Abstract
Background
Previous studies in piglets show a direct relationship between intestinal mass and arginine (Arg) synthesis. We aimed to study the effects of 75% intestinal resection on whole-body Arg synthesis.
Methods
Piglets were allocated to sham or jejunocolic (JC) surgery and to enteral nutrition (EN) at 20% [sham (n = 8), JC (n = 10)], or 40% [sham (n = 4), JC (n = 5)]. A gastric tube was placed for EN and a venous catheter for parenteral nutrition and blood sampling. On day 6, a primed bolus and constant infusion of Arg m + 2 label and proline m + 1 label was delivered. In addition, 40% EN piglets received a citrulline (Cit) m + 3 tracer. Blood sampling was undertaken and whole-body Arg synthesis was calculated. On day 7, intestinal length was measured, and samples were collected for gene expression (PCR quantification) and histopathology.
Results
On Day 7, sham piglets showed intestinal lengthening compared to JC (p = 0.02). Whole-body Arg synthesis was similar between groups (p = 0.50). Adjusting for absolute small intestinal length, JC piglets had greater Arg synthesis (p = 0.01). Expression of arginosuccinase was upregulated in the jejunum of JC compared to sham on 20% EN (p = 0.03).
Conclusion
This demonstrates for the first-time adaptive changes in intestinal Arg synthesis following intestinal resection.
Impact
-
The intestine makes a critical contribution to whole-body arginine synthesis, particularly in neonates, a human population at risk for short bowel syndrome. Therefore, we studied intestinal arginine synthesis in a neonatal piglet model of short bowel syndrome and demonstrated adaptive changes in the intestine that may preserve whole-body arginine synthesis, despite loss of intestinal mass.
-
This research adds new information to our understanding of the effects a massive intestinal resection has on amino acid metabolism during neonatal development.
Similar content being viewed by others
Introduction
Short bowel syndrome (SBS) is the leading cause of intestinal failure (IF) in children. IF results in the inability to absorb sufficient nutrients for normal growth and survival.1 Preterm infants are the most vulnerable, having an increased risk of developing necrotizing enterocolitis (NEC), a leading cause of SBS in this population.1 One accepted definition of SBS comes from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, and states that SBS is defined as a parenteral nutrition (PN) requirement for more than 60 days following intestinal resection, or when the remnant intestine is less than 25% of what is expected for age.2 Following significant intestinal resection, the residual intestine undergoes both structural and functional changes that allow for independence from PN—a process called intestinal adaptation.3 In growing neonates and infants it has been shown that adaptation can take many years.4
The intestine is an important site for whole-body amino acid metabolism, particularly for glutamine (Gln), arginine (Arg), methionine, glycine, lysine, threonine, and citrulline (Cit).5 The small intestine contributes up to 60% of whole-body Arg synthesis and in the TPN-fed piglet model, mucosal mass and Arg synthesis are directly related.6 In preterm infants, growing children, and critically ill adults, Arg is considered a conditionally essential amino acid.7 It has important roles in immune function, ammonia detoxification, synthesis of polyamines, nitric oxide (NO) production, and creatine synthesis.8 Further, preterm infants with NEC have lower plasma Arg and Arg supplementation decreases the incidence of NEC in this population.9,10
The dietary precursors for intestinal Arg synthesis include proline (Pro), Gln, and Cit. We have shown in piglets that first pass intestinal metabolism of an enterally fed diet is responsible for 40–60% of Arg synthesis from Pro.11 The orally administered Gln increases synthesis of Cit and Arg more than the parenteral route.12 Unfortunately, when solely parenterally fed, Arg synthesis from Pro is reduced and insufficient to meet whole-body Arg requirements.13 Finally, Arg metabolism in the intestine of neonates and infants up until 3–5 years of age differs from other age groups. This is characterized by an initial increased expression followed by gradual reduction in the abundance of the arginosuccinase synthase enzyme (ASS) and the absence of arginase (I and II) enzymes, thus favouring the release of Arg from the intestine, rather than conversion to Cit in situ.14
For all the above reasons we hypothesized that neonates with IF and SBS who are dependent on PN, and with decreased metabolically active intestinal mass, will have significantly perturbed whole-body Arg metabolism and that increasing EN would improve Arg synthesis. However, there has been no study on the effect of IF and SBS on Arg metabolism in developing animals or humans to date. The purpose of this study was to assess the effect of 75% intestinal resection on Arg production from Pro. We used a neonatal piglet model of SBS, undergoing both EN and PN, and stable isotope tracer methodology to assess whole-body Arg synthesis. This experimental design mimics the common clinical scenario, following major neonatal intestinal resection, of an infant who is predominantly PN dependent, receiving trophic amounts of enteral nutrition (EN), while then gradually advancing EN prior to PN weaning.
Methods
The Faculty of Agriculture, Life and Environmental Sciences Animal Policy and Welfare Committee, University of Alberta, approved the research methods (AUP00000155). The project was conducted at a biosecure swine research center, conforming to guidelines of the Canadian Council of Animal Care.
Thirty male Landrace Large White cross-bred piglets aged 3–5 days old were all randomly assigned to receive either 20% or 40% of their total nutrient requirements by the enteral route. They were further allocated to either surgical sham, with no intestinal resection, or a distal intestinal resection (jejunocolic—JC). Piglets underwent isoflurane general anesthesia for surgical intervention (2–3%; Bensen Medical Industries Inc., Markham, Ontario, Canada). Surgery included a laparotomy incision followed by measurement of the small intestine from the ligament of Trietz to the cecum using a 3-0, 60 cm silk suture along the anti-mesenteric border with minimal traction. JC piglets underwent resection of the distal 75% of small bowel, including the ileum, ileocecal valve, and first portion of the colon, with a resultant jejunocolonic anastomosis. In all piglets, a 5-French left external jugular catheter (Braintree Scientific Inc., Braintree, MA, USA) was inserted for PN and a 10-French gastrostomy tube for EN. Following recovery from anesthesia all piglets were housed individually in metabolic cages that were temperature (25–28 °C) and light controlled (12 h on/off light cycle). Antibiotic prophylaxis and pain medications were initiated and maintained for 3 days post-operative.15 Additional broad-spectrum antibiotics were added if piglets showed signs and symptoms of clinical sepsis, as previously published.16
Diet
All piglets were given PN at 100% of their nutrient requirements, with the addition of 20% EN on day 2, and further advancement to 40% EN on day 3 for a subgroup, to mimic both minimal enteral and advancing EN. Both formulations were made in our laboratory and provided 16 g/kg/day amino acids, 10 g/kg/day lipid (Intralipid®) and 29 g/kg/day glucose (Table 1). To prevent osmotic diarrhea, glucose was replaced with polycose in the EN. The formula was previously published and validated.17 Post-operatively, PN was initiated at 50% and gradually increased to 100% by the following morning. An Arg-deficient PN diet was initiated on day 3 (0.6 g/kg/day Arg) and in order to maintain an isonitrogenous solution the concentration of l-alanine was increased. The EN solution contained adequate Arg (1.2 g/kg/day)
Serum amino acids measurement and isotope tracers
Serum amino acid profiles (Cit, Arg, Pro, ornithine, Gln, methionine, alanine, asparagine, glycine, leucine, lysine, phenylalanine, serine, tyrosine, valine) were obtained at baseline (day 0) and on day 7 using liquid chromatography-mass spectrometry (LC/MS/MS).
Tracer studies were conducted on day 6, after 72 h adaptation to the low Arg PN diet, and were given via the gastric catheter for a constant infusion of 6 h. All piglets received a primed bolus and constant infusion as follows: Arg m + 2 label (15N2 guanido-arginine primed 12 µmol/kg/h, constant 20 µmol/kg/h) and Pro m + 1 label (15N proline, primed 20 µmol/kg/h, constant 40 µmol/kg/h). In 40% fed piglets (JC = 5, Sham = 4) an additional Cit m + 3 (ureido-13C, primed 5 µmol/kg/h, constant 5 µmol/kg/h) tracer was given. Blood sampling was undertaken at −60, −30, 0, 60, 120, 180, 240, 270, 300, 330, and 360 min. Samples were deproteinized using methanol (500 µL), and were then centrifuged for 10 min. Supernatants were moved to derivatized vials and were nitrogen dried at 37 °C. The dried samples were then derivatized using 100 µL of 3.0 N HCl-butanol derivative reagent (Regis Technologies Inc., Morton Grove, IL) for 20 min at 55 °C, and re-dried using nitrogen at 37 °C. Finally, the amino acid samples were reconsitituted in 0.1% formic acid and analyzed using an API 4000 triple quadruple mass spectrometer (Applied Biosystems/MDS SCIEX) coupled with an HPLC system (Agilent Technologies Canada Inc., Mississauga, Canada) as previously described.18
Isotope calculations
Isotopic enrichment is expressed as molecule percent excess calculated as enrichment at plateau minus the background enrichment at baseline. Flux of the infused amino acids, Arg, Pro, or Cit was calculated using the equation:
where Q is the flux of the amino acid through the free amino acid pool, i is the rate of infusion of the tracer, Ei is the enrichment of the tracer in the infusion, and Ep is the enrichment of the tracer in the plasma plateau of the infused amino acids.
The conversion rates of the precursor infused isotope to the product was calculated by the formula
Every unit of Cit synthesis from Arg releases one NO molecule. NO was measured as % of Arg flux:
Quantitative real time polymerase chain reaction (qPCR)
TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) was used to isolate RNA from jejunal scrapings of intestinal tissue on day 7 and then treated with DNAse (ThermoScientific). RNA purity and quantity were assessed using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA) and reversed transcribed to cDNA with SuperScript II (Invitrogen). Specific primers and probes for porcine intestinal arginase I and II (ARG1 and ARG2), ornithine decarboxylase, ornithine aminotransferase, pyrroline-5-carboxylate reductase, and ASS were designed using IDT Software (Integrated DNA Technologies, San Diego, CA). Primer sequences are shown in Table 2. Porcine liver samples were taken and used as positive controls for ARG1 and ARG2 expression. qPCR reactions were carried out with RNAse-free water and TaqMan Universal Master Mix II (Applied Biosystems, Foster City, USA). All genes were normalized to the housekeeping gene Gapdh and to the mean of normal control values (same age untreated sow-fed piglets). An ABI Prism 7900 HT Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA) was used to assess gene expression. Samples were run in triplicates and an average taken; those with cycle thresholds >30 were interpreted as non-detectable and excluded, as previously published.19,20
Small intestinal measurements
On day 0 and day 7, while piglets were anesthetized, the small intestinal length was measured, as described above, including post resection in the JC group. On day 7, piglets were euthanized, the intestinal length re-measured, and intestinal samples were collected from the jejunum, ileum, and colon. Mucosal scrapings of a 20-cm jejunal segment were used to measure mucosal mass (jejunum scraping weight/20 cm × 1000/pig weight; expressed in mg/cm/kg). Intestinal samples were collected and preserved in formaldehyde for examination by a certified veterinary pathologist, blinded to the treatment group. Routine hematoxylin and eosin preparation was done and a Nikon Eclipse 80i microscope was used to measure villus height and crypt depth. Ten standard villus heights and related crypt depths were measured and the mean was determined.
Statistical analysis
Data are expressed according to normality as mean and standard error or median with interquartile range (25th–75th percentiles). Groups were compared by either ANOVA or Kruskal Wallis tests, with Tukey or Mann Whitney tests for post hoc analysis. Significance was set at P < 0.05. Sow-fed controls (N = 5) are provided as a reference for data, other than isotope measurements, but are not included in the analysis. Three negative samples were excluded from isotope data due to sampling error.
Results
Piglet outcomes (Table 3)
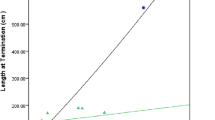
There was no difference in piglet age and size at baseline and no difference in growth over the course of the trial. Intestinal lengthening was only observed in the sham (20% fed sham 29.9 cm and JC −10.4 cm; 40% fed sham 28.1 cm and JC −3.9 cm; SE 6.2; P = 0.01). Small bowel weight was significantly higher in the sham group than in JC regardless of the amount of EN (20% sham 24.7 g/kg and JC 7.3 g/kg; 40% fed sham 25.3 g/kg and JC 6.8 g/kg; SE 1.8; P = 0.0001). Mucosal mass was significantly higher in 20% fed JC vs sham but not in those fed 40% diets (20% fed sham 29.2 mg/cm/kg and JC 38.8 mg/cm/kg; 40% fed sham 31.8 mg/cm/kg and JC 35.5 mg/cm/kg; SE 1.3; P = 0.007). Jejunal villus height was similar among all groups (20% fed sham 0.68 mm and JC 0.63 mm; 40% fed sham 0.51 mm and JC 0.62 mm; SE 0.3; P = 0.26).
Serum amino acids (Tables 4 and 5)
Serum Arg (20% fed sham 55.1 µM and JC 40.4 µM; 40% fed sham 101.0 µM and JC 79.9 µM; SE 5.6; P = 0.0001) and Cit (20% fed sham 58.9 µM and JC 50.9 µM; 40% fed sham 94.8 µM and JC 61.8 µM; SE 4.3; P = 0.005) were significantly higher in 40% fed sham than other groups. Precursors, Pro (20% fed sham 209.0 µM and JC 304.1 µM; 40% fed sham 432.3 µM and JC 401.6 µM; SE 22.8; P = 0.001) and Ornithine (Orn) (20% fed sham 41.1 µM and JC 33.1 µM; 40% fed sham 70.7 µM and JC 47.9 µM; SE 3.7; P = 0.005) were higher in 40% sham, compared to 20% sham and JC (P < 0.05), while Gln was significantly higher in JC vs Sham regardless of % EN diet (20% fed sham 202.3 µM and JC 569.0 µM; 40% 285.0 µM and JC 651.4 µM; SE 46.8; P = 0.0001).
To evaluate the trial effects on serum amino acids and control for baseline values, we calculated the change that occurred from day 0 to day 7. Notably Arg, Cit, Pro, and Orn all declined over the trial period, while Gln declined only in the sham and was increased in the JC groups (20% fed sham −344.0 and JC 211.8; 40% fed sham −452.7 and JC 92.4; SE 80.8; P = 0.02).
Isotope tracers (Tables 6 and 7)
Arg Flux (20% fed sham 1047.7 µmol/kg/h and JC 1776.5 µmol/kg/h; 40% fed sham 1356.7 µmol/kg/h and JC 1296.0 µmol/kg/h; SE 125.1; P = 0.15) was not statistically different between groups. Fractional Pro>Arg (20% fed sham 0.13 µmol/kg/h and JC 0.17 µmol/kg/h; 40% fed sham 0.31 µmol/kg/h and JC 0.17 µmol/kg/h; SE 0.02; P = 0.03) was significantly higher in sham 40% than other groups. Molar conversion of Pro to Arg (Q Pro>Arg) (20% fed sham 128.4 µmol/kg/h and JC 293.7 µmol/kg/h; 40% fed sham 395.6 µmol/kg/h and JC 226.2 µmol/kg/h; SE 37.7; P = 0.09) appeared higher in JC in 20% fed, but did not reach statistical significance. When correcting for the differences in intestinal length, Pro to Arg conversion was higher in JCs than shams regardless of diet (20% fed sham 0.22 µmol/kg/h/cm and JC 2.22 µmol/kg/h/cm; 40% fed sham 0.65 µmol/kg/h/cm and JC 1.54 µmol/kg/h/cm; SE 0.27; P = 0.013).
Analysis of the subgroup that received the Cit tracer allowed for assessment of conversion of Pro to Cit and Arg to Cit. In this subgroup, Cit flux was higher in Sham (40% fed sham 110.4 µmol/kg/h and JC 73.7 µmol/kg/h; SE11.05; P = 0.18). Molar conversion of Pro to Cit (Q Pro > Cit) (40% fed sham 402.1 µmol/kg/h and JC 246.6 µmol/kg/h; SE 36.8; P = 0.02) was higher in sham. However, when adjusted for intestinal length JC conversion was higher (40% fed sham 0.66 µmol/kg/h/cm and JC 1.68 µmol/kg/h/cm; SE 0.20; P = 0.001). Similarly, while Q Arg > Cit conversion (40% fed sham 35.8 µmol/kg/h and JC 22.1 µmol/kg/h; SE 6.1; P = 0.3) was not different, when corrected for intestinal length in the JC the conversion was significantly higher (40% fed sham 0.06 µmol/kg/h/cm and JC 1.5 µmol/kg/h/cm; SE 0.02; P = 0.04). As a result, NO synthesis was doubled in JC piglets when adjusted for intestinal length (NO as % of ARG flux/cm (40% fed Sham 0.004 and JC 0.012; SE 0.005; P = 0.04).
Quantitative PCR
As expected, ARG1 and ARG2 were not quantifiable in the neonatal piglet intestine. The key enzymes for intestinal Arg synthesis are shown in Table 8. Of note, ASS expression was significantly higher in JC 20% than sham 20% (20% fed sham 0.12 and JC 0.65; P = 0.027). While also higher in the JC piglets on the 40% diet, this did not reach statistical significance (sham 0.16 and JC 0.47; P = 0.46).
Discussion
We undertook an investigation of whole-body amino acid metabolism in neonates with SBS because neonatal SBS is a costly and serious problem,21 and because we know, based on our studies in piglets, that the intestine contributes 60% of whole-body Arg synthesis.11 A key difference for neonates is the greater capability of the intestine to produce Arg for entry into the plasma amino acid pool compared to older subjects. In fact, children younger than 3–5 years have all the enzymes required for Arg production solely in the intestine.14 Hence, the neonate with intestinal resection may have insufficient endogenous production of Arg to meet the metabolic demands to respond to inflammation, urea clearance and NO production.
Despite the above, to date, there are few data on the limitations on whole-body Arg if the intestine is compromised in length. Most studies to date have focused on NEC in premature infants, given the observation of low plasma Arg levels preceding NEC and the reduction in prevalence of NEC with Arg supplementation.10 In a premature piglet intraluminal model of NEC, Lorenzo et al.22 allocated piglets to receive either Arg as an NO substrate or N-omega-nitro-l-arginine methyl ester (l-NAME) as an inhibitor of NO synthase.22 In this model, the Arg supplemented group showed less transmural damage, while l-NAME had higher grade NEC on histopathology. Similarly, a systematic review of the available clinical literature, with inclusion of 235 neonates, reported a 59% reduction of NEC with supplementation of Arg.23 Of note, this review only included two publications, so clearly more studies are required.
Because the intestine plays such an important role in Arg synthesis, it is unknown if significant resection of the intestine will hinder whole-body Arg synthesis and contribute to the morbidity, including limited growth, observed in SBS infants. In this study, we chose to use a neonatal piglet model of SBS without ileum, as this better represents clinical SBS in infants, where the ileum is usually lost or resected.24 We have previously shown that this JC model will develop true IF, with a dependence on PN, and limited potential for structural adaptation.4 The majority of infants with IF are provided early trophic nutrition to enhance adaptation and will subsequently wean from PN according to the tolerance for EN. Previously, it has been shown in piglets that 40% of nutrient requirements delivered enterally is the minimal amount necessary to support growth of mucosal mass and this would generally be considered above trophic amounts of nutrition.25 Therefore, we studied both 20% and 40% EN to mimic trophic nutrition and enteral advancement. However, we did not see a major impact of the 40% advancement on mucosal adaptation as jejunal scraping and mucosal mass were not significantly different in 40% vs. 20% EN fed, while 20% EN fed were higher in the JC group.
Regardless, when comparing the JC model to the sham we know that the JC had one quarter of the gut length. While we had anticipated that this would have a negative impact on whole-body Arg synthesis, we found that even with this massive reduction in intestinal length, Arg synthesis was in fact similar between both the JC and sham. Therefore, this would suggest that the JC SBS piglets were able to adapt to massive resection and upregulate intestinal Arg synthesis, so as to maintain similar levels to the non-resected sham. This hypothesis is supported by our molecular data, showing an increase in ASS expression in the jejunal tissue of the JC piglets, at least with 20% minimal trophic nutrition. It is plausible the 20% EN was sufficient to reach maximum endogenous ARG synthesis,26 and thus with 40% EN saturation occurred and no upregulation was required. Furthermore, upregulated synthesis was made evident when adjusting synthesis from enteral Pro for the piglet intestinal lengths. The JC showed a 5.5-fold increase in Arg synthesis from Pro over the sham, regardless of the amount of enteral diet fed. However, we acknowledge that we did not perform portal catheterization and thus strictly speaking have not isolated the intestinal synthesis of Arg. Nevertheless, given we did identify key enzymatic differences within the intestine, it is reasonable to assume these contribute to measured whole-body Arg synthesis from the dietary precursor Pro. To reiterate, this was equivalent between both groups, despite large differences in intestinal mass and no other model system differences, including similar PN delivery.
Another finding that supports the upregulation of intestinal Arg and more limited Cit synthesis in SBS was observed utilizing a Cit tracer, where there was very limited conversion of Arg back to Cit as compared to Pro > Arg conversion. Since the Arg to Cit pathway releases NO, it is of importance to note that production was doubled in JC piglets compared to sham when taking into effect intestinal length as it is the main source of Arg in neonates.27
Wilkinson et al.11 compared Arg-deficient vs Arg-sufficient diets and the different synthesis that occurs using both intragastric and intraportal tracer infusions, to isolate the intestinal contribution, in neonatal piglets.11 They found that whole-body Arg synthesis was upregulated in those piglets given the Arg-deficient diet; however, the intestinal contribution was not increased. While they did find that the intestine was responsible for 50–60% of whole-body Pro to Arg conversion, it is assumed another site was responsible for the upregulation that occurred and this cannot be excluded in our own study. Further studies are required to understand the whole-body adaptations in Arg metabolism that occur, given a limited dietary supply of Arg and/or massive intestinal resection in neonates. This study is the first to provide data that the intestine contributes to such adaptation, by increased enzymatic expression of ASS.
While this is the first study of the impact of SBS anatomy on Arg metabolism in the piglet, Dejong et al. studied a rat model of SBS with 75% intestine resection to assess the effect of resection on Cit and Arg tissue concentration, and renal uptake and release. Using Cit and Arg radioactive isotope tracers in sham, 75% resected, and fasted control rats, they showed a reduction of Arg renal synthesis by 10% in the SBS group, while whole-body Arg synthesis was not dramatically altered.28 Thus, the kidney is not responsible for the upregulation to maintain whole-body Arg levels; therefore, intestinal upregulation is plausible.
A notable finding in our study was the decline in serum Arg in all groups over the trial period. Given equivalent synthesis from dietary Pro, this suggests either increased Arg utilization or insufficient whole-body synthesis to maintain the serum amino acid level. Arg is an important substrate for the production of NO, which modulates intestinal muscle relaxation, regulates mucosal blood flow, and maintains barrier function.23 Other notable metabolic fates of Arg include ammonia detoxification, and creatine and polyamine synthesis. Overall, all amino acids that are utilized by the urea cycle declined equivalently in all our study groups, with the exception of Gln, which interestingly was higher in JC than Sham groups. As the major fuel of the intestine it is possible that the increase in Gln in the JC piglet was driven by decreased utilization in the intestine, because of shorter intestinal length, with increased utilization expected in the sham given the longer length. Perhaps consistent with this theory, there was lower serum Gln in the 40% fed JC than the 20% fed JC, while there was slightly less loss of length at day 7 with the increased EN.
A limitation of this study was the small sample size in the 40% fed JC group, which may have limited our ability to identify significant differences from the 20% EN group. A further limitation was the inability to more accurately infer the intestinal contribution in whole-body Arg synthesis, given we did not perform portal vein sampling for isotope enrichment studies. However, as aforementioned, we did assess key Arg enzymatic changes in the jejunal tissue of the intestine and our findings do suggest that intestine plays an important role in the regulation of whole-body Arg synthesis, following massive intestinal resection. Measurement of the protein, rather than merely mRNA expression, would have also provided additional confirmatory information.
In conclusion, whole-body Arg synthesis was similar in resected and un-resected piglets due to upregulation of synthesis by the intestine and possibly by other organs. Overall, PN had a reducing effect on serum amino acids and the role of further supplementation of these important amino acids in this vulnerable population to aid normal growth and development warrants further investigation.
References
Amin, S. C., Pappas, C., Iyengar, H. & Maheshwari, A. Short bowel syndrome in the NICU. Clin. Perinatol. 40, https://doi.org/10.1016/j.clp.2012.1012.1003 (2013).
Merritt, R. J. et al. Intestinal rehabilitation programs in the management of pediatric intestinal failure and short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 65, 588–596 (2017).
Tappenden, K. A. Intestinal adaptation following resection. JPEN J. Parenter. Enteral Nutr. 38, 23S–31S (2014).
Belza, C., et al. Predicting intestinal adaptation in pediatric intestinal failure: a retrospective cohort study. Ann Surg. 269, 988–993 (2019).
Wang, W. W., Qiao, S. Y. & Li, D. F. Amino acids and gut function. Amino Acids 37, 105–110 (2009).
Urschel, K. L., Shoveller, A. K., Pencharz, P. B. & Ball, R. O. Arginine synthesis does not occur during first-pass hepatic metabolism in the neonatal piglet. Am. J. Physiol. Endocrinol. Metab. 288, E1244–E1251 (2005).
Morris, J. S. M. Arginine: beyond protein. Am. J. Clin. Nutr. 83, 508S–512S (2006).
Wu, G. Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17 (2009).
Polycarpou, E. et al. Enteral L‐arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates. J. Parenter. Enteral Nutr. 37, 617–622 (2013).
Richir, M. C. et al. Low plasma concentrations of arginine and asymmetric dimethylarginine in premature infants with necrotizing enterocolitis. Br. J. Nutr. 97, 906–911 (2007).
Wilkinson, D. L. et al. Arginine synthesis is regulated by dietary arginine intake in the enterally fed neonatal piglet. Am. J. Physiol. Endocrinol. Metab. 287, E454–E462 (2004).
Melis, G. C. et al. The feeding route (enteral or parenteral) affects the plasma response of the dipetide Ala-Gln and the amino acids glutamine, citrulline and arginine, with the administration of Ala-Gln in preoperative patients. Br. J. Nutr. 94, 19–26 (2007).
Urschel, K. L., Evans, A. R., Wilkinson, C. W., Pencharz, P. B. & Ball, R. O. Parenterally fed neonatal piglets have a low rate of endogenous arginine synthesis from circulating proline. J. Nutr. 137, 601–606 (2007).
Kohler, E. S. et al. The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes. BMC Dev. Biol. 8, 107 (2008).
Lim, D. W. et al. Synergy of glucagon-like peptide-2 and epidermal growth factor coadministration on intestinal adaptation in neonatal piglets with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G390–G404 (2017).
Josephson, J. et al. Parenteral soy oil and fish oil emulsions: impact of dose restriction on bile flow and brain size of parenteral nutrition-fed neonatal piglets. JPEN J. Parenter. Enter. Nutr. 39, 677–687 (2015).
Wykes, L. J., Ball, R. O. & Pencharz, P. B. Development and validation of a total parenteral nutrition model in the neonatal piglet. J. Nutr. 123, 1248–1259 (1993).
Urschel, K. L., Rafii, M., Pencharz, P. B. & Ball, R. O. A multitracer stable isotope quantification of the effects of arginine intake on whole body arginine metabolism in neonatal piglets. Am. J. Physiol. Endocrinol. Metab. 293, E811–E818 (2007).
Beggs, M. R. et al. Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. Am. J. Physiol. Ren. Physiol. 313, F629–F640 (2017).
Beggs, M. R. et al. TRPV6 and Cav1.3 mediate distal small intestine calcium absorption before weaning. Cell Mol. Gastroenterol. Hepatol. 8, 625–642 (2019).
Oliveira, C. et al. Change of outcomes in pediatric intestinal failure: use of time-series analysis to assess the evolution of an intestinal rehabilitation program. J. Am. Coll. Surg. 222, 1180–1188 e1183 (2016).
Di Lorenzo, M., Bass, J. & Krantis, A. Use of l-arginine in the treatment of experimental necrotizing enterocolitis. J. Pediatr. Surg. 30, 235–241 (1995).
Mitchell, K. et al. Arginine supplementation in prevention of necrotizing enterocolitis in the premature infant: an updated systematic review. BMC Pediatr. 14, 226 (2014).
Chandra, R. & Kesavan, A. Current treatment paradigms in pediatric short bowel syndrome. Clin. J. Gastroenterol. 11, 103–112 (2018).
Burrin, D. G. et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am. J. Clin. Nutr. 71, 1603–1610 (2000).
Ball, R. O., Urschel, K. L. & Pencharz, P. B. Nutritional consequences of interspecies differences in arginine and lysine metabolism. J. Nutr. 137, 1626S–1641S (2007).
Wu, G. & Knabe, D. A. Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol. 269, R621–629 (1995).
Dejong, C. H., Welters, C. F., Deutz, N. E., Heineman, E. & Soeters, P. B. Renal arginine metabolism in fasted rats with subacute short bowel syndrome. Clin. Sci. 95, 409–418 (1998).
Acknowledgements
This work was fully funded by the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
P.W.W. and J.M.T. contributed to conception/design of the research; M.L., G.S., P.W., P.N.N., M.R., P.B.P., M.R.B., R.T.A., R.O.B., J.M.T., and P.W.W. contributed to acquisition, analysis, or interpretation of the data; M.L. and J.M.T. drafted the manuscript. All authors critically revised the manuscript, read and approved the final manuscript, and agree to be fully accountable for ensuring the integrity and accuracy of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lansing, M., Slim, G., Wizzard, P. et al. Intestinal resection affects whole-body arginine synthesis in neonatal piglets. Pediatr Res 89, 1420–1426 (2021). https://doi.org/10.1038/s41390-020-01139-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01139-1
- Springer Nature America, Inc.