer Prostatic Dis 2020;23:62–65.
Cheung D, Finelli A. Magnetic resonance imaging diagnosis of prostate cancer: promise and caution. CMAJ. 2019;191:E1177–E1178.
Richstone L, Bianco F, Shah H, Kattan M, Eastham J, Scardino P, et al. Radical prostatectomy in men aged >or=70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101:541–6.
Zhang P, Qian B, Shi J, Xiao Y. Radical prostatectomy versus brachytherapy for clinically localized prostate cancer on oncological and functional outcomes: a meta-analysis. Transl Androl Urol 2020;9:332–43.
Crew B. A closer look at a revered robot. Nat Suppl. 2020;580:S5–S7.
Correa R, Loblaw A. Stereotactic body radiotherapy: hitting harder, faster, and smarter in high-risk prostate cancer. Front Oncol. 2022;12:889132.
Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–95.
Yu T, Zhang Q, Zheng T, Shi H, Liu Y, Feng S, et al. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11:e0154499.
Martinez A, Demanes J, Vargas C, Schour L, Ghilezan M, Gustafson G. High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2010;33:481–8.
Skowronek J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer—between options. J Contemp Brachytherapy. 2013;5:33–41.
Schaeffer E, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2023;21:1067–96.
Moideen N, Crook J, Araujo C, Batchelar D, Canovas F, Halperin R, et al. A randomised phase III trial comparing health-related quality of life after low dose rate (LDR) or high dose rate (HDR) prostate brachytherapy boost combined with external beam pelvic radiotherapy (EBRT). Int J Radiat Oncol Biol Phys. 2022;174:S4.
Acknowledgements
We thank Beata Coffey (BC), Information Specialist at the Royal Society of Medicine, for her assistance in developing a search strategy and performing the literature search. We also thank Alka Singh (AS), Associate Consultant at Mtech Access, and Jodie Worrall (JW), Associate Director at Mtech Access, who in their capacity as second reviewers independently screened reports, assessed them for eligibility and helped select reports for inclusion by consensus with the authors.
Funding
The study was funded by Becton, Dickinson and Company but they took no part in the design of the study, the collection and analysis of data, the preparation of the manuscript or the decision to publish.
Author information
Authors and Affiliations
Contributions
Benedict Stanberry (BS) jointly conceived the design of the study with Nikki Webber-Jones (NWJ). BS then developed and submitted the protocol. Together with BC he jointly developed the search strategy and BC performed the literature search. BS, AS and JW independently screened reports, assessed them for eligibility and selected reports for inclusion by consensus. BS wrote the manuscript and NWJ critically reviewed it.
Corresponding author
Ethics declarations
Competing interests
BS declares that he has received compensation from Becton, Dickinson and Company for participating in conferences and workshops. NWJ works for Becton, Dickinson and Company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stanberry, B., Webber-Jones, N. Low-dose-rate brachytherapy as a primary treatment for localised and locally advanced prostate cancer: a systematic review of economic evaluations. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00817-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00817-z
- Springer Nature Limited