Abstract
Background
Retzius-sparing robot-assisted radical prostatectomy (RARP) is not yet universally accepted due to still limited functional data and some concerns on oncological safety compared to the standard one. We assessed perioperative, pathological and early functional outcomes in patients with clinically localised prostate cancer treated with Retzius-sparing versus standard RARP.
Methods
A single-surgeon cohort of 207 consecutive patients undergoing RARP was analysed. A later study group of 102 patients receiving the Retzius-sparing approach was compared with an earlier control group of 105 patients receiving the standard one. Urinary continence recovery 1 week after catheter removal was the primary study outcome. Urinary continence recovery 1, 2, 3 and 6 months after catheter removal, potency recovery 6 months postoperatively, rate of perioperative complications and positive surgical margins were secondary study outcomes.
Results
Patients in the study group reported significantly higher urinary continence recovery rates 1 week (91.2% vs. 54.3%, p < 0.001), 1 month (92.2% vs. 66.7%, p < 0.001), 2 months (95.1% vs. 74.3%, p < 0.001), 3 months (96.1% vs. 83.8%, p = 0.01), but not 6 months (97% vs 90.5%, p = 0.09) after catheter removal compared to controls. Potency recovery rates 6 months after catheter removal were significantly higher in the study than the control group (68.2% vs 51.6%, p = 0.03). On multivariable analyses, the Retzius-sparing approach was an independent predictor of 1-week urinary continence recovery, but not of 6-month potency recovery. There were significant differences neither in perioperative complication rate (9.8% in the study vs. 14.3% in the control group, p = 0.28) nor in positive surgical margin rate (9.8% in the study vs. 8.6% in the control group, p = 0.75).
Conclusions
In a comparative study, we observed a significant improvement in immediate urinary continence, but not in early potency recovery, using the Retzius-sparing compared to the standard approach for RARP, with no increase in perioperative complication and positive surgical margin rate.
Similar content being viewed by others
Introduction
In the last decades, robot-assisted radical prostatectomy (RARP) has mostly been performed using a transperitoneal approach, while the extraperitoneal, transperineal and transvesical routes have been less popular. Most robotic surgeons have been performing transperitoneal RARP using a purely anterior approach, starting the dissection with the incision of the parietal peritoneum lateral to the umbilical ligaments, releasing the bladder and developing the Retzius space [1]. Conversely, others have adopted a mixed posterior/anterior approach according to the Montsouris technique [2], by starting the dissection posteriorly via a retrovesical incision in order to isolate the vas and the seminal vesicles before proceeding to the standard anterior approach. Regardless of these different approaches, refinements in surgical technique have been mostly directed to optimising functional outcomes, primarily urinary continence recovery. Some Authors have emphasised the preservation of key structures, such as bladder neck, cavernous nerves, puboprostatic ligaments, and membranous urethra, while others have focused on restoration of anatomical support to the urethra [3].
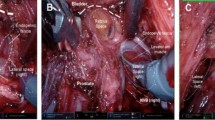
In 2010 Galfano et al. [4] described an original transperitoneal technique consisting of a fully posterior approach leaving the Retzius space untouched (i.e., Retzius-sparing RARP), with the aim to preserve the anterior anatomical structures involved in urinary continence mechanisms and to facilitate bladder neck preservation. However, the limited working space with unprecedented anatomical landmarks and close proximity to ureters, the concerns on a possibly higher risk of positive surgical margins (PSMs), and the unclear advantage in functional outcomes mostly in the long term, were soon advocated as factors impeding the widespread dissemination of the technique. Indeed, only 10% of robotic surgeons currently use the Retzius-sparing approach for RARP [5].
Most studies comparing Retzius-sparing and standard RARP focused on assessing urinary continence recovery, with a consistent benefit observed for the former in terms of immediate and early recovery, which was not uniformly confirmed at longer follow-up in recent meta-analyses including both randomised and non-randomised studies [6,7,8,9].
Only few studies have compared Retzius-sparing with standard RARP in terms of potency recovery, with two meta-analyses failing to show significant differences [7, 8]. Likewise, data on oncology safety are still limited, with some authors arguing for a potentially increased risk of overall PSMs after Retzius-sparing RARP [6] that was not replicated by more recent data when stratified by pT [7, 9].
Of note, all these data should be scrutinised with caution because they have been generated by few relatively small-sized randomised clinical trials (RCTs) with significant biases as to randomisation methods and/or patient allocation, or by prospective non-randomised studies with selective reporting as to functional or oncological outcomes.
Based on literature data available at that time, in late 2019 we planned to conduct a comparative non-randomised study to assess perioperative, pathological and early functional outcomes in men with clinically localised prostate cancer undergoing Retzius-sparing versus standard RARP.
Patients and methods
Patients
A prospectively maintained database for RARP patients collecting clinical, oncological, and functional data has been active at the University of Messina Urologic Section since May 1, 2017. From January 1, 2020 to December 31, 2021, all consecutive patients scheduled for RARP at our Department were treated with a fully posterior Retzius-sparing approach (study group). The control group consisted of patients treated with RARP with a standard anterior approach in the immediately preceding period, i.e., from January 1, 2018 to December 31, 2019. The University of Messina Ethics Committee approved the protocol and the collection of data for the current study (N. 94/19).
Preoperative urinary incontinence, previous urethral or prostatic endoscopic procedures, open simple prostatectomy, and concomitant neurological diseases were exclusion criteria. All patients agreed to participate and authorised data collection for scientific purposes. Patients with intermediate- or high-risk disease had computed tomography of abdomen and bone scan for staging purpose.
Surgical technique
All procedures were performed by a single, expert robotic surgeon beyond the learning curve for the standard anterior approach and naïve to the Retzius-sparing one. To minimise the learning curve effect, the first 15 cases performed with the Retzius-sparing approach were excluded from the analysis.
All cases were operated on under general anaesthesia using a 4-arm Xi Da Vinci robotic platform. Retzius-sparing RARP was performed according to the technique described by Galfano et al. [4], while standard anterior RARP was performed according to a technique initially described in 2012 [10] and modified in 2021 [11]. An extended pelvic lymphadenectomy was performed in all patients with high-risk disease and in those with intermediate-risk disease in whom the estimated risk for positive lymph nodes was >5% according to the updated Briganti nomogram [12].
Postoperative management
A third-generation cephalosporin and low-molecular-weight heparin plus elasto-compressive stockings were used for prophylaxis of infections and thromboembolic events, respectively. A cystogram was performed on postoperative day (POD) 3 or 4 to evaluate the presence of any leakage from the vesicourethral anastomosis (VUA). The catheter was immediately removed in presence of a watertight VUA or if there was minimal extravasation of contrast medium (<5% of the infused volume). Conversely, if a significant leakage was detected, a further cystogram was scheduled within 1–2 weeks, according to the severity of the leakage.
Data collection and study outcomes
The following variables were extracted from the database: age on RARP, body mass index (BMI), Charlson comorbidity score, preoperative total serum PSA level, prostate volume on preoperative MRI, presence of median prostate lobe, clinical tumour stage according to the 2009 TNM staging system, International Society of Urological Pathology (ISUP) grade group on biopsy, and D’Amico risk group [13]. Moreover, the following intraoperative parameters were recorded: operative room (OR) time, estimated blood loss (EBL), and complications. Surgical specimens were processed according to the classical Stanford protocol and reviewed by experienced pathologists, and the following parameters were recorded: pathological tumour and lymph node stage according to 2009 TNM system, ISUP grade group and surgical margins status. PSMs were defined as tumour extending to the inked surface of prostate specimens. PSMs were further stratified into focal or non-focal based on the 3-mm length cut-off.
Post-operative complications observed within 90 days from surgery were recorded and graded according to the Clavien–Dindo classification [14]. Grade 1–2 complications were considered as minor, grade 3 to 5 complications were classified as major.
Urinary continence was evaluated at 1 week, and 1, 2, 3 and 6 months after catheter removal using the modified self-administered item number 1 of the International Consultation of Incontinence Questionnaire - Urinary Incontinence Short Form (ICIQ-UI SF) instrument: “How often do you leak urine during the last week?”. Patients reporting no leak were considered continent [15]. Patients reporting some leak were asked to answer to the following modified item of the Expanded Prostate Cancer Index Composite (EPIC) questionnaire: “How many pads or adult diapers per day did you usually use to control leakage during the last week?” [16]. Patients reporting the use of only safety pads were also considered continent.
Pre- and post-operative potency status was evaluated using the Sexual Health Inventory for Men (SHIM) [17]. Patients with a SHIM score ≥17 and/or erections sufficient for sexual intercourse (≥2 on question 2) with or without phosphodiesterase type 5 (PDE5) inhibitors were considered potent.
As an early oncological outcome, PSA persistence, defined as total PSA values >0.2 ng/ml two months postoperatively, was assessed, as well the receipt of adjuvant treatments.
Urinary continence recovery at 1 week after catheter removal was the primary study outcome. Urinary continence recovery at 1, 2, 3 and 6 months after catheter removal, potency recovery at 6 months after catheter removal, and rate of perioperative complications and PSMs were secondary study outcomes. Predictors of 1-week urinary continence recovery and 6-month potency recovery were also assessed.
Statistical analysis
The sample size was calculated with a formal power analysis using the version 3.1.9.4 G* Power software. Assuming an α significance level of 5%, a power of 95% and an 1:1 allocation ratio, 89 patients per group were needed to ensure an adequate study power to detect a 25% difference in urinary continence rate 1 week after catheter removal. Considering a 10% maximum dropout rate for functional assessment at 6 months postoperatively, approximately 100 patients per group were finally required.
Parametric continuous variables were reported as mean ± standard deviation, whereas median and interquartile range (IQR) was used for non-parametric continuous variables. Student’s t-test, Mann–Whitney U-test and Pearson’s chi-squared test were used to compare continuous parametric, non-parametric, and categorical variables as appropriate.
Binary logistic regression was used to perform multivariable analyses aimed at identifying predictors of 1-week urinary continence recovery and 6-mo potency recovery. For the former, patients using ≥1 pad were considered as events, and covariates were age, BMI, prostate volume, nerve-sparing surgery (not performed, unilateral or bilateral), bladder-neck preservation (performed or not performed) and type of approach (Retzius-sparing or standard). For the latter, patients undergoing a nerve-sparing surgery and with a SHIM score <17 were considered as events, and covariates were age, BMI, prostate volume, preoperative SHIM score, nerve-sparing surgery (unilateral or bilateral), and type of approach (Retzius-sparing or standard).
All clinical records were imported into a dedicated database and analysed using SPSS v.23.0 (IBM Corp., Armonk, NY, USA) software. All reported p values are two-sided and statistical significance was set at p < 0.05.
Results
The study group included 102 consecutive patients undergoing Retzius-sparing RARP and the control group included 105 patients undergoing standard RARP. The two groups were comparable for all demographic, clinical and pathological variables, except for preoperative potency rate, which was significantly higher in patients in the study group (p < 0.001) (Table 1).
Median OR time was 180 min (IQR 158–185) in the study, and 180 min (IQR155–187) in the control group (p = 0.06). Median EBL was 100 ml (IQR 50–150) in the study and 100 ml (IQR 50–150) in the control group (p = 0.90). No intraoperative complications were observed in both groups.
In the study group a watertight VUA was documented in 96 (94.1%) patients, while a minimal or a significant leakage was detected in 5 (4.9%) and 1 (1%) cases, respectively. In the control group, VUA was watertight in 92 (87.6%) cases, and there was minimal leakage in 10 (9.5%) and a significant leakage in 3 (2.9%) cases, respectively. No significant differences in VUA quality were detected (p = 0.10). Transurethral catheter was removed after a median time of 5 (IQR 4–5) days in both the study and control group (p = 0.66).
Ninety-day post-operative complications were observed in 10 (9.8%) in the study, and in 15 (14.3%) patients in the control group (p = 0.28). No major complications were reported in the study group, while 3 (2.9%) patients had grade 3 and 2 (1.9%) grade 4 complications in the control group.
Patients in the study group reported significantly higher urinary continence recovery rates 1 week (91.2% vs. 54.3%, p < 0.001), 1 month (92.2% vs. 66.7%, p < 0.001), 2 months (95.1% vs. 74.3%, p < 0.001) and 3 months (96.1% vs. 83.8%, p = 0.01) after catheter removal compared to patients in the control group. Conversely, no difference between the groups was observed at the 6-month follow-up (97% vs 90.5%, p = 0.09) (Table 2). Supplementary Table 1 shows the ICIQ-UI SF scores and pad use reported by patients in the two groups at different follow-up time points. Only patients reporting the use of >2 daily pads had a score in the range of severe bother, regardless of the surgical technique. On multivariable analysis, Retzius-sparing approach was independently and significantly associated with decreased 1-week urinary incontinence rates (odds ratio [OR] 0.157, 95% confidence intervals [CI] 0.065–0.378, p < 0.001) (Table 3).
A nerve-sparing procedure was performed in 88 (86%) patients in the study, and 64 (61%) patients in the control group (p < 0.001). Details are reported in Table 4. One hundred fifty-two preoperatively potent patients undergoing nerve-sparing surgery reached a minimum follow-up of 6 months (88 in the study and 64 in the control group). Sixty/88 (68.2%) patients in the study, and 33/64 (51.6%) in the control group were potent 6 months after catheter removal (p = 0.03). In detail, in the study group, 41/60 (68.3%) patients had spontaneous erections, while 19/60 (31.7%) had erections assisted by PDE5 inhibitors. In the control group, 16/33 (48.5%) patients reported spontaneous erections, while 17/33 (51.5%) had erections assisted by PDE5 inhibitors (p = 0.06). On multivariable analysis, age (OR 1.141, 95% CI 1.067–1.220, p < 0.001) and nerve-sparing surgery (OR 0.270, 95% CI 0.107–0.699, p = 0.007) were the two independent and significant predictors of 6-month potency recovery (Table 5).
No differences were observed in terms of pT (p = 0.1), ISUP grade group (p = 0.12) and PSMs rate (p = 0.81). In detail, PSMs were detected in 10 (9.8%) patients in the study (4 focal and 6 non-focal) and in 9 (8.6%) patients in the control group (2 focal and 7 non-focal) (p = 0.75). Pelvic lymph node invasion was observed in only one patient (1%) in the control group. Details on pathological data are shown in Table 6.
PSA persistence 2 months after surgery was observed in 4 (4.6%) patients in the study, and 8 (7.8%) patients in the control group (p = 0.36), with no significant difference in proportion of adjuvant treatments (3 patients in the study, 6 patients in the control group, p = 0.18).
Discussion
Our study showed that Retzius-sparing RARP was significantly associated with improved immediate (1-week) urinary continence recovery, but not early (6-month) potency recovery, on multivariable analysis, compared to the standard approach. Urinary continence recovery at 6 months was comparable with the two techniques. Moreover, the Retzius-sparing approach did not compromise the oncologic safety of the procedure, being associated with similar PSM rates as the standard one.
The Retzius-sparing approach via the Douglas space can be considered an evolution of the Montsouris technique originally described by Guillonneau et al. in 2000 for laparoscopic prostatectomy [2]. Preservation of the anterior anatomical structures involved in the urinary continence mechanisms represents the most important anatomical rationale justifying the Retzius-sparing approach [4]. The first RCT published in 2017 clearly demonstrated a significant advantage in terms of immediate urinary continence recovery for Retzius-sparing vs standard RARP [18]. In this trial, the 1-week continence rate was 71% in patients treated with a posterior approach and 48% in those receiving the standard one. The difference was still present 3 months after surgery, whereby a urinary continence recovery rate of 95% with the Retzius-sparing and 86% with the standard approach were reported. However, this RCT failed to demonstrate significant differences between the two approaches at 6- and 12-mo follow-up [19]. Similarly, two other RCTs showed significant advantages in favour of Retzius-sparing RARP in terms of immediate and 3-mo urinary continence recovery, but did not report significant differences at 6- and 12-mo follow-up [20, 21]. Conversely, some non-randomised comparative studies reported significant differences in terms of 6-mo and 12-mo urinary continence recovery in favour of Retzius-sparing RARP [22, 23]. The most recent meta-analysis showed a significant advantage in terms of 1-mo, 3-mo, 6-mo and 12-mo urinary continence recovery for Retzius-sparing RARP [9]. Our data showed a significant advantage in favour of Retzius-sparing approach only in the initial 3-mo follow-up period. This data could be explained by the high rates of 6-mo urinary continence recovery in our control group composed by patients who underwent a standard approach using the urethral fixation technique [11]. Interestingly, in 2022 Turkolmez et al. reported equivalent urinary continence recovery rates at different follow-up time points in a study comparing Retzius-sparing with anterior Retzius-repairing RARP. In detail, the 1-, 3-, 6- and 12-mo urinary continence rates were 52.5%, 95%, 97.5% and 97.5% after Retzius-repairing RARP, and 61.5%, 97.5%, 97.5% and 97.5% after Retzius-sparing RARP (all non-significant p values) [24].
While data about urinary continence outcome are widely reported in the literature, very few studies assessed potency recovery when comparing Retzius-sparing versus standard RARP [19, 22, 25,26,27]. Moreover, interpretation of potency outcomes was subject to methodological issues, above all due to criteria used for definition. Only a single RCT analysed potency recovery rates at different follow-up time points showing comparable results between the two techniques. In details, 3-mo, 6-mo and 12-mo potency rates were 43.7%, 59.4%, and 86% after Retzius-sparing, and 36.7%, 64.8%, and 69% after standard RARP (all non-significant p values) [19]. In 2021 Egan et al reported a 12-mo potency rate of 65.7% with Retzius-sparing, and 62.9% with standard RARP (p = 0.72) [22]. In 2021, Umari et al prospectively compared a large series of patients who underwent Retzius-sparing or standard RARP showing similar erectile function scores at different time points within the first 12-mo follow-up [25]. Finally, in two more recent, but smaller, comparative studies, Deng et al [26] and Tahra et al [27] also reported a comparable 12-mo potency recovery rate for the two approaches. All these data were confirmed by two recent meta-analyses [7, 8]. In line with all these data, our study failed to show that Retzius-sparing approach was significantly associated with higher 6-month potency recovery rates compared to standard RARP on multivariable analysis. Rather, bilateral nerve-sparing surgery and younger age were the two independent predictors of potency recovery. It remains, however, questionable whether the higher rate of nerve-sparing surgery in the study group is due to a truly more favourable anatomical plane with Retzius-sparing allowing the preservation of cavernous nerves posterolaterally or to a greater commitment of the treating surgeon when exposed to a novel technique.
In the early experience with Retzius-sparing RARP some urologists expressed concerns on a possibly increased risk in PSMs [6, 28]. Checcucci et al in their meta-analysis reported a significant increase in overall PSM rate for Retzius-sparing vs. standard RARP [6], which was not confirmed by more recent RCTs [20, 21]. Two recent meta-analyses of comparative studies reported a significantly higher risk of PSMs in pT2 only [7] and pT3 only [9] cases for Retzius-sparing vs. standard RARP. Our study showed comparable stage-stratified PSMs rate thus reassuring that Retzius-sparing RARP is a safe oncological procedure. We emphasise these findings especially considering the significantly higher proportion of patients undergoing nerve sparing in the study group as well as the relatively small number of initial Retzius-sparing cases excluded from the analysis.
There is no consensus regarding the length and steepness of the learning curve in Retzius-sparing RARP, especially for surgeons who are proficient with other RARP approaches. In a recent multi-centre, multi-surgeon study by Galfano et al. [29], surgeons were stratified according to their experience with Retzius-sparing RARP in two categories, i.e., initial and expert, based on the cut-off of 25 procedures. Moreover, the same study showed that functional and oncological outcomes were not negatively affected in the first 50 cases when surgeons experienced with standard RARP transitioned to the Retzius-sparing approach. In view of the above study, and considering the high robotic expertise of the treating surgeon in our study, we arbitrarily decided to consider the first 15 cases as his initial experience. We acknowledge, nonetheless, that certain outcomes in the study group could have been influenced by the surgeon learning curve.
The main limitation of our study is the single-centre, single-surgeon, non-randomised design. Moreover, although clinical records were prospectively collected as per institutional research policy, the control group was not exactly contemporary to the study group. However, the present study represents the real-life evolution of our clinical practice for RARP.
In conclusion, our study showed that Retzius-sparing RARP was significantly associated with improved immediate (1-week) urinary continence recovery, but not early (6-month) potency recovery, on multivariable analysis, compared to the standard approach. Urinary continence recovery at 6 months was comparable with the two techniques. Moreover, we did not observe an increased risk of stage-stratified PSMs with the Retzius-sparing approach, thus reassuring on the oncological safety of this technique. Larger, multi-surgeon studies outside centres of excellence and with longer follow-up are needed to further assess the potential advantages of this approach.
Data availability
The datasets created and analyzed in this study are not publicly available. Requests for data can be addressed to the corresponding author (GG).
References
Martini A, Falagario UG, Villers A, Dell’Oglio P, Mazzone E, Autorino R, et al. Contemporary techniques of prostate dissection for robot-assisted prostatectomy. Eur Urol. 2020;78:583–91.
Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643–9.
Checcucci E, Pecoraro A, De Cillis S, Manfredi M, Amparore D, Aimar R, et al. The importance of anatomical reconstruction for continence recovery after robot assisted radical prostatectomy: a systematic review and pooled analysis from referral centers. Minerva Urol Nephrol. 2021;73:165–77.
Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. 2010;58:457–61.
Gragg P, Sellers CL. Twitter. Law Libr J. 2010;102:325–30.
Checcucci E, Veccia A, Fiori C, Amparore D, Manfredi M, Di Dio M, et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int. 2020;125:8–16.
Barakat B, Othman H, Gauger U, Wolff I, Hadaschik B, Rehme C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: which technique is more beneficial for prostate cancer patients (MASTER study)? A systematic review and meta-analysis. Eur Urol Focus. 2022;8:1060–71.
Liu J, Zhang J, Yang Z, Liu Q, Zhang W, Qing Z, et al. Comparison of Retzius-sparing and conventional robot-assisted laparoscopic radical prostatectomy regarding continence and sexual function: an updated meta-analysis. Prostate Cancer Prostatic Dis. 2022;25:47–54.
Chung DY, Jung HD, Kim DK, Lee MH, Lee SW, Paick S, et al. Outcomes of Retzius-sparing versus conventional robot-assisted radical prostatectomy: a KSER update series systematic review and meta-analysis. PLoS One. 2022;17:e0268182.
Ficarra V, Gan M, Borghesi M, Zattoni F, Mottrie A. Posterior muscolofascial reconstruction incorporated into urethrovescical anastomosis during robot-assisted radical prostatectomy. J Endourol. 2012;26:1542–5.
Ficarra V, Rossanese M, Crestani A, Alario G, Mucciardi G, Isgrò A, et al. Robot-assisted radical prostatectomy using the novel urethral fixation technique versus standard vesicourethral anastomosis. Eur Urol. 2021;79:530–6.
Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–7.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30.
Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Dalela D, Jeong W, Prasad MA, Sood A, Abdollah F, Diaz M, et al. A pragmatic randomized controlled trial examining the impact of the Retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2017;72:677–85.
Menon M, Dalela D, Jamil M, Diaz M, Tallman C, Abdollah F, et al. Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the retzius sparing and standard approaches. J Urol. 2018;199:1210–7.
Qiu X, Li Y, Chen M, Xu L, Guo S, Marra G, et al. Retzius-sparing robot-assisted radical prostatectomy improves early recovery of urinary continence: a randomized, controlled, single-blind trial with a 1-year follow-up. BJU Int. 2020;126:633–40.
Asimakopoulos AD, Topazio L, De Angelis M, Agrò EF, Pastore AL, Fuschi A, et al. Retzius-sparing versus standard robot-assisted radical prostatectomy: a prospective randomized comparison on immediate continence rates. Surg Endosc. 2018;33:2187–96.
Egan J, Marhamati S, Carvalho FLF, Davis M, O’Neill J, Lee H, et al. Retzius-sparing robot- assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: single-surgeon series and step-by-step guide. Eur Urol. 2021;79:839–57.
Sayyid RK, Simpson WG, Lu C, Terris MK, Klaassen Z, Madi R. Retzius-sparing robotic-assisted laparoscopic radical prostatectomy: a safe surgical technique with superior continence outcomes. J Endourol. 2017;31:1244–50.
Turkolmez K, Akpinar C, Kubilay E, Suer E. Retzius-Sparing vs modified anatomical structure preserving and Retzius-Repairing Robotic-Assisted Radical Prostatectomy: a prospective randomized comparison on functional outcomes with a 1-year follow-up. J Endourol. 2022;36:1214–22.
Umari P, Eden C, Cahill D, Rizzo M, Eden D, Sooriakumaran P. Retzius-sparing versus standard robot-assisted radical prostatectomy: a comparative prospective study of nearly 500 patients. J Urol. 2021;205:780–90.
Deng W, Jiang H, Liu X, Chen L, Liu W, Zhang C, et al. Transvesical Retzius-Sparing versus standard robot-assisted radical prostatectomy: a retrospective propensity score-adjusted analysis. Front Oncol. 2021;11:687010.
Tahra A, Sen UT, Sobay R, İnkaya A, Kucuk EV, Boylu U. Comparison of Retzius-sparing versus standard robot-assisted radical prostatectomy for prostate cancer. Actas Urol Esp. 2022;46:293–300.
Stonier T, Simson N, Davis J, Challacombe B. Retzius-sparing robot-assisted radical prostatectomy (RS-RARP) vs standard RARP: it’s time for critical appraisal. BJU Int. 2019;123:5–7.
Galfano A, Secco S, Dell’Oglio P, Rha K, Eden C, Fransis K, et al. Retzius-sparing robot-assisted radical prostatectomy: early learning curve experience in three continents. BJU Int. 2021;127:412–7.
Author information
Authors and Affiliations
Contributions
Conceptualisation: VF. Data curation: MR, MG, MF, LM, GM, MM. Investigation: VF. Resources: VF. Formal Analysis: VF. Writing Original draft: VF. Writing Review & Editing: GG. Supervision: VF, GG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ficarra, V., Rossanese, M., Gilante, M. et al. Retzius-sparing vs. standard robot-assisted radical prostatectomy for clinically localised prostate cancer: a comparative study. Prostate Cancer Prostatic Dis 26, 568–574 (2023). https://doi.org/10.1038/s41391-022-00625-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00625-3
- Springer Nature Limited
This article is cited by
-
Single port robot-assisted radical and simple prostatectomy: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2024)
-
Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) during robot-assisted radical prostatectomy: a systematic review and meta-analysis of comparative studies
Prostate Cancer and Prostatic Diseases (2024)
-
Retzius-sparing vs. posterior urethral suspension: similar early-phase post-robotic radical prostatectomy continence outcomes
Journal of Robotic Surgery (2024)
-
Retzius sparing robot-assisted radical prostatectomy: optimizing functional results
World Journal of Urology (2024)