Abstract
Background
The aim of this study was to describe the trajectory of oscillatory mechanics from the first week of life to term equivalent and evaluate whether oscillatory mechanics are associated with simultaneous lung disease in infants ≤32 weeks gestation.
Methods
In this observational, longitudinal study, we enrolled 66 infants. Forced oscillations were applied using a neonatal mechanical ventilator (Fabian HFOi) that superimposed oscillations (10 Hz, amplitude 2.5 cmH2O) on a positive end-expiratory pressure (PEEP). Measurements were performed at 5-7-9 cmH2O of PEEP or the clinical pressure ±2 cmH2O; they were repeated at 7, 14, 28 post-natal days, and 36 and 40 weeks post-menstrual age (PMA).
Results
The mean (range) gestational age of study participants was 29.2 (22.9–31.9) weeks. Nineteen infants (29%) developed bronchopulmonary dysplasia (BPD). Respiratory system reactance was significantly lower (lower compliance), and respiratory system resistance was significantly higher in infants with developing BPD from 7 post-natal days to 36 weeks PMA. All oscillatory mechanics parameters were significantly associated with the simultaneous respiratory severity score (p < 0.001 for all).
Conclusions
Serial measurements of oscillatory mechanics allow differentiating lung function trajectory in infants with and without evolving BPD. Oscillatory mechanics significantly correlate with the severity of simultaneous lung disease.
Impact
-
The results of the present study suggest that respiratory system reactance, as assessed by respiratory oscillometry, allows the longitudinal monitoring of the progression of lung disease in very premature infants.
-
This paper describes for the first time the trajectory of oscillatory mechanics in very preterm infants with and without evolving bronchopulmonary dysplasia from the first week of life to term equivalent.
-
Serial respiratory oscillometry measurements allow the identification of early markers of evolving bronchopulmonary dysplasia and may help personalizing the respiratory management strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bronchopulmonary dysplasia (BPD) is the most common complication of prematurity, with an incidence of 38% in infants born <30 weeks’ gestation.1 BPD represents a continuum of different diseases—airway obstruction, alveolar and pulmonary vascular disease—which may not all be present in all patients at all times.2 Phenotype diversity and the presence of non-mutually exclusive disease traits make it necessary to apply tailored ventilatory and therapeutic strategies. Moreover, BPD is typically diagnosed or staged at 36 weeks post-menstrual age (PMA), but chronic lung disease of prematurity evolves over time, starting from the first days of life. Indeed, identifying functional markers of specific treatable traits over time would facilitate personalized prevention and treatment of the disease. However, the lack of non-invasive bedside tools suitable for serial infant lung function testing makes such personalized approach challenging in clinical practice.
Respiratory oscillometry is a non-invasive method for assessing lung mechanics. Oscillometry does not need patient co-operation, and is, therefore, particularly attractive for use in infants. Respiratory oscillometry has been successfully used in healthy infants,3,4,5,6,7 including in the first days of life, and infants assisted with different respiratory support modes. Moreover, respiratory oscillometry is now available at the bedside, making it an attractive tool for assessing lung function trajectory in preterm infants at risk of BPD. Oscillometry mechanics could differentiate between preterm and term infants,8 healthy infants and infants with transient tachypnea of the newborn,9 preterm infants with respiratory distress or evolving BPD,10 and preterm infants with and without BPD at 36 weeks PMA.11 Moreover, the relationship between oscillatory mechanics parameters and positive end-expiratory pressure improves the prognostication of respiratory outcomes12 and differs in infants with different stages of lung disease.10 However, we lack longitudinal data on the same cohort of very preterm infants.
To fill this knowledge gap, we performed serial oscillatory mechanics measurements in very preterm infants aiming at (i) describing the trajectory of oscillometry parameters in BPD and non-BPD patients; (ii) characterizing the relationship between oscillometry parameters and airway opening pressure at different stages of lung disease; (iii) assessing the association between oscillometry parameters and simultaneous lung disease severity.
Methods
Study design and participants
This was an observational, single-centre study conducted at Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy, between November 2020 and October 2022. The local ethics committee (nr. 3804/21) approved the study, and written informed consent was obtained from parents before enrollment. Infants below 32.0 weeks gestational age (GA) were eligible if they had no major congenital malformations.
Lung function measurements
Infants were studied in the supine position. Infants on non-invasive respiratory support were studied during unsedated quiet sleep or quiet awake, according to European Respiratory Society (ERS)/American Thoracic Society (ATS) standards13 and the ATS/ERS workshop report on the assessment of respiratory mechanics and lung function in the neonatal and paediatric intensive care units.14 We applied respiratory oscillometry using a mechanical ventilator (Fabian HFOi, Vyaire) that superimposed sinusoidal pressure oscillations (amplitude 2.5 cmH2O, frequency 10 Hz) on a positive end-expiratory pressure (PEEP). During conventional invasive mechanical ventilation, the ventilator automatically switched to CPAP for 3–5 s at a CPAP equal to the PEEP in use; during high-frequency oscillatory ventilation, the ventilator changed the oscillatory frequency to 10 Hz and the amplitude to 2.5 cmH2O for 3–5 s. In non-intubated patients, measurements were performed using a face mask with the ventilator operating in dual-limb CPAP mode. Airways opening pressure and flow were measured using the ventilator sensors, sampled at 250 Hz, and exported for off-line analysis.
Study protocol
Measurements were performed on 7 (±1), 14 (±2), and 28 (±2) days of life and at 36 (±1) and 40 (±1) weeks PMA. Lung mechanics were assessed at different PEEP levels. During non-invasive respiratory support or conventional mechanical ventilation, we applied PEEPs of 5, 7, and 9 cmH2O; during high-frequency oscillatory ventilation, we performed the measurements at the clinical continuous distending pressure and the clinical distending pressure ±2 cmH2O. Each pressure was applied for 1 min during non-invasive respiratory support or 3 min during invasive respiratory support, followed by at least 2 oscillometry measurements. Vital parameters were monitored continuously, and the fraction of inspired oxygen was adjusted to keep the saturation in the target range.
Data analysis
The pressure and flow signals were processed using a least-squares algorithm to compute respiratory system resistance and reactance (Rrs and Xrs, respectively). Data collected during non-invasive respiratory support were compensated for leaks and the shunt of the face mask, as described before.15 Data collected during invasive ventilation were corrected for the contribution of the tracheal tube. Briefly, the resistance and reactance of the tracheal tube (Rett and Xett, respectively) were measured in vitro using a mechanical analogue of the infant respiratory system obtained by combining a linear resistance and a rigid bottle (R = 21 cmH2O*s/L, X = −21 cmH2O*s/L). Rett and Xett of the tubes used in the study (internal diameter = 2.5, 3.0, 3.5 and 4.0 mm) were calculated by subtracting the resistance and reactance of the intubated and non-intubated system.16
For each measurement, we manually discarded data segments affected by obvious artefacts (e.g., large leaks, flow breaking). Xrs z-scores were calculated from PMA and weight z-score using a prediction equation specific for very preterm infants.17 We used the nearest neighbour algorithm for the imputation of missing or discarded data.
Clinical data and outcomes
Demographic and clinical data of study participants were extracted from the electronic medical records. We defined BPD as the need for any respiratory support at 36 weeks of PMA.18 We extracted the mean values over 24 h of the fraction of inspired oxygen (FIO2) and mean airway pressure (MAP) from the clinical records for each time point. Such parameters were used to compute the respiratory severity score (RSS = FIO2 × MAP). We attributed a MAP of 3 cmH2O to infants on high flow nasal cannula, because in our unit, we use such respiratory support mode for infants requiring a CPAP lower than 5 cmH2O.
The primary outcome was the trajectory of Xrs in infants with and without BPD; we also assessed the trajectories of Rrs and Xrs z-score. Secondary outcomes were: (i) the difference in Xrs and Rrs at different PEEP levels and (ii) the association between oscillometry parameters and simultaneous RSS.
Sample size estimates and statistical analysis
Aiming for 95% power at the 5% significance level, we calculated a minimum required sample size of 54 patients to compare lung mechanics in infants with and without BPD, using two-way ANOVA with 5 repeated measurements assuming an effect size of 0.3911 and a correlation between repeated measurements of 0.5.
We compared demographics and clinical data between infants with and without BPD using t test for continuous variables and the Chi-square test for dichotomous variables. We assessed the association between respiratory oscillometry parameters and airway pressure using mixed-effects linear models. Intercepts were allowed to vary for each subject, while airway pressure and BPD classification were modelled as fixed effects. We built separate models for Rrs and Xrs at each time point. We applied two-way ANOVA for repeated measurements with BPD classification and time as factors to compare respiratory oscillometry parameters in infants with and without BPD at different times. We used Tukey test as a post hoc test after ANOVA. We used linear mixed-effects models clustering on the individual subject to assess the difference in the trajectory of oscillatory mechanics over time in infants with and without BPD. We used Spearman correlation to evaluate the correlation between respiratory oscillometry parameters and simultaneous RSS. p Values < 0.05 were considered statistically significant. Data were analysed using SigmaPlot v11 (Systat Software, Inc., San Jose, California) and Matlab R2019b (MathWorks, Natick, MA).
Results
Between November 2020 and October 2022, we screened 78 infants for eligibility. Three infants were excluded for congenital malformations, four because they were transferred, three due to unavailability of staff, and two died within the observation period; 66 infants were included in the study. Table 1 summarises the characteristics of the included infants. Study participants had a mean (range) GA of 29.2 (22.9–31.9) weeks and a mean (range) birth weight of 1260 (450–1870) g. Nineteen (29%) infants developed BPD. As expected, BPD patients had significantly lower GA and birth weight and received longer respiratory support and supplemental oxygen than patients who did not develop BPD. Twelve infants received a 9-day course of dexamethasone (cumulative dose = 2.64 mg/kg). Fifteen infants received inhaled budesonide around 36 weeks PMA, including five who had received dexamethasone before. Supplemental Table 1 reports body weight and respiratory support mode at each time point.
We attempted 295 measurements. One measurement was skipped due to surgery, one due to exacerbation, and one due to the unavailability of staff. Two hundred eighty-nine measurements (98%) were of satisfactory data quality, confirming the high feasibility of the measurements.
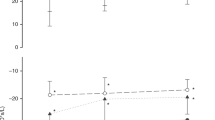
Figure 1 shows the trajectory of oscillometry parameters evaluated at the lowest airway pressure; Fig. 2 shows the relationship between Rrs and Xrs vs pressure in infants with and without BPD over time. Rrs at the lowest pressure was significantly higher in BPD than in non-BPD patients at all time points but 40 weeks PMA; it decreased significantly over time at a similar rate in the two groups (Fig. 1 and Supplemental Table 2). Rrs decreased with pressure at all time points; the reduction of Rrs with pressure was steeper in BPD than in non-BPD patients at 7 post-natal days (Fig. 2 and Supplemental Table 3). Xrs at the lowest pressure was significantly lower in BPD than in non-BPD patients at all time points, the difference being more marked in the early stages of lung disease. Xrs increased significantly over time, especially in BPD patients (Fig. 1 and Supplemental Table 2). Xrs z-score was higher in BPD than in non-BPD patients at all time points and decreased significantly in BPD patients (Fig. 1 and Supplemental Table 2). The relationship between Xrs and pressure changed over time and differed between BPD and non-BPD patients. Xrs presented a significant reduction with increasing pressure at 7 days of life, especially in BPD patients (Supplemental Table 3). Xrs significantly increased with pressure at 36 and 40 weeks PMA; the increase in Xrs with pressure was significantly steeper in BPD than in non-BPD patients at 36 weeks PMA (Fig. 2 and Supplemental Table 3).
a Respiratory system resistance, b respiratory system reactance, c respiratory system reactance in z-scores. Data are expressed as mean and SD. Rrs and Xrs respiratory system resistance and reactance at the lowest PEEP level, BPD bronchopulmonary dysplasia, 7 d, 14 d, and 28 d 7, 14, and 28 post-natal days, respectively, 36 w PMA and 40 w PMA 36 and 40 weeks post-menstrual age, respectively. *p < 0.05 vs BPD within the same time point.
a–e Respiratory system resistance, f–j respiratory system reactance, a, f: 7 post-natal days (7 d), b, g: 14 post-natal days (14 d), c, h: 28 post-natal days (28 d), d, i: 36 weeks post-menstrual age (36 w PMA), e, j: 40 weeks post-menstrual age (40 w PMA). Data are expressed as mean and SD. Rrs and Xrs respiratory system resistance and reactance, PEEP positive end-expiratory pressure, BPD bronchopulmonary dysplasia. Infants on high-frequency oscillatory ventilation were studied at clinical mean airway pressure ±2 cmH2O and not at the PEEP levels indicated on the x-axis.
Figure 3 compares respiratory oscillometry parameters in infants receiving different levels of respiratory support, which were used as a proxy of lung disease severity. Rrs and Xrs z-score were progressively higher, and Xrs was lower (more negative) as the intensity of respiratory support increased. Rrs and Xrs z-score presented a positive relationship with simultaneous respiratory severity score (rho = 0.28, p < 0.001; rho = 0.58, p < 0.001), while Xrs presented a negative correlation (rho = −0.65, p < 0.001).
a Respiratory system resistance, b respiratory system reactance, c respiratory system reactance in z-scores. Rrs and Xrs respiratory system resistance and reactance at the lowest CPAP level, no RS no respiratory support, HFNC high flow nasal cannula, nCPAP nasal CPAP or non-invasive ventilation, invasive invasive mechanical ventilation. The lines within the boxes are the medians, the boundaries of the boxes represent Q1 and Q3, the whiskers indicate 10th and 90th percentiles, closed circles indicate individual patients. Rrs and Xrs respiratory system resistance and reactance at the lowest PEEP level. *p < 0.05 vs no RS, §p < 0.05 vs HFNC, +p < 0.05 vs nCPAP.
Discussion
The main findings of our study are: (1) Xrs differed significantly between BPD and non-BPD patients from the first week of life to term equivalent, even after normalizing for the patient’s growth; (2) Rrs and Xrs were significantly correlated with the severity of simultaneous lung disease, as estimated by the intensity of the respiratory support requirement. Moreover, the relationship between Xrs and pressure changed over time and differed between BPD and non-BPD patients. Rrs tended to reduce with increasing pressure at all time points. On average, Xrs decreased with increasing pressure in the first week of life, while it increased with pressure at 36 and 40 weeks PMA. These findings suggest that respiratory oscillometry allows clinicians to monitor the progression of the respiratory conditions of very preterm infants at risk of BPD and has the potential to help identify early signs of evolving BPD and to optimize and adjust the respiratory support strategy matching it with lung function.
Relationship between respiratory oscillometry and positive airway pressure
In non-BPD patients, Rrs tended to decrease with increasing pressure at all time points; Xrs did not change significantly with increasing pressure at all time points between 7 days of life and 36 weeks PMA. Such behaviour may be associated with an increase in lung volume over the linear portion of the pressure-volume curve (e.g. without relevant lung volume recruitment or overdistension), and it is consistent with the behaviour observed in term infants without lung disease.10 At 40 weeks PMA, the reduction of Rrs was associated with a slight but significant increase in Xrs with increasing pressure, suggesting that the reduction in airway resistance allowed the pressure oscillations to reach a larger portion of the lung periphery. We can hypothesize that a slight increase in Xrs with increasing pressure might be determined by inhomogeneous peripheral airway obstruction reversible with pressure (see BPD patient below). Such behaviour was not clinically relevant as the patient did not need respiratory support, but it is not completely physiological, confirming that very preterm infants may not reach a fully normal lung function even if they do not develop bronchopulmonary dysplasia.
In infants with evolving BPD, the relationship between oscillometry parameters and pressure differed at different time points. At 7 days of life, Rrs and Xrs decreased significantly with increasing pressure. Such behaviour has already been described in RDS patients in the first week of life10,12 and can be interpreted as tissue distension with a stenting effect on the airways in a stiff lung without efficient volume recruitment with increasing pressure. At 36 weeks PMA, we observed a marked increase in Xrs with increasing pressure mirrored by a marked reduction in Rrs. Similar behaviour has been described in infants with evolving BPD10 and can be interpreted as peripheral airway collapse (e.g., accumulation of interstitial fluids due to inflammation and reduced elastic recoil) reversible by applying positive pressure and increasing functional residual capacity. At 40 weeks PMA, Rrs was similar in BPD and non-BPD patients, while Xrs presented a similar relationship against pressure in the two groups with a shift towards lower values in BPD patients.
Xrs presented a stronger association with respiratory outcomes than Rrs. Such results are in agreement with the behaviour observed in the first post-natal days15,19 and at 36 weeks PMA11 and suggest that Xrs better reflects the underlying prematurity-related lung disease, which mainly affects lung periphery. Indeed, Rrs mainly accounts for the resistance of the upper and central airways, while Xrs accounts for the elastic properties of the respiratory system and is more sensitive to peripheral phenomena, including peripheral airway collapse.
Not all measurements were performed at the same pressure levels to not deviate too much from the clinical respiratory support settings. However, the variability in the range of applied pressure does not invalidate our results because we changed the pressure level around the clinical one, which was carefully optimized. Moreover, since the relationship between respiratory oscillometry parameters and pressure differs among patients and time points, applying the same pressure range at all evaluations would pool together different conditions.
Clinical implications
The results of this study suggest that respiratory oscillometry can be applied at the bedside to monitor the trajectory of lung function in preterm infants at risk of BPD. Serial measurements of oscillatory mechanics would allow the identification of early signs of developing BPD and the correct timing to adjust the respiratory support strategy matching it with the evolving lung function. Interestingly, the trajectory of Xrs in BPD and non-BPD patients is consistent with that of lung aeration, as assessed by lung ultrasound.20 This similarity is logical, as lung aeration is related to the patency of the alveoli and peripheral airways. Future studies are warranted to investigate the correlation between the two measurements and whether they might provide complementary information about neonatal lung disease.
Moreover, assessing lung mechanics by respiratory oscillometry at different pressure levels increases the specificity of measurements to the underlying pathophysiology and may allow the optimization of the respiratory support strategy. The observation that the relationship between oscillatory mechanics parameters changed over time and based on the severity of lung disease has important implications for phenotyping lung disease and the patients’ clinical management.
We speculate that the behaviour characterized by a marked reduction in Rrs and Xrs (which becomes more negative) at increasing pressure, characteristic of patients with evolving BPD in the first weeks of life, is associated with a restrictive phenotype characterized by small lungs that do not increase their volume with increasing pressure and are, therefore, susceptible to lung tissue overdistension. In patients manifesting such behaviour, it is reasonable to limit the applied pressure. The behaviour characterized by an increase in Xrs mirrored by a reduction in Rrs with increasing pressure, typical of infants with chronic lung disease or established BDD, may be associated with an obstructive phenotype characterized by peripheral airway collapse reversible with pressure. Such behaviour, in turn, may be due to reduced local airway transmural pressures secondary to alveolar simplification or interstitial oedema or by reduced elastic recoil and tethering of the parenchyma on the small airways.21,22 We further speculate that if changes in Rrs and Xrs vs pressure are particularly steep, the airway collapse may affect larger airways preventing the forced oscillations from reaching larger portions of the lung. Patients with such phenotype are likely prone to develop dynamic hyperinflation associated with increased intrinsic PEEP. These patients often require high pressure levels to keep the airways patent and overcome the intrinsic PEEP. In our cohort, infants with evolving BPD tended to manifest a restrictive phenotype in the early stages of lung disease, followed by an obstructive phenotype. Infants who did not develop BPD did not manifest a restrictive phenotype in the first weeks of life but presented a relationship between Xrs and pressure suggestive of mild peripheral airway obstruction. Assessing oscillatory mechanics at different pressure levels may increase the specificity of the measurement to the underlying pathophysiology, allowing the optimization and individualization of the respiratory support strategy.
Technical considerations
Since very preterm infants are likely to receive invasive and non-invasive respiratory support during their NICU stay, we pooled data on intubated and non-intubated patients. We corrected the Rrs and Xrs values measured in intubated patients by subtracting the contribution of the tracheal tube. In this way, Rrs and Xrs values reflect the mechanical properties of the respiratory system downstream of the trachea, as opposed to the values measured in non-intubated patients that also include the contribution of the upper airways. In infants, the nasal pathway contributes for approximately half of the total airway resistance and most of the respiratory system inertance.23 Pilot measurements performed on the same subjects pre- and post-extubation suggest that the applied correction leads to comparable results between measurements performed during invasive or non-invasive respiratory support. However, it is possible that measurements in intubated patients are affected by the loss of the resistance and inertia of the nasal pathway after tube correction. Further cross-sectional studies are needed to allow for more sound comparison of results obtained during invasive vs non-invasive respiratory support.
Body position can affect lung function in infants with respiratory distress or evolving BPD.24 In the present study, body position was standardized, and patients were studied supine because the position of the head and neck in prone position can largely affect the results in patients on non-invasive respiratory support. Patients were supine for at least 15 minutes before the studies because measurements were performed after care rounds. A previous study reported that 15-min pronation produced a slight decrease in Rrs and no effect on Xrs in intubated preterm infants.25 However, we cannot exclude that in infants who had been prone for longer before the study, body position did not affect Rrs and Xrs values.
In the present study, measurements were performed with commercial equipment to improve the generalizability of results. To date, the intended application of respiratory oscillometry within the device is the titration of positive end-expiratory pressure in patients on invasive respiratory support. Measurements on non-invasive respiratory support were processed off-line to correct for unintentional leaks and remove possible artefacts. In the future, software, including the automatic correction for leaks and real-time quality control, can further improve respiratory oscillometry feasibility and allow its application in non-intubated infants in the clinical routine.
Strengths and limitations of the study
A strength of this study is the recruitment of very preterm infants from 23 weeks of GA, receiving both invasive and non-invasive respiratory support, including patients with severe neonatal lung disease. The single-centre study design limits the generalizability of results due to potential differences in respiratory management among centres. The low number of BPD patients prevented the possibility of stratifying for BPD severity. Some infants received systemic or inhaled post-natal corticosteroids. Such interventions may have affected the trajectory of lung function in the BPD group at 28 days and 36 weeks PMA. However, the changes in lung function were associated with an improvement in the clinical condition and a reduction in the respiratory support requirement. Finally, we used respiratory support metrics and BPD classification as proxies of lung disease severity, which are not specific to the underlying pathophysiology and are subject to variability in unit policies. However, to date, they remain the only readily available measures of lung disease severity and outcome.
Conclusion
Respiratory oscillometry is feasible in very preterm infants with different respiratory conditions, managed with different respiratory support modes, from the first week of life to term equivalent. Oscillatory mechanics parameters, and Xrs in particular, have value in monitoring the trajectory of lung function in infants with evolving BPD. Serial oscillatory mechanics measurements may identify changes in lung functions early and help adjust the respiratory support strategy based on the pathophysiology of the underlying lung disease.
Data availability
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Doyle, L. W. et al. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 118, 108–113 (2006).
Collaco, J. M. & McGrath-Morrow, S. A. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann. Am. Thorac. Soc. 15, 530–538 (2018).
Gray, D. M. et al. Intra-breath measures of respiratory mechanics in healthy African infants detect risk of respiratory illness in early life. Eur. Respir. J. 53, 1800998 (2019).
Gray, D. et al. Determinants of early-life lung function in African infants. Thorax 72, 445–450 (2017).
Radics, B. L. et al. Respiratory oscillometry in newborn infants: conventional and intra-breath approaches. Front. Pediatr. 10, 867883 (2022).
Hantos, Z. et al. Assessment of respiratory mechanics with forced oscillations in healthy newborns. Pediatr. Pulmonol. 50, 344–352 (2015).
Gray, D. et al. Lung function and exhaled nitric oxide in healthy unsedated African infants. Respirology 20, 1108–1114 (2015).
Travers, C. P. et al. Non-invasive oscillometry to measure pulmonary mechanics in preterm infants. Am. J. Respir. Crit. Care Med. 204, 485–488 (2021).
Klinger, A. P. et al. Non-invasive forced oscillometry to quantify respiratory mechanics in term neonates. Pediatr. Res. 88, 293–299 (2020).
Dellacà, R. L. et al. Relationship between respiratory impedance and positive end-expiratory pressure in mechanically ventilated neonates. Intensive Care Med. 39, 511–519 (2013).
Zannin, E. et al. Oscillatory mechanics at 36 weeks post-menstrual age as markers of lung disease in preterm infants: a cohort study. Eur. Respir. J. 59, 2103023 (2022).
Veneroni, C., Wallström, L., Sindelar, R. & Dellacaʼ, R. L. Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr. Res. 85, 312–317 (2018).
Bates, J. H., Schmalisch, G., Filbrun, D. & Stocks, J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur. Respir. J. 16, 1180–1192 (2000).
Peterson-Carmichael, S. et al. An official American Thoracic Society/European Respiratory Society Workshop Report: evaluation of respiratory mechanics and function in the pediatric and neonatal intensive care units. Ann. Am. Thorac. Soc. 13, S1–S11 (2016).
Zannin, E., Neumann, R. P., Dellacà, R. & Schulzke, S. M. Forced oscillation measurements in the first week of life and pulmonary outcome in very preterm infants on noninvasive respiratory support. Pediatr. Res. 86, 382–388 (2019).
Dorkin, H. L. et al. Respiratory system impedance from 4 to 40 Hz in paralyzed intubated infants with respiratory disease. J. Clin. Investig. 72, 903–910 (1983).
Zannin, E. et al. Oscillatory mechanics in very preterm infants on continuous positive airway pressure support: reference values. Pediatr. Pulmonol. 58, 746–752 (2023).
Jensen, E. A. et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants an evidence-based approach. Am. J. Respir. Crit. Care Med. 200, 751–759 (2019).
Veneroni, C., Wallström, L., Sindelar, R. & Dellacaʼ, R. L. Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr. Res. 85, 312–317 (2019).
Loi, B. et al. Lung ultrasound to monitor extremely preterm infants and predict bronchopulmonary dysplasia a multicenter longitudinal cohort study. Am. J. Respir. Crit. Care Med. 203, 1398–1409 (2021).
Colin, A. A., McEvoy, C. & Castile, R. G. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 126, 115 (2010).
Henschen, M., Stocks, J., Brookes, I. & Frey, U. New aspects of airway mechanics in pre-term infants. Eur. Respir. J. 27, 913–920 (2006).
Hall, G. L., Hantos, Z., Sly, P. D. & Wildhaber, J. H. Contribution of nasal pathways to low frequency respiratory impedance in infants. Thorax 57, 396 (2002).
Loi, B. et al. Respiratory and haemodynamic effects of 6h-pronation in neonates recovering from respiratory distress syndrome, or affected by acute respiratory distress syndrome or evolving bronchopulmonary dysplasia: a prospective, physiological, crossover, controlled. eClinicalMedicine 55, 101791 (2023).
Vendettuoli, V. et al. Positional effects on lung mechanics of ventilated preterm infants with acute and chronic lung disease. Pediatr. Pulmonol. 50, 798–804 (2015).
Acknowledgements
We acknowledge Abedulrhman S Abdelfattah for their support in performing the measurements.
Author information
Authors and Affiliations
Contributions
E.Z. has contributed to the study design, data acquisition and data analysis and drafted the first version of the manuscript. C.R. contributed to the study design, data acquisition and clinical interpretation of results and approved the final manuscript as submitted. G.D. contributed to the data collection and critically revised the manuscript. R.D. supervised data acquisition and analysis and critically revised the manuscript. M.L.V. contributed to the study design and the clinical interpretation of the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.D. reports that Politecnico di Milano received research grants from Vyaire and licensed a patent for using FOT to assess lung volume recruitment to Vyaire. C.R. has received honoraria for lectures from Vyaire. The other authors have nothing to disclose. None of the authors received any form of payment to produce the manuscript.
Ethics approval and consent to participate
The local ethics committee (nr. 3804/21) approved the study and written informed consent was obtained from parents before enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rigotti, C., Zannin, E., Dognini, G. et al. Oscillatory mechanics trajectory in very preterm infants: a cohort study. Pediatr Res 94, 1998–2004 (2023). https://doi.org/10.1038/s41390-023-02724-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02724-w
- Springer Nature America, Inc.