Abstract
The highest incidence of sepsis across all age groups occurs in neonates leading to substantial mortality and morbidity. Cardiovascular dysfunction frequently complicates neonatal sepsis including biventricular systolic and/or diastolic dysfunction, vasoregulatory failure, and pulmonary arterial hypertension. The haemodynamic response in neonatal sepsis can be hyperdynamic or hypodynamic and the underlying pathophysiological mechanisms are heterogeneous. The diagnosis and definition of both neonatal sepsis and cardiovascular dysfunction complicating neonatal sepsis are challenging and not consensus-based. Future developments in neonatal sepsis management will be facilitated by common definitions and datasets especially in neonatal cardiovascular optimisation.
Impact
-
Cardiovascular dysfunction is common in neonatal sepsis but there is no consensus-based definition, making calculating the incidence and designing clinical trials challenging.
-
Neonatal cardiovascular dysfunction is related to the inflammatory response, which can directly target myocyte function and systemic haemodynamics.
Similar content being viewed by others
Introduction
Sepsis has been declared a global health priority by the World Health Organisation.1 The highest sepsis incidence across all age groups occurs in neonates, affecting an estimated 22 per 1000 live births with a mortality ranging from 3 to 19% and an unquantified burden of long-term neurological impairment.2,3 However, these data are imprecise due to the absence of internationally accepted unified diagnostic criteria or a definition of neonatal sepsis which has led to difficulty in both diagnosis and in forming an evidence-based approach to treatment.4 Sepsis-induced cardiovascular dysfunction includes myocardial dysfunction; vasoregulatory failure, which may lead to systemic vasodilation or vasoconstriction; and pulmonary hypertension (PHT), which is seen in approximately 50% of septic neonates.5 Neonatal sepsis may induce a low cardiac output state or a hyperdynamic circulation.6 Cardiovascular dysfunction complicating sepsis increases mortality and has therefore been extensively studied in the adult and paediatric literature.7,8 However, there remains a dearth of studies in the neonatal literature which specifically address the cardiovascular consequences of sepsis.
In this review, we summarise these data to date focussing on the mechanisms and pathophysiology of sepsis-induced cardiovascular dysfunction in neonates. Intrauterine infection is a major cause of early preterm birth and is present in approximately a quarter of all preterm deliveries.9 Thus, we also examine the relationship between intra-uterine infection, the fetal inflammatory response syndrome and fetal cardiovascular dysfunction. Challenges inherent to the assessment and management of cardiovascular dysfunction in neonatal sepsis, goal-directed therapy, and the importance of an individualised patient approach are discussed in the second and third papers of this series.
Definitions of neonatal sepsis and cardiovascular dysfunction
Sepsis is a syndrome identified by a constellation of signs and symptoms in a patient with a suspected infection. Sepsis was previously defined as the systemic inflammatory response syndrome (SIRS) that occurs during infection. However, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) has recently defined sepsis in adults as life-threatening organ dysfunction caused by a dysregulated response to infection,10 moving away from the SIRS definition. Such organ dysfunction is identified by an acute increase in the sequential organ failure assessment (SOFA) score consequent to infection. The International Consensus defined septic shock as a subset of sepsis in which particularly profound circulatory, cellular and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone.10 Clinically, the Sepsis-3 definition correlated septic shock, in the adult population, with a vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and a serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolaemia. This combination of findings is associated with a hospital mortality rate of over 40%. The definition of sepsis for children is also in flux.11 In 2005, the International Pediatric Sepsis Consensus Conference (IPSCC) definitions were introduced in order to enable enrolment in a clinical trial; however, these definitions have never been validated. Concerns exist regarding the application of the Sepsis-3 definitions to children, due to the difference in pathophysiological mechanisms in paediatric sepsis and the fact that the paediatric SOFA score has not yet been well validated.12,13
Similarly, there remains no consensus definition of sepsis for neonates, in whom clinicians still predominantly rely on microbiological results rather than organ dysfunction.14,15,16,17 A recent systematic review catalogued the current definitions of sepsis in 80 published randomised controlled trials and found a diverse range of definitions used.4 These were based on microbiological culture, laboratory tests and clinical signs in contrast to the adult and paediatric definitions, which use organ dysfunction. A positive microbial culture remains the gold standard of diagnosis in many units, despite data from two large randomised controlled trials demonstrating culture-negative sepsis rates of 46% and 56% in preterm infants treated for clinically suspected sepsis.18,19 These data pose further questions regarding the definition of ‘culture-negative sepsis’.
Therefore in adult and paediatric medicine, there has been a paradigm shift to define sepsis by organ dysfunction, including cardiovascular dysfunction, which is characterised by the SOFA score. In neonates, an electronic health record-automated neonatal SOFA score was recently proven to predict mortality in very low birth weight (VLBW) neonates with late-onset sepsis (LOS)20 (Table 1). This has been evaluated in a multicentre cohort including 653 preterm infants with LOS and found to predict mortality, implying generalisability and the potential to serve as the foundation for a consensus definition of neonatal sepsis.21 The cardiovascular section of the neonatal SOFA score is based on the requirement for inotropes and/or systemic corticosteroid treatment. Medications considered as inotropic or vasoactive included dopamine, dobutamine, epinephrine, norepinephrine, vasopressin, milrinone, and phenylephrine. The vasoactive inotropic score (VIS) has also been used as a surrogate to define cardiovascular dysfunction. Using the maximum score (VISmax), based on the maximum dose load of vasoactive medications, has been shown to predict mortality in premature infants,22,23 and in neonates with sepsis.24 The problem with such definitions is that they score the use, rather than the need, for such therapy and therefore instead of directly evaluating the cardiovascular dysfunction, they assess the response of the clinician. NICUs using such therapies early or more generously will seem to have ‘sicker’ babies. Nevertheless, the validity of a neonatal SOFA score needs to be established for neonates of all gestational ages with suspected sepsis of both early- and late-onset, and including those with negative cultures but where there is a high index of clinical suspicion. A validated score for neonatal sepsis would address a critical unmet need for an objective operational definition for cardiovascular dysfunction and for sepsis in neonates to align international investigators and aid future international prospective clinical trials. Further work is also currently being undertaken in order to standardise outcome reporting in neonatal sepsis trials by developing a core outcome set.16
Epidemiology
The epidemiology of cardiovascular dysfunction in neonatal sepsis is difficult to describe due to the lack of consensus definitions. However, the use of vasoactive medications is often used as a surrogate, taking into account the limitations already described, and has clearly been associated with increased mortality among septic neonates. Among infants with fatal sepsis, 40% received vasoactive medications in one study.25 In another large prospective study, 26% of neonates with sepsis had hypotension that was treated with catecholamine and this more than tripled mortality when present.26 Hypotension requiring catecholamine treatment is more common in EOS than LOS. When defining septic shock as hypotension or need for fluid bolus or vasoactive drugs, there was a 40% mortality rate in one neonatal cohort, with 19% of survivors suffering severe sequelae at 18-month follow-up (including neurodevelopmental sequelae and short gut syndrome secondary to complications of necrotising enterocolitis).27
If we consider myocardial dysfunction specifically, there have been no large prospective echocardiography studies which have documented the prevalence of myocardial dysfunction in neonatal sepsis and how this relates to outcomes. The results of the studies to date, which have been variable, are discussed below.
Sepsis-induced cardiovascular dysfunction
The neonatal cardiovascular response to sepsis differs from that seen in older children and adults and may result in a hyperdynamic or a hypodynamic circulation. The hyperdynamic response (‘warm shock’) is characterised by decreased systemic vascular resistance (SVR) and increased heart rate (HR) and cardiac output (CO), resulting in bounding pulses, hypotension and a brisk capillary refill time. The increase in cardiac output is dependent on adequate pre-load and preserved myocardial function. The hypodynamic phase (‘cold shock’) is characterised by decreased cardiac output and increased SVR, clinically detectable as cool, pale peripheries, reduced volume pulses and prolonged capillary refill time. Adult patients typically have a biphasic response, the initial period being hyperdynamic followed by a hypodynamic period. In adults, severe peripheral vasodilation is likely a major determinant of mortality28 and often requires vasopressors. Paediatric patients can present as either hyperdynamic or hypodynamic, but the majority have an initial high SVR and low cardiac output state.29 In paediatric patients, myocardial dysfunction appears to be the major predictor of mortality.30,31
Comparatively, in neonates, the haemodynamic features of sepsis are less well defined, complicated by the fact that blood pressure is a poor marker of systemic blood flow in this patient group and values for low blood pressure are not well defined for all gestational ages.32 However there are some significant differences established in neonates compared to older children and adults; for example, there is more severe circulatory impairment and more evident PHT. There is even less literature available describing the haemodynamic pattern of sepsis specifically in preterm or VLBW neonates, but it suggests that they predominantly present with a warm shock, hyperdynamic phenotype, postulated to be in part due to the immaturity of the neonatal autonomic nervous system compounding the vasoregulatory failure seen with sepsis,6,33,34,35,36,37 exacerbated by relative adrenal insufficiency in some cases. One study of preterm infants with a median gestation of 27 weeks and LOS demonstrated, using echocardiography, that after initial volume infusion, the infants had relatively high left and right ventricular output, low SVR and normal blood pressure.6 Survivors maintained low SVR and adequate ventricular output, while the non-survivors had lower initial blood pressures and cardiac outputs that decreased substantially on subsequent measurements. These findings have been replicated.35 Adrenal insufficiency and decreased vascular responsiveness to circulating catecholamines can contribute to vasopressor-resistant shock in both term and preterm infants.38,39,40,41 In premature infants, lower cortisol levels following ACTH testing are associated with adverse outcomes.42 Relative adrenal insufficiency as a contributor to hypotension is supported by the fact that hydrocortisone administration rapidly improves cardiovascular status in preterm infants with volume- and pressor-resistant hypotension.43,44,45
Preterm infants are predisposed to PHT due to their baseline elevated pulmonary vascular resistance (PVR) as a result of a lower capacity vascular bed and the fact that they often have respiratory co-morbidities.46 In one recent study, almost half of term and preterm infants with LOS had echocardiographic evidence of PHT.5 This was significantly increased compared to non-septic controls. The proportion of PHT increased with increasing gestational age. Another study, which included 67 infants with culture-positive LOS with a median gestational age of 33 weeks, reported increased pulmonary artery pressures in a quarter of patients.37 Neonatal animal models of Group B streptococcus shock documented increased PVR, SVR and mesenteric vascular resistance with decreased cardiac output.47,48 Sepsis-induced acidosis and hypoxaemia, as well as inflammatory cytokines including TNF-alpha and toxic metabolites from altered nitric oxide metabolism can increase PVR, pulmonary artery pressure and maintain patency of the ductus arteriosus (PDA).49,50 This is most commonly seen in EOS and is associated with tricuspid regurgitation and hepatomegaly. Raised PVR can cause acute right ventricular failure. This affects the left ventricle (LV) in two ways. Firstly, decreased pulmonary venous return and therefore reduced LV preload impairs systolic function via the Frank–Starling mechanism. Secondly, dilatation of the right ventricle in the setting of raised PVR directly impairs left ventricular systolic and diastolic function by displacement of the interventricular septum due to ventricular inter-dependence. Ultimately, this leads to reduced left ventricular output and systemic hypoperfusion.
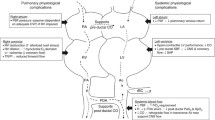
Changes in SVR depending on whether an infant is in the hypodynamic or hyperdynamic state can cause profound myocardial dysfunction. For example, vasodilation decreases venous return to the right side of the heart and subsequently to the left, the resulting tachycardia reduces diastolic filling time, and ultimately, the left ventricular output is impaired. In a hypodynamic state, the raised SVR may directly reduce the LV systolic and diastolic function and cause increased end-systolic and end-diastolic left ventricular pressures (see Fig. 1).
Cytokines, products of the complement cascade, nitric oxide release and decreased response to catecholamines lead to vasoregulatory dysfunction and myocardial dysfunction. Arterial and venodilation results in hypotension and pooling of blood in the capacitance veins leading to low preload and shock. Myocardial dysfunction secondary to reduced preload, mitochondrial impairment and abnormalities in calcium-sensing leads to ventricular dilation and dysfunction. Factors unique to neonates such as increased pulmonary vascular resistance in response to inflammatory mediators causing pulmonary hypertension, right-to-left shunts at patent ductus arteriosus (PDA) and patent foramen ovale (PFO) contribute to hypoxaemia, right ventricular (RV) afterload and exacerbate cardiac dysfunction.
Newborns demonstrate several differences in cardiac developmental physiology which affect their ability to cope with sepsis and its cardiovascular effects. For example, the neonatal heart has fewer contractile elements compared to older children and adults.51 In the heart, Type I collagen mainly provides rigidity, and Type III collagen, elasticity.52 The neonatal heart has a high content of collagen compared to the number of myocytes and an increased Type I collagen/Type III collagen ratio, which results in more stiff, less compliant ventricles, which are less able to increase stroke volume or cardiac contractility in the case of sepsis.52 This is further limited by the fact the neonatal ventricular myocardium has a high basal contractile state.53,54 These issues cause relative diastolic dysfunction and are compounded by the increased heart rate of neonates. The neonatal heart is therefore at the flatter end of the Frank–Starling curve compared to older children and adults and therefore a similar change in preload will result in a less significant increase in stroke volume.55 Preterm infants are even more vulnerable due to a low myocardial contractile reserve,56 exposing them to increased risk of hypotension and low-cardiac output states. The LV myocardium comprises circumferential fibres in the mid-wall layer and longitudinal fibres in the endocardial and epicardial layers. Collectively, these layers allow the base of the heart to rotate in a clockwise motion while the apex simultaneously rotates in an anticlockwise motion. The net effect of these two forces is myocardial twist, a ‘wringing’ motion that is thought to support both systolic (during ‘twisting’) and diastolic (during ‘untwisting’) left ventricular function. Preterm infants have been reported to demonstrate increased twist and LV torsion (LV twist indexed to LV length) compared to term infants.57 This is thought to be a compensatory mechanism for the decreased ventricular compliance in preterm infants to increase stroke volume. Other features of immaturity of neonatal myocardium also render it more vulnerable to dysfunction during sepsis including a higher water content, greater surface-to-volume ratio, immature sarcoplasmic reticulum and a reliance on extracellular calcium stores.58 These mechanisms explain why neonates, and especially premature neonates, are particularly sensitive to changes in afterload such as that which may occur with increased PVR (affecting the right ventricle) or raised SVR (affecting the left ventricle) and which are compounded by the negative impact of inflammation on myocardial contractility.59
Adults with sepsis or septic shock may demonstrate myocardial dysfunction along a continuum of isolated diastolic dysfunction to both systolic and diastolic failure of the left and/or right ventricle, which is not ischaemic in nature and completely reversible.60,61,62,63 This has come to be termed sepsis-induced myocardial dysfunction (SIMD) or sepsis-induced cardiomyopathy; a transient myocardial dysfunction in septic patients. There are no formal diagnostic criteria to date; however, experts agree on several key features in the adult population including acute and reversible changes (dependent on survival), global biventricular dysfunction (systolic and/or diastolic), left ventricular dilatation, diminished response to fluid resuscitation and catecholamines.64 These features occur in the presence of sepsis and in the absence of an acute coronary syndrome. In adults, ventricular function generally returns to normal within 7–10 days.65 The prevalence of SIMD in adult patients with sepsis or septic shock ranges from 10 to 70% due to differing definitions of both sepsis and SIMD in the literature.66 For example, in one series of adult patients with septic shock, a third had decreased LV systolic function with a mean LV ejection fraction of 38%.67 However, ejection fraction may not be an accurate measure of sepsis-induced myocardial dysfunction, and this may underestimate the true prevalence. Interestingly, in an adult post-mortem study of patients who died of sepsis and/or septic shock, over half demonstrated myocardial injury.68 In adults, the presence of myocardial dysfunction in sepsis is associated with a significantly increased mortality rate of 70–90% compared to 20% mortality in those without myocardial dysfunction.69,70 In one paediatric study 71% of patients with septic shock had SIMD; diastolic dysfunction was more common than systolic and the mortality was higher for patients with SIMD compared to those with septic shock but preserved myocardial function.71
The prevalence of neonatal myocardial dysfunction in sepsis is unknown due to the lack of definitions of both neonatal sepsis and neonatal myocardial dysfunction in sepsis. There is also a dearth of studies in the literature which have evaluated echocardiographic findings in neonatal sepsis and the results are variable. Regarding fractional shortening and ejection fraction in neonatal sepsis compared to controls; some studies have demonstrated a significant reduction in these functional parameters,72,73 while others have not.35,37,74,75,76,77 One neonatal study showed that of the septic infants, left ventricular fractional shortening was significantly higher in the survivors compared to those who died.75 Left ventricular diastolic dysfunction based on mitral inflow patterns has been demonstrated in term and preterm neonates with sepsis compared to non-septic controls.75,76 Advanced echocardiography techniques such as tissue doppler imaging and myocardial deformation imaging by speckle tracking echocardiography have demonstrated reduced right and left ventricular systolic function in septic neonates compared to the healthy controls.74,77 This is despite conventional echocardiography parameters of cardiac index, fractional shortening and atrioventricular inflow velocity patterns showing no significant difference between the two groups, suggesting that these advanced techniques are more sensitive to detect early myocardial damage in neonatal sepsis.
In order to improve the reporting of neonatal myocardial dysfunction seen in sepsis, we suggest a definition of neonatal sepsis-induced myocardial dysfunction as acute globally reduced biventricular systolic and/or diastolic dysfunction, associated with diminished response to catecholamines, often associated with pulmonary hypertension, and completely reversible dependent on survival. This proposed definition is based on the paucity of studies to date which have described the features of myocardial dysfunction in neonatal sepsis but there is a need for further echocardiography studies to improve the characterisation of the condition. Differences compared to the adult definition include the removal of both LV dilatation and decreased responsiveness to fluid therapy as features. This is because LV dilatation has not been consistently reported in the neonatal sepsis literature, perhaps due to the higher contractile state of the neonatal heart and its relative stiffness. The approach to fluid therapy is different in neonatal sepsis compared to adults and therefore response to fluid therapy is not as relevant. This definition would require echocardiography to enable objective evaluation of myocardial dysfunction; however, neonatologist-performed functional echocardiography is increasingly becoming a standard assessment tool in the neonatal unit and enables accurate and reproducible measurements.
Pathophysiological mechanisms of sepsis-induced cardiovascular dysfunction
Sepsis is characterised by an inappropriate immune response to infection. The pathophysiology of cardiovascular dysfunction in sepsis involves a complex interplay of host and pathogen processes. Injection of lipopolysaccharide (LPS) in human adult volunteers has demonstrated significant myocardial depression and left ventricular dilatation within 5 h.78 The underlying pathophysiology which leads to cardiovascular dysfunction in sepsis is complex and incompletely understood. There are three important categories to consider: myocardial dysfunction, vasoregulatory dysfunction, and complicating factors associated with the neonatal period.
Mechanisms of myocardial dysfunction include decreased right ventricular preload (secondary to vasodilation and increased vascular permeability), altered myocardial microcirculation with endothelial disruption and maldistribution of blood flow,79,80 circulating myocardial depressant factors (cytokines, components of the complement cascade),81 abnormalities of beta-adrenergic signal transduction causing attenuated adrenergic response,82 mitochondrial dysfunction and abnormal intracellular calcium regulation,83 myocardial oedema leading to impaired myocardial compliance and function,84,85,86 cardiomyocyte injury and cell death,87,88 and reduced right ventricular function secondary to increased afterload in the context of PHT. Vasoregulatory dysfunction includes vasodilation (seen in ‘warm shock’), vasoconstriction (seen in ‘cold shock’) and endothelial dysfunction which is associated with impaired endothelium-derived nitric oxide release. The peripheral vasodilation is multifactorial but critical mechanisms which lead to vascular smooth muscle relaxation are excess nitric oxide production, activation of potassium-ATP channels, which are directly activated by hyperlactataemia and vasopressin deficiency89 (see Fig. 2). Complicating factors associated with the neonatal period include the presence of a patent ductus arteriosus, the risk of persistent pulmonary hypertension of the newborn, and developmental differences in haemodynamic responses based on gestational age.
Release of cytokines and other mediators in response to sepsis results in endothelial dysfunction, increased vascular permeability and loss of vasomotor response to catecholamines. The loss of glycocalyx (lining shown in green) covering the endothelium, increases the risk for thrombosis and tissue ischaemia. Increased permeability results in capillary leak and tissue oedema. Release of vasodilators such as nitric oxide and poor response to catecholamines leads to vasodilation and shock.
The immune system detects microorganisms through recognition of pathogen-associated molecular patterns (PAMPs). PAMPs include LPS, lipoteichoic acid, flagellin and DNA in bacteria; RNA in viruses; and mannan in fungi.90 Pattern-recognition receptors (PRRs), for example, toll-like receptors (TLRs), are expressed on the surface of host cells and recognise and bind to PAMPs.91 In binding to PAMPs, PRRs initiate host immune responses.92,93 These receptors also recognise endogenous damage-associated molecular patterns (DAMPs) and respond by activating the innate immune system. In infection, these mechanisms induce a pro-inflammatory milieu which is usually balanced by anti-inflammatory cytokines. However, in sepsis, there is a dysregulated and imbalanced pro-inflammatory response. Pro-inflammatory cytokines which play a major role in sepsis include tumour necrosis factor-alpha (TNF-alpha), interleukin-1-beta (IL-1-beta), interleukin-6 (IL-6) and interleukin-8 (IL-8).94 Alterations in nitric oxide metabolism in sepsis produce toxic metabolites which cause adjacent tissue damage and further amplify the inflammatory response.95 Cellular injury liberates further DAMPs, mitochondrial DNA (mtDNA) and adenosine triphosphate (ATP), which all contribute to the activation of the immune system and a vicious pro-inflammatory cycle.90 These products of the sepsis cascade mediate cardiovascular dysfunction directly (for example, by disrupting myocyte calcium traffic and thus decreasing contractility: TNF-alpha, IL-1-beta, nitric oxide) and indirectly (by perpetuating the inflammatory cascade).96 The inflammatory response to sepsis differs in neonates compared to older children and adults. Crucially, term and preterm neonates have a diminished capacity to produce anti-inflammatory cytokines as a compensatory response in sepsis.97 They also have an enhanced pro-inflammatory response. In one in vitro study which evaluated the percentage of IL-6 and IL-8 positive monocytes following an endotoxin challenge, flow cytometry demonstrated a higher percentage of IL-6 and IL-8 positive cells in the monocytes from neonates compared to those from adults, with those from preterm neonates having the highest percentage of IL-8 positive cells.98 Furthermore, the increase in pro-inflammatory cytokines occurs faster and is more pronounced in the neonatal compared to the adult cells. Compared to older children and adults, neonates demonstrate both quantitative and qualitative differences in their immunity, which may at least partially explain their increased susceptibility to sepsis. Comprehensive reviews explore these differences in detail.99,100
In vitro studies have demonstrated significant depression of adult myocyte cells with increasing levels of TNF-alpha and IL-1-beta.81 Exposing the hearts of rats to endotoxin leads to increased production of nitric oxide and its metabolite peroxynitrite as well as superoxide, a free radical, in association with reduced myocardial activity.101 LPS-induced sepsis in rat models leading to an increase in TNF-alpha has been associated with increased cardiomyocyte apoptosis associated with decreased ventricular contractility suggesting apoptosis as another TNF-alpha-induced mechanism along with negative inotropy for decreased myocardial function in sepsis.87 However, in neonatal rat models, the cardiomyocyte apoptosis was not observed, suggesting this mechanism of myocardial dysfunction may not be relevant in the neonatal population.102 Myocardial calcium homoeostasis, pivotal for myocyte contraction, is affected in sepsis ultimately leading to impaired contractility. Animal studies of sepsis have demonstrated multiple mechanisms of altered myocyte calcium homoeostasis including myocyte inhibition of L-type calcium channels, inhibition of sarcoplasmic reticulum calcium pump, an increase in ryanodine receptor calcium leak and a decrease in myofilament calcium sensitivity.103
Glycocalyx is a gel-like layer covering the luminal surface of vascular endothelial cells. In sepsis, glycocalyx is degraded by pro-inflammatory cytokines including TNF-alpha and IL-1.79 This leads to microvessel thrombosis, further increase in inflammation and increased permeability of the endothelium resulting in myocardial oedema when glycocalyx of coronary arteries and intramyocardial vasculature is damaged79 (see Fig. 2). Myocardial oedema is thought to play a direct role in myocardial dysfunction by decreasing myocardial compliance and contractility. Myocardial oedema is present in rat models of sepsis84 and has been more recently demonstrated in humans by magnetic resonance imaging (MRI).86
However, all sepsis is not the same and it is likely that different organisms affect the cardiovascular system via different pathophysiological mechanisms. This is exemplified by a study which demonstrated a significantly higher cardiac output state in neonates with Gram-negative sepsis compared to those with Gram-positive sepsis, suggesting different host responses to different pathogens.36 There is, however, a paucity of research which delineates the contrasting haemodynamic effects of bacterial versus viral sepsis, or the particular effects of individual pathogens. There are some well-known associations. For example, myocarditis is a prominent feature in neonatal enterovirus sepsis, occurring in approximately a third of patients and often associated with arrhythmias.104 In contrast, no patients in a recent large review series had myocarditis in association with neonatal adenovirus infection.105 However, in disseminated disease when vasoregulatory dysfunction led to hypotension, there was an 80% mortality rate. Cardiac complications during neonatal viral sepsis secondary to acute SARS-CoV-2 infection are rare but include myocardial dysfunction and arrhythmia. This is in contrast to multisystem inflammatory disorder temporally related to SARS-CoV-2 infection in which cardiac sequelae including myocarditis, coronary artery anomalies and arrhythmias are common.106
Intrauterine infection and cardiovascular dysfunction
Intrauterine infection occurs when pathogens invade the amniotic cavity leading to histological chorioamnionitis and activation of the fetal immune system. The fetal inflammatory response syndrome (FIRS) is characterised by an elevation of inflammatory mediators in the fetal blood stream with the potential to progress to multi-organ dysfunction.107,108,109 Intrauterine infection is a major cause of early preterm birth. It is present in approximately a quarter of all preterm births and increases in frequency the earlier the gestation.9 Chorioamnionitis is often subclinical and difficult to diagnose early. It increases the risk of preterm delivery and is a strong predictor of chronic lung disease,110,111 periventricular leukomalacia (PVL)112 and cerebral palsy.113,114 Although the exact cause of neurodisability following chorioamnionitis is unclear, it is hypothesised that FIRS leading to alterations in cardiovascular function may contribute, as well as a systemic vasculitis. One study demonstrated that histological evidence of chorioamnionitis was associated with elevated cord blood IL-6 and IL-1beta, increased newborn heart rate and decreased blood pressure.115 Furthermore, the cord blood IL-6 concentration correlated inversely with newborn systolic, mean and diastolic blood pressures. A mouse model of intra-amniotic LPS causing an acute inflammatory reaction demonstrated increased IL-6 in amniotic fluid and LPS-induced expression of IL-1beta and TNF-alpha in the fetal myocardium along with echocardiographic markers of cardiovascular compromise including reduced cardiac output.116
Recent sheep studies have evaluated the cardiovascular changes which occur during fetal inflammatory response secondary to LPS-induced maternal septicaemia.117 In this model, there was a statistically significant peak of IL-6 following LPS injection. The group demonstrated that fetal heart rate variability monitoring was able to track the temporal profile of fetal inflammation and discriminate between normal and inflammatory processes over time. Changes in fetal heart rate alone were not sufficient to predict inflammation; however, changes in fetal heart rate variability were able to predict fetal inflammation as early as 1 h following LPS injection in LPS-induced sepsis without signs of shock. This is in keeping with human neonatal studies, which have shown that decreased heart rate variability and alteration in other heart rate characteristics precedes a clinical diagnosis of necrotising enterocolitis,118 sepsis, meningitis and death.119,120,121 Contrastingly, another recent sheep study demonstrated increased short-term variability during histological chorioamnionitis following LPS injection.122 The authors hypothesised that this might be due to the activation of the parasympathetic nervous system anti-inflammatory pathway as a compensatory mechanism in the setting of FIRS.123 Activation of the parasympathetic nervous system increases variability. Further studies are required to elucidate whether fetal heart rate characteristics monitoring in humans could identify subclinical fetal inflammatory syndrome in chorioamnionitis leading to earlier interventions and reduced morbidity.
Fetal echocardiography studies, although limited, suggest changes to the fetal heart in patients with PPROM and with documented intra-amniotic infection, for example, higher compliance of the left ventricle,124 and higher left ventricular Tei index (an echocardiographic measure suggesting reduced myocardial systolic and diastolic function).125 A recent study in nonhuman primates with Ureaplasma parvum intra-amniotic infection demonstrated increased vascular impedance in the umbilical and fetal pulmonary arteries and a decrease in fetal cardiac output compared to controls.126 Notably, these haemodynamic changes improved with maternal azithromycin therapy.
These limited data suggest similar cardiovascular dysfunction is seen antenatally in association with the fetal inflammatory response as that which is seen postnatally in association with sepsis. Further research is required in order to determine the optimal cardiovascular management of these patients following birth during the transitional period. Another pertinent question for further research is whether antenatal cardiovascular parameters should be included in the organ dysfunction definition for early-onset neonatal sepsis.
Conclusion
The highest incidence of sepsis across all age groups is seen in neonates, in whom it causes significant morbidity and mortality. Sepsis-induced cardiovascular dysfunction occurs commonly in the neonatal population, and yet, partly due to a lack of consensus definitions, there is a paucity of data available regarding the condition, including its prevalence, and prognosis. This review highlights the urgency to form an international consensus definition for both neonatal sepsis and for cardiovascular dysfunction complicating neonatal sepsis to allow for further research in both areas in order to improve neonatal outcomes. Animal and human studies have outlined the pathophysiological mechanisms involved. Further echocardiographic studies are required in neonatal cohorts in order to define myocardial dysfunction and provide reference ranges and cut-off values. Specific questions for further study include the relationship between the fetal and post-natal inflammatory response syndrome. The assessment and management of cardiovascular dysfunction complicating neonatal sepsis are discussed in the second and third papers of this series.
References
Reinhart, K. et al. Recognizing sepsis as a global health priority — a WHO resolution. N. Engl. J. Med. 377, 414–417 (2017).
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 6, 223–230 (2018).
Schlapbach, L. J. et al. Impact of sepsis on neurodevelopmental outcome in a Swiss national cohort of extremely premature infants. Pediatrics 128, e348–e357 (2011).
Hayes, R. et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr. Res. 93, 1141–1148 (2023).
Deshpande, S. et al. Pulmonary hypertension in late onset neonatal sepsis using functional echocardiography: a prospective study. J. Ultrasound 25, 233–239 (2022).
de Waal, K. & Evans, N. Hemodynamics in preterm infants with late-onset sepsis. J. Pediatr. 156, 918–922.e1 (2010).
Habimana, R. et al. Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute Crit. Care 35, 57–66 (2020).
Prusakowski, M. K. & Chen, A. P. Pediatric sepsis. Emerg. Med. Clin. North Am. 35, 123–138 (2017).
Gonçalves, L. F., Chaiworapongsa, T. & Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 8, 3–13 (2002).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801 (2016).
Morin, L. et al. The current and future state of pediatric sepsis definitions: an international survey. Pediatrics 149, e2021052565 (2022).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 171, e172352 (2017).
Schlapbach, L. J., Straney, L., Bellomo, R., MacLaren, G. & Pilcher, D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 44, 179–188 (2018).
Molloy, E. J. & Bearer, C. F. Paediatric and neonatal sepsis and inflammation. Pediatr. Res. 91, 267–269 (2022).
Molloy, E. J. et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr. Res. 88, 2–4 (2020).
Henry, C. J. et al. Neonatal sepsis: a systematic review of core outcomes from randomised clinical trials. Pediatr. Res. 91, 735–742 (2022).
McGovern, M., Giannoni, E., Kuester, H., Turner, M. A., van den Hoogen, A. & Bliss, J. M. et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr. Res. 88, 14–26 (2020).
ELFIN Trial Investigators Group. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 393, 423–433 (2019).
The International Neonatal Immunotherapy Study (INIS) Collaborative Group. Treatment of neonatal sepsis with intravenous immune globulin. N. Engl. J. Med. 365, 1201–1211 (2011).
Wynn, J. L. & Polin, R. A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 88, 85–90 (2020).
Fleiss, N. et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw. Open 4, e2036518 (2021).
Aziz, K. B. et al. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J. Perinatol. 41, 2337–2344 (2021).
Kharrat, A. et al. Validity of the vasoactive-inotropic score in preterm neonates receiving cardioactive therapies. Early Hum. Dev. 173, 105657 (2022).
Demirhan, S., Topcuoglu, S., Karadag, N., Ozalkaya, E. & Karatekin, G. Vasoactive inotropic score as a predictor of mortality in neonatal septic shock. J. Trop. Pediatr. 68, fmac100 (2022).
Wynn, J. L. et al. Timing of multiorgan dysfunction among hospitalized infants with fatal fulminant sepsis. Am. J. Perinatol. 34, 633–639 (2017).
Giannoni, E. et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J. Pediatr. 201, 106–114.e4 (2018).
Kermorvant-Duchemin, E., Laborie, S., Rabilloud, M., Lapillonne, A. & Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 9, 186–191 (2008).
Groeneveld, A. B., Nauta, J. J. & Thijs, L. G. Peripheral vascular resistance in septic shock: its relation to outcome. Intensive Care Med. 14, 141–147 (1988).
Ceneviva, G., Paschall, J. A., Maffei, F. & Carcillo, J. A. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics 102, e19 (1998).
Pollack, M. M., Fields, A. I. & Ruttimann, U. E. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit. Care Med. 13, 454–459 (1985).
Mercier, J.-C., Beaufils, F., Hartmann, J.-F. & Azema, D. Hemodynamic patterns of meningococcal shock in children. Crit. Care Med. 16, 27–33 (1988).
El-Khuffash, A. F. & McNamara, P. J. Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin. Fetal Neonatal Med. 16, 50–60 (2011).
Cerritelli, F. et al. A review on the vagus nerve and autonomic nervous system during fetal development: searching for critical windows. Front. Neurosci. 15, 721605 (2021).
Galland, B. C., Taylor, B. J., Bolton, D. P. G. & Sayers, R. M. Heart rate variability and cardiac reflexes in small for gestational age infants. J. Appl. Physiol. (1985) 100, 933–939 (2006).
Saini, S. S., Kumar, P. & Kumar, R. M. Hemodynamic changes in preterm neonates with septic shock: a prospective observational study. Pediatr. Crit. Care Med. 15, 443–450 (2014).
Deshpande, S., Suryawanshi, P., Chaudhary, N. & Maheshwari, R. Cardiac output in late onset neonatal sepsis. J. Clin. Diagn. Res. https://doi.org/10.7860/JCDR/2017/30312.10871 (2017).
Yengkhom, R. et al. Point of care neonatal ultrasound in late-onset neonatal sepsis. J. Neonatol. 35, 59–63 (2021).
Briegel, J., Jochum, M., Gippner-Steppert, C. & Thiel, M. Immunomodulation in septic shock: hydrocortisone differentially regulates cytokine responses. J. Am. Soc. Nephrol. 12, S70–S74 (2001).
Soliman, A. T. et al. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism 53, 209–214 (2004).
Ng, P. C. Adrenocortical insufficiency and refractory hypotension in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 101, F571–F576 (2016).
Scott, S. M. & Watterberg, K. L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 37, 112–116 (1995).
Huysman, M. W. A., Hokken-Koelega, A. C. S., De Ridder, M. A. J. & Sauer, P. J. J. Adrenal function in sick very preterm infants. Pediatr. Res. 48, 629–633 (2000).
Seri, I., Tan, R. & Evans, J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 107, 1070–1074 (2001).
Ng, P. C. et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 117, 367–375 (2006).
Higgins, S., Friedlich, P. & Seri, I. Hydrocortisone for hypotension and vasopressor dependence in preterm neonates: a meta-analysis. J. Perinatol. 30, 373–378 (2010).
Kharrat, A. & Jain, A. Hemodynamic dysfunction in neonatal sepsis. Pediatr. Res. 91, 413–424 (2022).
Meadow, W. L. & Meus, P. J. Unsuspected mesenteric hypoperfusion despite apparent hemodynamic recovery in the early phase of septic shock in piglets. Circ. Shock 15, 123–129 (1985).
Meadow, W. L. & Meus, P. J. Early and late hemodynamic consequences of group B beta streptococcal sepsis in piglets: effects on systemic, pulmonary, and mesenteric circulations. Circ. Shock 19, 347–356 (1986).
Davis, A. L. et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit. Care Med. 45, 1061–1093 (2017).
Truog, W. E., Gibson, R. L., Henderson, W. R. & Redding, G. J. Tumor necrosis factor-induced neonatal pulmonary hypertension: effects of dazmegrel pretreatment. Pediatr. Res. 27, 466–471 (1990).
Anderson, P. A. W. The heart and development. Semin. Perinatol. 20, 482–509 (1996).
Marijianowski, M. M. H., van der Loos, C. M., Mohrschladt, M. F. & Becker, A. E. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 23, 1204–1208 (1994).
Crepaz, R., Pitscheider, W., Radetti, G. & Gentili, L. Age-related variation in left ventricular myocardial contractile state expressed by the stress velocity relation. Pediatr. Cardiol. 19, 463–467 (1998).
Rowland, D. G. & Gutgesell, H. P. Noninvasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am. J. Cardiol. 75, 818–821 (1995).
Vrancken, S. L., van Heijst, A. F. & de Boode, W. P. Neonatal hemodynamics: from developmental physiology to comprehensive monitoring. Front. Pediatr. 6, 87 (2018).
Rudolph, A. Myocardial growth before and after birth: clinical implications. Acta Paediatr. 89, 129–133 (2000).
Smith, A. et al. Comparison of left ventricular rotational mechanics between term and extremely premature infants over the first week of age. Open Heart 8, e001458 (2021).
Singh, Y., Katheria, A. C. & Vora, F. Advances in diagnosis and management of hemodynamic instability in neonatal shock. Front. Pediatr. 6, 2 (2018).
Drosatos, K. et al. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr. Heart Fail. Rep. 12, 130–140 (2015).
Poelaert, J., Declerck, C., Vogelaers, D., Colardyn, F. & Visser, C. A. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 23, 553–560 (1997).
Kimchi, A. et al. Right ventricular performance in septic shock: a combined radionuclide and hemodynamic study. J. Am. Coll. Cardiol. 4, 945–951 (1984).
Parker, M. M. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 100, 483 (1984).
Bouhemad, B. et al. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit. Care Med. 36, 766–774 (2008).
L’Heureux, M., Sternberg, M., Brath, L., Turlington, J. & Kashiouris, M. G. Sepsis-induced cardiomyopathy: a comprehensive review. Curr. Cardiol. Rep. 22, 35 (2020).
Vieillard-Baron, A. et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 36, 1701–1706 (2008).
Beesley, S. J. et al. Septic cardiomyopathy. Crit. Care Med. 46, 625–634 (2018).
Vieillard-Baron, A., Prin, S., Chergui, K., Dubourg, O. & Jardin, F. Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am. J. Respir. Crit. Care Med. 168, 1270–1276 (2003).
Torgersen, C. et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth. Analg. 108, 1841–1847 (2009).
Parrillo, J. E. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113, 227 (1990).
Merx, M. W. & Weber, C. Sepsis and the heart. Circulation 116, 793–802 (2007).
Jain, A., Sankar, J., Anubhuti, A., Yadav, D. K. & Sankar, M. J. Prevalence and outcome of sepsis-induced myocardial dysfunction in children with ‘sepsis’ ‘with’ and ‘without shock’—a prospective observational study. J. Trop. Pediatr. 64, 501–509 (2018).
Yang, C. et al. NT-Pro-BNP and echocardiography for the early assessment of cardiovascular dysfunction in neonates with sepsis. Medicine (Baltimore) 101, e30439 (2022).
Alzahrani, A. K. Cardiac function affection in infants with neonatal sepsis. J. Clin. Trials 7, 329 (2017).
Abdel-Hady, H. E., Matter, M. K. & El-Arman, M. M. Myocardial dysfunction in neonatal sepsis: a tissue Doppler imaging study. Pediatr. Crit. Care Med. 13, 318–323 (2012).
Fahmey, S. S., Hodeib, M., Refaat, K. & Mohammed, W. Evaluation of myocardial function in neonatal sepsis using tissue Doppler imaging. J. Matern. Fetal Neonatal Med. 33, 3752–3756 (2020).
Tomerak, R. H., El-Badawy, A. A., Hussein, G., Kamel, N. R. M. & Razak, A. R. A. Echocardiogram done early in neonatal sepsis: what does it add? J. Investig. Med. 60, 680–684 (2012).
Awny MM, Abd-Rab-Alrasol OT, Al Biltagi MA, Al-Asy HM, El-Mahdy HS. Cardiac functions by tissue Doppler and Speckle tracking echocardiography in neonatal sepsis and its correlation with sepsis markers and cardiac troponin-T. J. Pediatr. Neonatal Care 5, 11–12 (2016).
Kumar, A. et al. Experimental human endotoxemia is associated with depression of load-independent contractility indices. Chest 126, 860–867 (2004).
Uchimido, R., Schmidt, E. P. & Shapiro, N. I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care 23, 16 (2019).
De Backer, D., Orbegozo Cortes, D., Donadello, K. & Vincent, J.-L. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 5, 73–79 (2014).
Kumar, A., Kumar, A., Paladugu, B., Mensing, J. & Parrillo, J. E. Transforming growth factor-β1 blocks in vitro cardiac myocyte depression induced by tumor necrosis factor-α, interleukin-1β, and human septic shock serum. Crit. Care Med. 35, 358–364 (2007).
Bernardin, G., Strosberg, A. D., Bernard, A., Mattei, M. & Marullo, S. Beta-adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med. 24, 1315–1322 (1998).
Jarkovska, D. et al. Cellular mechanisms of myocardial depression in porcine septic shock. Front. Physiol. 9, 726 (2018).
Yu, P. et al. Myocardial collagen changes and edema in rats with hyperdynamic sepsis. Crit. Care Med. 25, 657–662 (1997).
Chagnon, F., Bentourkia, M., Lecomte, R., Lessard, M. & Lesur, O. Endotoxin-induced heart dysfunction in rats: assessment of myocardial perfusion and permeability and the role of fluid resuscitation. Crit. Care Med. 34, 127–133 (2006).
Vasques-Nóvoa, F. et al. Myocardial edema: an overlooked mechanism of septic cardiomyopathy? Shock 53, 616–619 (2020).
Comstock, K. L. et al. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J. Mol. Cell. Cardiol. 30, 2761–2775 (1998).
Carlson, D. L., Willis, M. S., White, D. J., Horton, J. W. & Giroir, B. P. Tumor necrosis factor-α-induced caspase activation mediates endotoxin-related cardiac dysfunction. Crit. Care Med. 33, 1021–1028 (2005).
Landry, D. W. & Oliver, J. A. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 345, 588–595 (2001).
Kakihana, Y., Ito, T., Nakahara, M., Yamaguchi, K. & Yasuda, T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J. Intensive Care 4, 22 (2016).
O’Hare, F. M., William Watson, R. & Molloy, E. J. Toll-like receptors in neonatal sepsis. Acta Paediatr. 102, 572–578 (2013).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Adib-Conquy, M. & Cavaillon, J.-M. Réponse inflammatoire et anti-inflammatoire de l’hôte au cours du sepsis. Pathol. Biol. 60, 306–313 (2012).
Soriano, F. G., Lorigados, C. B., Pacher, P. & Szabó, C. Effects of a potent peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis. Shock 35, 560–566 (2011).
Greer, J. Pathophysiology of cardiovascular dysfunction in sepsis. BJA Educ. 15, 316–321 (2015).
Schultz, C. et al. Immature anti-inflammatory response in neonates. Clin. Exp. Immunol. 135, 130–136 (2003).
Schultz, C. et al. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr. Res. 51, 317–322 (2002).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Kollmann, T. R., Levy, O., Montgomery, R. R. & Goriely, S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37, 771–783 (2012).
Khadour, F. H. et al. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am. J. Physiol. Heart Circ. Physiol. 283, H1108–H1115 (2002).
Hickson-Bick, D. L. M., Jones, C. & Buja, L. M. The response of neonatal rat ventricular myocytes to lipopolysaccharide-induced stress. Shock 25, 546–552 (2006).
Hobai, I. A., Edgecomb, J., LaBarge, K. & Colucci, W. S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 43, 3–15 (2015).
Zhang, M. et al. Clinical characteristics of severe neonatal enterovirus infection: a systematic review. BMC Pediatr. 21, 127 (2021).
Ronchi, A., Doern, C., Brock, E., Pugni, L. & Sánchez, P. J. Neonatal adenoviral infection: a seventeen year experience and review of the literature. J. Pediatr. 164, 529–535.e4 (2014).
Pawar, R. et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel). 8, 572 (2021).
DiGiulio, D. B. et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 64, 38–57 (2010).
Gotsch, F. et al. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol. 50, 652–683 (2007).
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213, S29–S52 (2015).
Kalikkot Thekkeveedu, R., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir. Med. 132, 170–177 (2017).
Perniciaro, S. et al. Early- and late-respiratory outcome in very low birth weight with or without intrauterine inflammation. Am. J. Perinatol. 37, S76–S83 (2020).
Resch, B. et al. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leucomalacia. Eur. J. Pediatr. 159, 663–670 (2000).
Grether, J. K. & Nelson, K. B. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 278, 207–211 (1997).
Yoon, B. H. et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol. 177, 19–26 (1997).
Yanowitz, T. D. et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr. Res. 51, 310–316 (2002).
Rounioja, S. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction: a mouse model for fetal inflammatory response. Cardiovasc. Res. 60, 156–164 (2003).
Durosier, L. D. et al. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol. Meas. 36, 2089–2102 (2015).
Stone, M. L. et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J. Perinatol. 33, 847–850 (2013).
Griffin, M. P. & Moorman, J. R. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 107, 97–104 (2001).
Griffin, M. P. et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 116, 1070–1074 (2005).
Weitkamp, J.-H. et al. Meningitis, urinary tract, and bloodstream infections in very low birth weight infants enrolled in a heart rate characteristics monitoring trial. Pediatr. Res. 87, 1226–1230 (2020).
Kyozuka, H., Yasuda, S., Hiraiwa, T., Nomura, Y. & Fujimori, K. The change of fetal heart rate short-term variability during the course of histological chorioamnionitis in fetal sheep. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 32–37 (2018).
Garzoni, L., Faure, C. & Frasch, M. G. Fetal cholinergic anti-inflammatory pathway and necrotizing enterocolitis: the brain-gut connection begins in utero. Front. Integr. Neurosci. 7, 57 (2013).
Romero, R. et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 16, 146–157 (2004).
Letti Müller, A. L. et al. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet. Gynecol. 36, 26–31 (2010).
Kelleher, M. A. et al. Maternal azithromycin therapy for Ureaplasma parvum intraamniotic infection improves fetal hemodynamics in a nonhuman primate model. Am. J. Obstet. Gynecol. 223, 578.e1–578.e11 (2020).
Author information
Authors and Affiliations
Consortia
Contributions
S.M.D and E.J.M: manuscript design, manuscript draft. All authors contributed to editing, including revising the paper for important intellectual content and approved the final draft. S.L: created figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duignan, S.M., Lakshminrusimha, S., Armstrong, K. et al. Neonatal sepsis and cardiovascular dysfunction I: mechanisms and pathophysiology. Pediatr Res 95, 1207–1216 (2024). https://doi.org/10.1038/s41390-023-02926-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02926-2
- Springer Nature America, Inc.