Abstract
Objectives
To assess whether Medicare expenditures for men with incident prostate cancer, treated in Accountable Care Organizations (ACOs) differ from those of men treated in non-ACOs.
Methods
Using the 20% Medicare sample, total charges for 1 year following an initial diagnosis of prostate cancer were abstracted from Medicare claims. Prostate cancer expenditures were calculated by subtracting total charges from the year prior to diagnosis. Propensity score weighting was used to balance baseline characteristics of men treated in ACOs and non-ACOs, and between treatment modalities (radiation, prostatectomy, and expectant management). A propensity score weighted regression model was then used to estimate mean expenditures for men with prostate cancer treated in ACOs and non ACOs and to test the association between ACO status and costs.
Results
We identified 3297 men treated in ACOs for localized prostate cancer versus 24,088 in the non-ACO cohort. The weighted total charges for each treatment modality were $32,358 (radiation), $27,662 (prostatectomy), and $11,134 (expectant management). In our propensity score weighted regression model, the association between charges and ACO status was not significant, nor was the interaction between treatment type and costs. This was true both overall, and in a stratified analysis by treatment type.
Conclusions
There was no significant difference in Medicare spending on prostate cancer care based on provider ACO affiliation, regardless of treatment type. Although the effects of ACOs on clinical care are complex, this study adds to a growing body of evidence suggesting that ACOs fail to achieve significantly lower charges in certain clinical settings.
Similar content being viewed by others
Introduction
Accountable Care Organizations (ACOs) are delivery and payment model designed to encourage efficient, high-value health care, and to encourage integrated delivery of care [1]. Although traditional fee-for-service payment models reward individual providers based on the number and complexity of services provided, the ACO model rewards groups of providers with financial incentives for providing high-quality, coordinated care [2]. The Medicare Shared Savings Program, the nation’s largest ACO, now covers 10.5 million Medicare beneficiaries [3]. In the Shared Savings Program, ACOs receive financial bonuses if they are able to achieve specific quality and financial benchmarks [4].
While Medicare fee-for-service spending was lower in ACO-treated patients [5], the effect varies by care setting: There were greater reductions in low-value services were found in independent primary care groups compared to hospital-affiliated organizations [6] and organizations which historically provided more low-value care [7].
Although much research on ACOs has focused on primary care and preventive services, subspecialty cancer care is characterized by high costs and variations in practice [8, 9]. It may therefore benefit from alternative payment models that encourage efficiency, standardization, and value-based practice [10]. Prostate cancer is an example of a cancer which might be sensitive to value-based payment models. The disease incurs high population-level costs, with multiple possible treatment options, large variation in practice, and potential for over-treatment [11,12,13]. Although the Shared Savings Program includes no prostate cancer-specific benchmarks, cancer care may benefit from “spillover” where overall care improves at institutions that invest to improve coordination and value-based approaches [14].
In prostate cancer care, two recent studies found no changes in PSA screening in ACOs [15, 16]. However, this latter finding may be because prostate cancer screening was not included among the 31 quality metrics within the Shared Savings Program. One noteworthy finding from Borza et al. is that ACOs also may reduce prostate cancer over-treatment of men unlikely to benefit [17]. However, it is not known whether the total expenditures for men with prostate cancer who do decide to undergo treatment are lower in ACOs. In this setting, we compared typical costs between ACOs versus non-ACO’s for men with prostate cancer. We hypothesized that costs of each treatment modality (radiation, surgery, and expectant management) would be lower in ACOs compared to non-ACOs.
Materials and methods
Data source
The data source for the study is the 20% random sample of Medicare claims. We used the Provider-level Research Identifiable File (available from the CMS) to identify ACOs that participated in the Medicare Shared Savings Program in 2013 [18]. Medicare claims data were used to obtain fee-for-service reimbursement data for primary care services (for purposes of ACO attribution).
Study population
Our study cohorts consist of men 67 and older, continuously enrolled in Medicare Part A and B in 2013 and at least 2 years prior with a diagnosis of prostate cancer (ICD-9 = 185). This age range was chosen to ensure that we would have at least 2 years to assess for pre-existing comorbidities and to exclude pre-existing prostate cancer (so as to focus on incident prostate cancer).
Main outcome
The main outcome was the total 1-year charges for prostate cancer care during the year after diagnosis. This was calculated using a previously utilized approach [11]. Costs attributable to prostate cancer care were inferred by subtracting the total Medicare charges of the 12-month period immediately before diagnosis from Medicare charges in the 12 months after diagnosis. This was done to account for baseline spending and to account for the broadest possible number of charges which could be attributed to prostate cancer. We included outpatient, carrier (physician), and inpatient claims, but excluded skilled nursing and long-term care costs, which generally concern the claims for elderly in long-term care facilities and which may artificially inflate total costs [19]. In addition, the number of patients requiring rehab after prostate cancer treatment is low suggesting that the majority of prostate cancer charges are comprised of either outpatient or inpatient charges [20].
Exposure variable
The predictor variable in our analysis was the ACO status of the beneficiary’s primary care provider, ascertained using the following methodology. First, we identified individual ACOs within the Medicare Shared Savings Program based on taxpayer identification numbers in the ACO Provider-level Research Identifiable File. Provider taxpayer identification numbers listed in 2013 were categorized as belonging either to a Shared Savings Program ACO or to a non-ACO.
Second, we assigned beneficiaries to ACOs or non-ACOs using primary care services. We modified the Medicare Shared Savings Program algorithm as suggested by McWilliams et al. [6], to achieve a greater balance between the comparison groups. We did this by attributing beneficiaries based on outpatient primary care services (CPT 99201-99205, 99211-99215, G0402, G0438, and G0439) but not physician services provided in nursing facilities or other settings (CPT 99304-99318, 99324-99340, and 99341-99350) as these services are underrepresented in ACO contracts.
Our choice to focus on primary care providers rather than specialty care was done for two major reasons: First, there are many ACO networks which comprise only primary care providers and we wanted to capture as many possible ACO patients as feasible [1]. Second, ACOs are a team-based model with the primary care provider taking on a key leadership and coordination role [21]. Thus, from a mechanistic and practical perspective it was felt that primary care providers’ ACO status (rather than urologists/radiation oncologists) would be the most relevant for assessing the effectiveness of ACOs.
Covariates
For each beneficiary, the following covariates were assessed: age, race (White, Black, Asian, Hispanic, and other), Charlson-Deyo Comorbidity Index (CCI)—utilizing comorbidities in the year prior to diagnosis (0, 1, or ≥2), and geographic region (South, West, Midwest, Northeast). Treatment type was categorized into three groups based on CPT and ICD-9 procedural codes for radical prostatectomy, radiation, and a third group with no definitive surgery or radiation within 180 days of diagnosis was termed (expectant management). The 180-day cutoff was made because we looked at a single year of charges. If a treatment was performed towards the very end of that year it might not have enough time to alter spending and should be controlled for in our model. The reason we did not control for ADT or other systemic therapies is that some systemic treatments for prostate cancer may include oral agents which would have been captured under a prescription drug benefit (Medicare Part D) and thus not part of our data.
Statistical analyses
Observed differences in baseline characteristics between beneficiaries in the ACO and non-ACO group were controlled using a weighted propensity score analysis. The propensity (probability) of being in the ACO vs. non-ACO group as well as the probability of receiving each treatment modality were estimated using a multinomial logistic regression model including the above covariates. Each beneficiary was weighted by the inverse probability of being in the ACO group and year, with the goal of balancing observable characteristics between the groups [22]. Balance between the covariates in weighted groups was evaluated using chi-squared for categorical variables and linear regression for continuous variables [23].
The costs of treatment in ACO and non-ACO cohorts for each treatment modality were estimated using propensity score-weighted generalized estimating equations linear model (costs as a function of group) with empirical standard error estimates, with a main outcome of annual prostate cancer costs. This type of model provides correct estimates (means) as long as the underlying propensity model balances out confounders across groups. The propensity score-weighted generalized estimating equations estimate of the mean does not depend on any specific distribution of the outcomes and is robust to misspecification of within-subject (due to clustering) variance. To account for the effect of significant outliers, we employed propensity score weight trimming to diminish the impact of extremely large weights [24].
Estimated mean costs with 95% confidence intervals were reported for all three “treatment” types in ACO and non-ACOs. The association between ACO status and costs was evaluated by testing the effect of ACO status on mean costs. We also tested the interaction between ACO status and treatment modality by using interaction terms which combined treatment and costs. This was done in order to assess whether the effect of ACOs on treatment costs might vary in a statistically significant fashion between the three treatment categories.
All statistical analyses were performed with SAS 9.4 (SAS, Cary, NC) software. Two-sided statistical significance was defined as P > 0.05. Code available on request. To account for correlation within provider groups, statistical calculations were clustered by provider tax identification number. Ethical approval was obtained from the Brigham and Women’s Hospital IRB under a general protocol for research on trends in cancer care using Medicare Claims.
Results
Using the above selection criteria, we identified male Medicare beneficiaries within our 20% random Medicare sample, who were diagnosed with prostate cancer in 2013 and who had at least two preceding years of Medicare claims. This consisted of a total of 241,348 men with prostate cancer diagnoses (ICD-9 185) in 2013. After excluding those with prostate cancer in 2 years preceding the study year (2011 and 2012, in order to identify those only with newly diagnosed prostate cancer) we were left with a total cohort of 51,978 men. Of these, a total of 27,665 men had sufficient Medicare claims for relevant primary care services to allow for attribution of PCP status to either ACO- or non-ACO providers tax identification numbers using the attribution algorithm described above.
The characteristics of prostate cancer patients treated within ACO and non-ACOs are described in Table 1. On average, patients in ACOs were more commonly white, were more often treated in the northeastern United States, and had lower comorbidity burden. After propensity score weighting, both ACO and non-ACO groups did not differ significantly (p > 0.10 for all covariates).
In total, 78.7% of men received expectant management. Of men who received definitive treatment more men chose radiation therapy. See Table 1.
After weighting, there was no statistically significant difference in Medicare charges between men in ACO versus non-ACO cohorts, regardless of treatment category (p = 0.61).
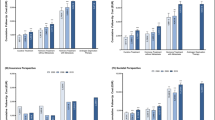
In our stratified analysis by treatment, the mean weighted charges for patients receiving prostatectomy was $27,662, which was significantly lower than the mean charges of men receiving radiation at $32,358. Both were higher than the mean charges in the expectant management cohort, which incurred average charges of $11,134 (p < 0.0001 for all modalities). Although there were large and statistically significant differences in costs between modalities (Table 2), the interaction of treatment and ACO status was not significantly associated with costs (p = 0.13 and p = 0.15). This suggests that the effect of ACOs on charges did not exert a differential effect depending on treatment modality. The difference in estimated costs of treating ACO and non-ACO treated men in each treatment category are shown in Fig. 1.
Discussion
In this retrospective study of Medicare beneficiaries, we found that there was no association between ACO status and total Medicare charges for prostate cancer care during the 1-year period following diagnosis. This was the case regardless of treatment modality. Although radiation incurred the highest costs, followed by radical prostatectomy and expectant management, the interaction between ACO status and treatment type was not significantly associated with cost.
Overall, our findings add to a growing, but inconsistent body of research on ACOs’ impact on prostate cancer care. For example, ACOs were not associated with improvements in several perioperative outcomes such as inpatient mortality and prolonged length of stay, but they were associated with lower readmission rates [25, 26]. Borza et al. found a secular trend for overall reduction in overtreatment for prostate cancer from 2010 to 2013 in Medicare beneficiaries but that ACO status did not impact an overall background trend of declining charges [17]. Interestingly, Hollenbeck et al. found that ACOs were associated with a significant trend towards increased spending in men treated surgically for prostate cancer. However in that study, the authors “attributed each patient to the practice affiliation of his primary urologist.” [14] In contrast, our work attributed ACO status based on tax identification number of primary care provider groups (e.g. where the patient received the majority of their primary care). Although both approaches have potential strengths, we felt that primary care services for ACO attribution was a better approach for multiple reasons. First, ACO participation by urologists remains low, resulting in a potential loss of many men with prostate cancer [27]. Second, much of the theory behind ACOs is based on an integrated delivery system with ACO affiliated primary care providers taking on a “team leader” role. This approach of attributing ACOs based on primary care providers has been used in other high-impact studies of ACOs, including studies on specialty and cancer care [5, 6, 17]. It is also the approach endorsed by CMS regarding calculation of financial payments for ACO quality benchmarks (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/Attribution-Fact-Sheet.pdf).
Our finding of significant variability in prostate cancer surgical costs (95% CI in ACOs of $8052–71,034) is also consistent with earlier work, which found a sixfold difference in direct costs between surgeons [12]. Although this prior work reported a significantly lower average cost for prostate surgery ($11,878 95% CI $11,804–11,952), the focus was on hospital line-item costs for the index hospitalization, not total costs. In addition, that study focused only on direct surgical costs including surgery and 90 days thereafter, whereas the current study encompasses all Medicare charges in the year of diagnosis for these patients. When compared with research published by Nguyen et al., which employed a similar methodology to assess costs of prostate cancer care using Medicare claims, our study found similar costs [11].
Other strengths include our use of the 20% random Medicare sample, which provides a true population-based sample of the US elderly. In addition, by using a propensity score weighted regression we could simulate the effects of random allocation into ACO and non-ACO cohorts and thereby estimate treatment-specific costs in ACO and non-ACO practice settings. Other assets include our use of a full year’s-worth of claims post-diagnosis to better compare costs between treatment groups. This may be more accurate when comparing treatments like radiation and surgery (with high one-time costs) against management strategies like active surveillance or watchful waiting (where costs may be more spread out across many visits).
Our findings should be considered in light of certain limitations. First, patients and providers were not randomized to ACO and non-ACO cohorts. Given that ACO participation is voluntary, there are likely to be intrinsic differences between ACO and non-ACO providers, even before contracts. Additionally, because our study design was cross-sectional, we were not able to control for providers’ baseline (pre-ACO) costs. Similarly, while we employed propensity score weighting to balance covariates between the groups of patients, there may well be unmeasured characteristics which are more common in ACO or non-ACO providers and which could be associated with high or low costs.
Another important limitation relates to our use of Medicare claims—which do not include information on tumor stage or grade. Finally, we are somewhat limited by the age of most Medicare beneficiaries. With few exceptions, Medicare serves mainly the elderly (65 and older). In contrast, a significant portion of the high costs and variability in prostate cancer costs are found in middle aged men [12]. This could explain why the proportion of men receiving expectant managment is high.
Finally, we attributed charges to prostate cancer care by comparing Medicare charges in the year before and after prostate cancer diagnosis. This has been used in prior studies [11]. We chose to do this based on the inherent limitations of attributing post-diagnosis claims to prostate cancer versus non-cancer related conditions. With that said, this method may fail to account for some natural year to year age-related progression in health care costs. For example, if ACOs better control non-prostate cancer health care costs, then it may mask an increase in prostate cancer cost controls or vice versa.
Regardless of these limitations, this work has several important policy implications. It adds to a growing body of evidence suggesting that the benefit of ACOs on certain areas of specialty care such as oncology, cardiology, and mental health may be limited [14, 28, 29]. Although there is generally widespread agreement that fee-for-service payments can result in perverse incentives, there is an ongoing debate about the best ways to achieve more value-based care delivery [30]. Certain researchers strongly advocate capitated systems such as ACOs. It is hoped that these may lead to more integrated care delivery which reduces costs.
Overall, the effect of ACOs on cancer care seems to be less than in chronic disease care (where care coordination and reduction in duplicated care may be significant). One reason may be related to the tendency for cancer care be performed within hospital settings. Another factor may be the tendency for cancer care be performed within hospital-integrated care settings. As noted above, a recent study found that the impact of ACOs was greatest in independent, provider-led primary groups (rather than hospital-integrated) provider groups [6]. If cancer care tends to be performed more in hospital-based settings, then this could attenuate potential benefits of ACOs. From a practical perspective, many cancer specialists work within cancer-focused referral centers. Thus, even though ACO-affiliated primary care providers are supposed to function as “team leaders” who can coordinate care, the actual decision making around cancer care (e.g. on matters such as repeating imaging, choosing high versus low-cost studies, and surgery versus radiation) may depend little on primary care provider ACO affiliation. This may further the case made by some that other alternative payment models make more sense in surgical and subspecialty care [31, 32].
Conclusions
In this cross-sectional study of United States Medicare beneficiaries with prostate cancer, we found that expenditures for men whose providers enrolled in the Medicare Shared Savings Program did not differ significantly from those treated by non-ACO providers. Although the effects of ACOs on clinical care are complex, this study adds to a growing body of evidence suggesting that ACOs may fail to achieve significantly lower costs in certain areas of specialty care.
References
Fisher ES, McClellan MB, Bertko J, Lieberman SM, Lee JJ, Lewis JL, et al. Fostering accountable health care: moving forward in medicare. Health Aff (Millwood). 2009;28:w219–31.
Devore S, Champion RW. Driving population health through accountable care organizations. Health Aff (Millwood). 2011;30:41–50.
MSSP ACO Fact Sheet: Centers for Medicare and Medicaid Services; 2017. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/All-Starts-MSSP-ACO.pdf.
Federal Register: Medicare Shared Savings Program: accountable care organizations: Centers for Medicare and Medicaid Services; 2015. p. 32691-845.
McWilliams JM, Chernew ME, Landon BE. Medicare ACO program savings not tied to preventable hospitalizations or concentrated among high-risk patients. Health Aff (Millwood). 2017;36:2085–93.
McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374:2357–66.
Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175:1815–25.
Leow JJ, Cole AP, Seisen T, Bellmunt J, Mossanen M, Menon M, et al. Variations in the costs of radical cystectomy for bladder cancer in the USA. Eur Urol. 2018;73:374–82.
Brooks GA, Li L, Sharma DB, Weeks JC, Hassett MJ, Yabroff KR, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105:634–42.
Ellimoottil C, Miller DC. Anticipating the effect of the Patient Protection and Affordable Care Act for patients with urologic cancer. Urol Oncol. 2014;32:55–8.
Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–24.
Cole AP, Leow JJ, Chang SL, Chung BI, Meyer CP, Kibel AS, et al. Surgeon and hospital level variation in the costs of robot-assisted radical prostatectomy. J Urol. 2016;196:1090–5.
Aizer AA, Gu X, Chen MH, Choueiri TK, Martin NE, Efstathiou JA, et al. Cost implications and complications of overtreatment of low-risk prostate cancer in the United States. J Natl Compr Canc Netw. 2015;13:61–8.
Hollenbeck BK, Kaufman SR, Borza T, Yan P, Herrel LA, Miller DC, et al. Accountable care organizations and prostate cancer care. Urol Pract. 2017;4:454–61.
Luckenbaugh AN, Hollenbeck BK, Kaufman SR, Yan P, Herrel LA, Skolarus TA, et al. Impact of accountable care organizations on diagnostic testing for prostate cancer. Urology. 2018;116:68–75.
Trinh QD, Sun M, Krasnova A, Ramaswamy A, Cole AP, Fletcher S, et al. Impact of accountable care organizations on prostate cancer screening & biopsies in the United States. Urol Pract. 2018. https://doi.org/10.1016/j.urpr.2018.07.003. [Epub ahead of print].
Borza T, Kaufman SR, Yan P, Herrel LA, Luckenbaugh AN, Miller DC, et al. Early effect of Medicare Shared Savings Program accountable care organization participation on prostate cancer care. Cancer . 2018;124:563–70.
Cole AP, Friedlander DF, Trinh QD Secondary data sources for health services research in urologic oncology. Urol Oncol. 2018;36:165.
Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV104–IV117.
Trinh QD, Bianchi M, Sun M, Sammon J, Schmitges J, Shariat SF, et al. Discharge patterns after radical prostatectomy in the United States of America. Urol Oncol. 2013;31:1022–32.
Lustig A, Ogden M, Brenner RW, Penso J, Westrich KD, Dubois RW. The central role of physician leadership for driving change in value-based care environments. J Manag Care Spec Pharm. 2016;22:1116–22.
Cole AP, Trinh QD. Secondary data analysis: techniques for comparing interventions and their limitations. Curr Opin Urol. 2017;27:354–9.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Hawken SR, Herrel LA, Ellimoottil C, Montgomery ZA, Ye Z, Miller DC. Understanding pre-enrollment surgical outcomes for hospitals participating in Medicare Accountable Care Organizations. Am J Surg. 2016;211:998–1004.
Borza T, Oerline MK, Skolarus TA, Norton EC, Dimick JB, Jacobs BL, et al. Association between hospital participation in medicare shared savings program accountable care organizations and readmission following major surgery. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002737. [Epub ahead of print].
Hawken SR, Herrel LA, Ellimoottil C, Ye Z, Hollenbeck BK, Miller DC. Urologist participation in medicare shared savings program accountable care organizations (ACOs). Urology. 2016;90:76–81.
Busch AB, Huskamp HA, McWilliams JM. Early efforts by medicare accountable care organizations have limited effect on mental illness care and management. Health Aff (Millwood). 2016;35:1247–56.
McWilliams JM, Najafzadeh M, Shrank WH, Polinski JM. Association of changes in medication use and adherence with accountable care organization exposure in patients with cardiovascular disease or diabetes. JAMA Cardiol. 2017;2:1019–23.
Miller HD. From volume to value: better ways to pay for health care. Health Aff (Millwood). 2009;28:1418–28.
Porter ME, Kaplan RS. How to pay for health care. Harv Bus Rev. 2016;94:18.
James BC, Poulsen GP. The Case for Capitation. (cover story). Harv Bus Rev. 2016;94:102–11.
Acknowledgements
Q.-D.T. is supported by the Brigham Research Institute Fund to Sustain Research Excellence, the Bruce A. Beal and Robert L. Beal Surgical Fellowship, the Genentech Bio-Oncology Career Development Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology (grant # 10202), a Health Services Research pilot test grant from the Defense Health Agency, the Clay Hamlin Young Investigator Award from the Prostate Cancer Foundation (grant # 16YOUN20), and an unrestricted educational grant from the Vattikuti Urology Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Q.-D.T. reports honoraria from Astellas, Bayer, and Jannseen and research funding from Bayer and Intuitive Surgical. A.S.K. reports consulting fees from Profound, Janssen, and ConfirmMDX. T.K.C. has received research funding from Pfizer and has an advisory role at Pfizer, Novartis, GlaxoSmithKline, Genentech, and Bayer. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cole, A.P., Krasnova, A., Ramaswamy, A. et al. Prostate cancer in the medicare shared savings program: are Accountable Care Organizations associated with reduced expenditures for men with prostate cancer?. Prostate Cancer Prostatic Dis 22, 593–599 (2019). https://doi.org/10.1038/s41391-019-0138-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0138-1
- Springer Nature Limited