Abstract
Most of the organs of the digestive tract comprise secretory epithelia that require specialized molecular machines to achieve their functions. As such anterior gradient (AGR) proteins, which comprise AGR1, AGR2, and AGR3, belong to the protein disulfide isomerase family, and are involved in secretory and transmembrane protein biogenesis in the endoplasmic reticulum. They are generally expressed in epithelial cells with high levels in most of the digestive tract epithelia. To date, the vast majority of the reports concern AGR2, which has been shown to exhibit various subcellular localizations and exert pro-oncogenic functions. AGR2 overexpression has recently been associated with a poor prognosis in digestive cancers. AGR2 is also involved in epithelial homeostasis. Its deletion in mice results in severe diffuse gut inflammation, whereas in inflammatory bowel diseases, the secretion of AGR2 in the extracellular milieu participates in the reshaping of the cellular microenvironment. AGR2 thus plays a key role in inflammation and oncogenesis and may represent a therapeutic target of interest. In this review, we summarize the already known roles and mechanisms of action of the AGR family proteins in digestive diseases, their expression in the healthy digestive tract, and in digestive oncology. At last, we discuss the potential diagnostic and therapeutic implications underlying the biology of AGR proteins.
Similar content being viewed by others
Introduction
The digestive system is composed of organs with specific differentiated cells, each of which has specific functions in the digestion process. Beyond these differences, all these organs show common secretory properties essential for homeostasis. Alterations in the secretory pathway have been implicated in oncogenesis. Digestive cancers, like most cancers, often result from a chronic inflammation that dysregulates cell metabolism, gene expression, and epigenetics. Chronic inflammation can be responsible for precancerous lesions, before the evolution toward invasive cancer, like Barrett metaplasia in the esophagus, inflammatory bowel diseases (IBD) in the gut, or pancreatic intraepithelial neoplasia (PanIN) in the pancreas. Among the cellular dysregulations that favor carcinogenesis, alterations of endoplasmic reticulum (ER) homeostasis and the subsequent adaptive mechanisms activated to cope with it were shown to significantly contribute to these processes. The ER is the first compartment of the secretory pathway that enables the proper biogenesis, folding, and maturation of secretory and transmembrane proteins [1]. Beyond its role in protein homeostasis, the ER also maintains calcium and lipid homeostasis. As part of the ER folding and quality control machinery, the protein disulfide isomerase (PDI) family is involved in the formation of inter and intra-molecular disulfide bridges in client proteins, productive protein folding, redox activity, and disulfide exchange through their thioredoxin-like domain [1,2,3]. Although the roles of most PDI family members in protein folding have been well characterized, their function in ER homeostasis control and proteostasis maintenance is still unclear [4]. Along with glutaredoxins and thioredoxins, PDIs are members of the thioredoxin (TRX) superfamily which are characterized by the presence of a thioredoxin fold [5]. So far, 20 PDIs have been identified [4, 6]. Most of them are located in the lumen of the ER, while others are transmembrane proteins (TMX 1 to TMX 5) [6]. PDIs are defined as “proteins that can react with cysteine side chains”, but it includes also “proteins that contain non-thiol-reactive thioredoxin-like domains with chaperone-like activities for ER folding and secretion of proteins” [7]. Therefore, the PDIs can be divided between thiol-non reactive (ERp27 and ERp29) and thiol-reactive proteins.
The anterior gradient (AGR) family is composed of three PDI-like proteins: AGR1 (also known as thioredoxin domain-containing protein 12 (TXNDC12)), AGR2 (protein disulfide isomerase family A member 17 (PDIA17)), and AGR3 (protein disulfide isomerase family A, member 18 (PDIA18)) [7, 8]. AGR1 is expressed in the ER and plays a role in the formation of mixed disulfide bonds with protein [9]. The expression of AGR2 is induced upon disruption of ER homeostasis, through the activation of the ATF6 and IRE1 arms of the unfolded protein response (UPR) [1]. AGR2 is shown to contribute to protein folding and maturation [10, 11] and to participate in the elimination of misfolded proteins through ER-associated degradation (ERAD) [12]. Several studies in the last decade have shown that AGR2 expression is associated with poor prognosis in solid cancers and may play pro-oncogenic roles through yet uncharacterized mechanisms. AGR3 is also an ER-resident protein involved in the formation of disulfide bonds but its expression is independent of ER stress [13]. Compared to AGR2, less is known about the functions and regulations of both AGR1 and AGR3 as well as their respective roles in the digestive tract. In this review, we cover the most recent data on the AGR family from biochemical and cell biology perspectives; we next connect these properties to the functions of these proteins in both normal and pathological gastrointestinal tract and lastly discuss the potential theranostic aspects related to the AGR family.
The AGR family
Genes, structures, and regulators
AGR2 is the most documented member of the AGR family. It was initially identified in the anterior region of the ectoderm of Xenopus laevis as XAG-2 [14,15,16]. XAG-2 plays important roles in endodermal patterning and in forebrain maturation by anteroposterior migration of the cement gland (mucus-secreting organs) during embryogenesis [14, 17]. In humans, AGR2 is also expressed during embryonic development in the endoderm-derived tissues like esophagus, trachea, lung, liver, stomach, and in the gut [18]. In adult human tissues, the highest levels of AGR2 expression are observed in the gastrointestinal tract from the stomach to the rectum, in the genitourinary tract (urinary bladder), and in the respiratory system (see Fig. 3). Human AGR2 is located on chromosome 7 [19] and is composed of 8 exons and 7 introns. Seven different AGR2 transcripts are reported in the Ensembl database. In cancer, the most recurrent AGR2 anomaly is amplification; only a few mutations or deletions were described [20]. AGR2 predominant isoform is 175 amino acid long resulting in a molecular weight of ~20 kDa. Three other isoforms have been described [B5MC07, C9J3E2, and H7C3Z9 (UniProt)] but so far, there is no information on their functions. Its structure is composed of an N-terminal cleavable signal peptide, a non-canonical thioredoxin domain CXXS, a peptide binding loop, and a C-terminal domain ER-retention (KTEL) motif (Fig. 1a, b) [1]. AGR1 has a high similarity with the thioredoxin domain CXXC motif in invertebrates, but is lost in higher vertebrates where it is replaced by the CXXC-containing protein TXNDC12 [21,22,23,24,25]. It is an 18 kDa ER-resident protein composed of 146 amino acid in its mature sequence (Human Protein Atlas [26]). AGR1 gene is located on chromosome 1 unlike AGR2 and AGR3. Similar to other PDI, it is involved in disulfide bond formation [24]. AGR2 and AGR3 are present in both amphibians and higher vertebrates [8]. AGR proteins ER-retention motifs differ (i.e., EDEL for AGR1, KTEL for AGR2, QSEL for AGR3) as do their thioredoxin-like motifs (Fig. 1a, b) [27]. Even though both proteins are very similar (>90% identity) and highly conserved in mammals (e.g., mouse and human—Fig. 1b), the role of AGR3 is less documented than AGR2 but its expression seems correlated with AGR2 and plays similar roles in some aspects, for instance promoting migration and metastasis [28]. However, the expression of AGR3 is independent of ER stress suggesting that the regulation of the two proteins differs from each other. AGR3 gene is located on chromosome 7p21.1, it is composed of 10 exons and can produce three different transcripts (AGR3-201, AGR3-202, AGR3-203). The molecular weight of AGR3-201 is 19.2 kDa and contains 166 amino acids (Human Protein Atlas [29]). AGR3 was first identified in breast cancer cell membranes and in breast tumors, where its expression correlates with estrogen receptor expression [30, 31]. It is expressed in airway epithelium where it is required for mucociliary clearance and for regulation of ciliary beat frequency [32]. It is also expressed in prostate, as well as stomach and liver tissues of the digestive tract [33].
a Phylogram representation of human and mouse AGR1, 2, and 3 proteins (performed using ClustalOmega). b Amino acid (aa) sequence alignment of human and mouse AGR1, 2, and 3 proteins. Three functional domains are indicated (signal peptide for the targeting to the ER; thioredoxin domain; peptide binding and ER retention). Polar and basic aa are indicated in pink, polar and acidic aa in blue, polar and neutral aa in green, non-polar and neutral in red. AA identity is indicated by “*”, conservation by “:”. c Structure of the E60–K64 interaction-dependent AGR2 dimer. d Structure of the C81 interaction-dependent AGR2 dimer.
AGR2 exists in monomeric or homodimeric forms [34]. AGR2 dimerizes through E60 and K64-dependent salt bridges, far from the thioredoxin domain [35, 36] (Fig. 1c). It was also reported that AGR2 could form cysteine 81-dependent dimers but the precise functions of these dimers remain elusive [37]. AGR2 was also found to form heterodimers with AGR3 in vitro, but again the functions associated with this complex are unknown [38] (Fig. 1d). AGR2 thioredoxin-like domain is found at position 81–84 (CXXS) and is conserved in mouse and human. It may be involved in the formation of mixed disulfide bridges with client proteins [39]. ER stress conditions were shown to induce AGR2 monomerization, which was correlated with its secretion to the extracellular milieu [34, 40]. Interestingly, AGR3 thioredoxin-like domain is not conserved in humans and mice, exhibiting a CXXS sequence in humans and a canonical CXXC site in mice (Fig. 1b). This might reflect a difference in AGR3 redox functions between the species. The AGR2 peptide binding loop (VDPSL, conserved in AGR3, Fig. 1b) interacts with proteins such as the AAA+ protein RuvBL2/Reptin [41]. The AGR2 C-terminal domain contains a non-canonical ER-retention motif (KTEL), that enables partial retention of AGR2 in the ER through KDEL receptors [27].
AGR proteins functions and subcellular locations
AGR proteins exhibit various subcellular locations, associated with different roles (Fig. 2). So far most if not all functions of AGR1 were associated with the ER localization [24] and in particular in the regulation of the ATF6 arm of the UPR [42, 43]. Even though AGR2 and AGR3 show all the features of ER-resident luminal proteins (i.e., signal peptide and ER-retention motif), both proteins are released in the extracellular environment and AGR2 was also found in the cytosol [44]. In the ER, AGR2 (erAGR2) participates in protein folding, maturation, and secretion. It is also involved in ER-associated protein degradation [1]. In particular, erAGR2 contributes to the productive folding of (i) mucins (MUC2, MUC5A/C) most likely through the formation of mixed disulfide bonds [13], (ii) low affinity, Ca2+-binding, multiple EF-hand proteins such as CALU and RCN1 [45,46,47,48], (iii) proteases such as Cathepsins B and D [45], (iv) adhesion molecules such as EpCAM [49], or (v) other PDIs [50]. In contrast to AGR2, the ER functions of AGR3 remain to be fully characterized. The contribution of AGR2 to the productive folding of proteins whose functions are associated with cancer development, thereby sustaining their functions upon overexpression might represent a pro-oncogenic feature (Table 1). In addition to the “canonical” ER localization of AGR2 and AGR3, both proteins were also found to be secreted extracellularly (eAGR2, eAGR3). Although the underlying mechanisms have not been studied for AGR3, it appears that ER stress is a driver of AGR2 secretion. Indeed, we demonstrated that upon ER stress induction either overexpression of AGR2 or dimer disruption (or both) correlated with enhanced AGR2 secretion [34]. AGR2 dimer regulation was found to depend on the p24 cargo receptor family member TMED2 [34]. It was also shown that the Cys81Ser mutation in the CXXS domain also prompted AGR2 secretion [51]. Moreover, AGR2 KTEL motif binds to KDEL-R in a non-canonical manner, enabling an easier secretion of AGR2 compared to other proteins KDEL-containing motifs [1, 52, 53]. This was confirmed upon the replacement of KTEL by KDEL in AGR2 which led to its ER sequestration [52]. The extracellular AGR2 (eAGR2), as well as eAGR3, plays multiple pathophysiological roles in inflammation, cell proliferation, angiogenesis, cell migration, and metastasis, through autocrine, paracrine, and maybe endocrine secretion (Table 1). The third subcellular localization in which AGR2 was detected is the cytosol. The mechanism by which AGR2 might relocate from the ER lumen to the cytosol was identified to be an ER stress-induced reflux of properly folded proteins initially identified in yeast and also found in mammalian cells [44]. Although not demonstrated yet for AGR3, this phenomenon is likely to occur in the same manner as for AGR2 as suggested by the large number (12 out of 39) of cytosolic/nuclear proteins found associated with AGR3 in large-scale protein–protein interaction experiments [54]. These observations remain however to be confirmed experimentally with minimized experimental biases. Thus far the most important studies on the role of cytosolic AGR2 (cAGR2) have focused on its capacity to inhibit p53 protein tumor suppressor activity. Indeed, beyond the demonstrated genetic alterations associated with alterations of p53 functions, AGR2 could exert a non-genetic inhibition of p53 [44, 55]. Indeed, AGR2-p53 co-immunoprecipitation experiments performed on the cytosolic fraction extracted from A549 cells, showed that inhibition of p53 phosphorylation and activity resulted from the interaction of p53 with AGR2 consecutive to reflux of AGR2 into the cytosol in response to treatment with tunicamycin, thapsigargin, Brefeldin-A, or etoposide, a Topoisomerase II inhibitor [44, 55]. Moreover, preventing the association of cAGR2 with p53 by using specific AGR2 nanobodies expressed in the cytosol, resulted in the restoration of p53 activation upon stress [44, 55]. Cytosolic p53-dependent pro-oncogenic roles of AGR2 might also be mediated indirectly, for instance through the Dual Specificity Phosphatase 10 (DUSP-10) [56], Tumor-Susceptibility-Gene 101 (TSG-101) [53] or RuvbL2/Reptin [41], but the underlying mechanisms remain to be identified. Cytosolic roles of AGR2, and possibly AGR3 might as well (as for p53) be associated with pro-oncogenic gains-of-functions that need to be experimentally validated in relevant models (Table 1).
AGR family proteins expression in the gastrointestinal tract
Organ specificity
Analyses of the literature and databases (Human Protein Atlas [57, 58]) showed that AGR2 mRNA and protein are expressed from the stomach to the rectum in a healthy human digestive tract with the highest expression detected in the ileum and colon [59]. It is mostly found in luminal mucosal cells, witnessing its secretion with mucins in the intestinal lumen [51]. We confirmed that the AGR2 protein is not detected in the liver and in the two Malpighian tissues of the digestive tract that are the esophagus and the anus, while it is strongly expressed in all the other parts of the digestive system including the pancreas and gallbladder (Fig. 3a). The highest expression of AGR2 is observed in both stomach, colorectal and ileum epithelia (Fig. 3a, b). AGR1 is ubiquitously expressed in all human tissues and, in the gastrointestinal tract it is particularly abundant in the liver and rectum (Human Protein Atlas [26, 22]). In contrast, AGR3 is highly and almost exclusively expressed in the digestive tube, liver, respiratory system and female organs (Human Protein Atlas [29]).
a AGR2 expression measured by immune-histochemistry. Black borders indicate images obtained in-house and orange borders indicate data from the Human Protein Atlas (https://www.proteinatlas.org/ [50, 51]). b AGR1, AGR2, and AGR3 gene expression data extracted from the Human Protein Atlas measured by RNA-sequencing in the colon, stomach, liver, and pancreas of healthy tissues.
Cell specificity
AGR2 is expressed in epithelial cells from various tissues. It is essential in the production of gel-forming mucins and for the protection of the gastrointestinal tract. Cells exhibiting the highest expression of AGR2 throughout the entire gastrointestinal tract, and especially in the rectum, colon, ileum, and stomach, are mucus-secreting cells, namely goblet cells and gastric mucocytes. Undifferentiated cells, Paneth cells, and enterocytes are other specialized cells with high expression of AGR2 in the digestive tube. Both Paneth and goblet cell deregulation, especially downregulation, are involved in IBD. Indeed, the loss of the protective mucus layer participates in the aggression of the intestinal epithelium by pathogens and is involved in the genesis and maintenance of these diseases [60]. AGR2 expression is also reported in intestinal enteroendocrine cells. In the gallbladder, biliary tract, and liver, AGR2 is specifically expressed by cholangiocytes. AGR1 is ubiquitously expressed in all organs; single cell analysis shows the highest expression for exocrine pancreatic cells, ductal cells, and pancreatic endocrine cells among all the digestive cells from an RNA single cell analysis. Regarding AGR3 mRNA expression, proximal enterocytes are the highest expressers, followed by Paneth cells, goblet cells, and distal enterocytes. There is a low expression of AGR3 in enteroendocrine cells, gastric mucocytes, exocrine glandular cells. A very weak signal is found for ductal cells, cholangiocytes, and pancreatic endocrine cells, while there is no expression in hepatocytes (Human Protein Atlas, Single cell [26, 57, 57]).
Roles in the gastrointestinal tract
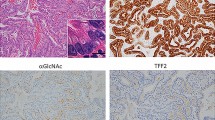
AGR2 is essential for the maintenance of homeostasis and the protection of the digestive tract, as it has a key role in mucus production, secretion, proliferation, and differentiation. AGR2 is known to interact with different mucins of the intestinal epithelial layer (MUC1, MUC2, MUC5AC, MUC5B) and is indispensable for their proper folding and secretion through its PDI-like domain [61]. AGR2 could be secreted with MUC2, but its role within the mucus layer in the intestinal lumen remains obscure [62]. In the stomach, AGR2 participates in calpain production and secretion, which is indispensable for mucosa protection [28]. It is also involved in mucus production in other organs such as lungs [60] or pancreas [63]. Mice knock-out for Agr2 shows large intestinal hyperplasia, from the antrum to the colon, causing multiple nutrient deficiencies and a shorter lifespan compared to Agr2+/+ mice (median survival 20 vs. 60 weeks) [64]. In the stomach of Agr2-/- mice, parietal and enteroendocrine cells are slightly present, and goblet cells expressing different gastric mucosal cell markers such as Trefoil factor family 2 (TFF2) and Griffonia Simplicifolia II (GSII)+ are overexpressed. As TFF2 and GSII markers normally disappear at birth, it is thought that AGR2 plays a key role in the maturation of gastric goblet cells. The gastric cells from Agr2-/- mice showed increased Ki67 expression and overexpressed SOX9 responsible for the maintenance of stem cell pluripotency. All these gastric features of Agr2-/- mice correspond to preneoplastic lesions known as spasmolytic protein expressing metaplasia [64]. In the intestine, goblet cells of Agr2-/- mice lose their normal morphology and functions, likely associated with defects in the production of mucus and MUC2 [59]. A dramatic expansion of the Paneth cell compartment and abnormal Paneth cell localization were observed in their small intestine. Mice developed severe terminal ileo-colitis with neutrophil infiltration in the lamina propria, hyperplasic submucosa and crypt base, Peyer’s patch hyperplasia, and granulomatous inflammation. Paneth, goblet, and progenitor cells experienced ER stress [61, 64, 65]. In conclusion, these experiments provide important information on the key roles of AGR2 in the digestive tract and especially its protective and anti-inflammatory roles.
AGR proteins in digestive cancers
AGR2 is overexpressed in numerous digestive solid cancers such as pancreas [66], biliary tract [67], esophagus [68], stomach [69], and colon [70, 71] cancers. Analysis using the pan-cancer TCGA data from cBioportal data sets reports mainly amplifications of AGR2, but also AGR3 and AGR1 in cancers, whereas few deletions are observed [72]. From a meta-analysis of 3285 cases of different solid tumors, AGR2 is associated with the occurrence of metastasis and a poor prognosis [73, 74]. Analysis of both the Human Protein Atlas and Cancer Genome Atlas [57] shows overexpression of AGR2 at both mRNA and protein levels in most digestive neoplasia, especially gastric, colorectal, pancreatic, and biliary tract adenocarcinoma with the highest overexpression in stomach cancer. Cholangiocarcinoma (CCA) data have been grouped with those of hepatocellular carcinoma (HCC), in which RNA and protein expression are poorly to not detected. However, a case-per-case analysis shows that for CCA, AGR2 is highly expressed. As AGR2 and AGR3 have similar roles in some aspects, we have compared their differential protein expression (tumors with high to moderate expression of proteins) in digestive cancers from the Human Protein Atlas (Fig. 4a, b). In intestinal and pancreatic cancers, high and moderate expression of AGR2 and AGR3 is noted in 80–70% of the cases, respectively. In stomach cancer, 100% of tumors have a high or moderate expression of AGR2, and 80% of tumors express AGR3. Among the liver tumors of the Human Protein Atlas, one-third express AGR2, whereas only 1 tissue sample out of 11 expresses AGR3. In the liver, all tumors expressing AGR2 and AGR3 are emanating from the biliary tract (Fig. 4a, b).
a Percentage of tumors from liver, pancreas, stomach, and intestinal sphere displaying a positive AGR2 (pink) or AGR3 (blue) immune-histochemistry staining (full circle = 100%). b AGR1, AGR2, and AGR3 gene expression data extracted measured by RNA-sequencing in the colon, stomach, liver, and pancreas of cancer tissues. Data extracted from the Human Protein Atlas (https://www.proteinatlas.org/ [50, 51]).
Esophagus adenocarcinoma
Although anomalies are very unlikely, amplification is the most recurrent anomaly in AGR2 and 3 genes in esophagus adenocarcinoma, and only one mutation in AGR1 gene was observed in this cancer subtype. Regarding esophageal squamous-cell carcinoma, no alterations of AGR1, 2, and 3 have been observed [20]. AGR2 is overexpressed in esophagus adenocarcinoma and in Barrett metaplasia [55, 75]. Overexpression of AGR2 in Barrett metaplasia may be a consequence of chronic ER stress secondary to permanent reflux of bile acid. Furthermore, immunohistochemical analysis of AGR2 has been found positive in 68 out of 69 esophagus adenocarcinoma but only focally in 15 out of 41 cases of squamous-cell carcinoma, making it a marker to differentiate them in combination with other markers in the context of esophageal carcinoma of uncertain differentiation [76]. Interestingly, in a whole genome array, Barrett metaplasia and esophagus adenocarcinoma tissues showed an increase of AGR2 mRNA, among other functional elements of the ER and Golgi apparatus and among secreted glycoproteins like MUC2B and MUC6, compared to healthy esophagus tissue. Among these genes, some of them are involved in the formation of a protective mucous membrane to protect the esophagus tissue against acidity. This indicates an increase in the glycosylation capacities of Barrett and esophagus adenocarcinoma compared to normal esophagus mucosa, and the potential implication of AGR2 in this increased glycosylation capacity [68].
Gastric adenocarcinoma
Analysis of the pan-cancer TCGA data indicates that amplification of AGR2 and 3 genes is the most common observed alteration with very few deletions or missense mutations, while nearly no missense mutations and no amplification anomaly are found for AGR1 [20]. Gastric adenocarcinoma cell lines, particularly SNU-520, HUG-1N, MKN-45, and IM-95 are the cancer cell lines with the highest expression of AGR2 in cbioportal.org [20] and Depmap.org [77]. A proteomic analysis comparing two gastric cancer cell lines with the differential potential of metastasis showed that AGR2 was the most differentially overexpressed protein, supporting the capacity of AGR2 to predict the risk of developing metastasis in gastric cancer [78]. In vitro analyses carried out on the gastric cancer cell line Tu-Kato III (TP53 deleted) showed that AGR2 favors cell growth and resistance to apoptosis under stress or hypoxia [79]. In a murine xenograft model of Tu-Kato-III treated with an AGR2 targeting miRNA, tumor growth was 8 fold lower and there were much less mice with peritoneal metastases than in the control group, suggesting AGR2 could contribute to gastric cancer aggressiveness and metastasis [79]. Expression of AGR2 has a prognostic value in gastric adenocarcinoma, especially in the early stages of the disease. In a retrospective study of 436 gastric cancer and 92 non-cancerous gastric samples [69] patients with low expression of tumor AGR2 had longer survival than those with high expression of tumor AGR2 in TNM stages I, II, and III groups, but not for those at stage IV. In this study, 46.8% of gastric cancer samples had a correlation between high expression of AGR2 and Cathepsin-D, a marker of tumorigenesis associated with the process of metastasis [80]. Increased tumor size, depth of invasion, tumor stage (based on UICC TNM classification) and positive vessel invasion, and lymphatic metastasis were also significantly associated with high expression of AGR2 [69]. However, another immunohistochemistry analysis performed on 145 biopsies of patients with gastric adenocarcinoma didn’t find a prognostic value of AGR2 [81]. There was even a tendency for a poorer prognosis for patients with low expression of AGR2. This may be due to a difference in the proportion of patients with stages I-II-III vs IV. AGR1 might act as a pro-oncogenic protein in gastric cancers. Its expression was measured by immunohistochemistry and qPCR in GC tissues of patients and found to be significantly more expressed in tumor tissues than in normal tissues. Moreover, AGR1 expression was found to be correlated with tumor size and poor clinical prognosis [82].
Primary liver cancers
Neither AGR2 nor AGR3 is expressed in hepatocytes and reports describing their roles in HCC or models of HCC might be due to specificities of the samples studied. A recent study based on the analysis of transcriptomic profiles of tissue specimen of patients with HCC or intrahepatic cholangiocarcinoma (iCCA) demonstrated the existence of 4 liver cancer (LC) subtypes (LC1–LC4) showing distinct genetic variations. LC1 was a typical HCC, whereas LC2 was iCCA-like HCC, LC3 was HCC-like iCCAs, and LC4 corresponded to iCCAs. Gene expression of AGR2 was absent in LC1 and appeared with the iCCA component, AGR2 being a marker of intrahepatic CCA-like HCC associated with TP53 mutation, ER stress, and unfavorable prognosis [83]. In the normal biliary tract, AGR2 is expressed in the mucus-secreting tall cells that cover the large bile ducts, and its expression was found enhanced in 100% of hilar and extra-hepatic cholangiocarcinoma (eCCA) and in 50% of the iCCA [67]. No data about the type of alteration are available on cbioportal.org. In addition, a cancer-specific isoform of AGR2 (AGR2vH) has been identified in metastatic CCA, conferring migrating and adhesion ability to metastatic cells [84]. The expression of AGR3 was analyzed on tissue sections of 74 patients diagnosed with either HCC or iCCA by immunohistochemistry. A negative or very weak heterogeneously expression of AGR3 was observed in HCC patients, whereas AGR3 expression was moderate or strong in iCCA tissues. Thus, AGR3 was proposed to be used as a complementary marker in liver cancers to distinguish between HCC and iCCA for diagnosis [85]. Fibrolamellar carcinoma (FLHCC), is a rare liver cancer that can arise in young patients without any previous liver disease. AGR2 was found often overexpressed in FLHCC [86]. At last, 75% of primary FLHCC and 75% of metastatic FLHCC were positive for AGR2 [87]. At last, AGR1 protein expression was evaluated in 106 human HCC tissues by immunohistochemistry and was significantly higher compared to corresponding adjacent normal tissues. In addition, its expression was correlated with HCC metastasis and AGR1 was found to promote metastasis through the induction of the epithelial–mesenchymal transition process [88]; however, the precise underlying molecular mechanisms remain unclear.
Pancreatic cancer
Few amplifications of AGR2 and AGR3 genes (<1%) have been demonstrated in pancreatic adenocarcinoma as well as very few missense mutations (<1%) have been observed for AGR2 [20]. So far, no alterations have been demonstrated for the AGR1 gene. Prolonged chronic ER stress induces the overexpression of AGR2 in normal Human Pancreatic Duct Epithelial cell line [66], and may be involved in the development of pancreatic adenocarcinoma. In LSL-KrasG12D;Pdx1-Cre (KC) mouse, the induction of AGR2 expression precedes the formation of PanIN, whereas PanIN do not develop in Agr2-/- mice, suggesting the involvement of AGR2 in the development of pancreatic neoplasms [66]. In humans, AGR2 is highly expressed in PanIN compared to normal pancreatic tissue, and its expression is higher in PanIN than in invasive pancreatic adenocarcinoma [45, 89,90,91]. According to a retrospective analysis of 57 biopsy samples, AGR2 mRNA expression is 14 times higher in pancreatic adenocarcinoma than in normal pancreatic or pancreatitis tissues, with a similar tendency for AGR2 protein expression [92]. These data thus suggest that AGR2 overexpression caused by ER stress is an early event in pancreatic cancer genesis and may be important for the progression from normal pancreas tissue to invasive pancreatic cancer. The prognostic value of AGR2 in pancreatic cancer is debated, with discordant results [89, 90]. A high expression was found to correlate with poor prognosis in one study [89], whereas in another study, a low expression related to poor prognosis only using univariate analysis, but could not be confirmed using multivariate analysis [91]. However, a high expression of AGR2 may be correlated to poor differentiation of pancreatic ductal adenocarcinoma (PDAC), and therefore to pancreatic cancer aggressiveness [91]. In pancreatic cancer cell line MPanc-96, AGR2 is involved in cell proliferation and invasion, resistance to gemcitabine, and to apoptosis. In an in vivo murine orthotopic pancreas tumor model, the silencing of AGR2 resulted in a 4-fold reduction of tumor volume compared to control, tumor sensitization to gemcitabine, and fewer metastases in the group treated with gemcitabine and silenced for AGR2 [92]. Interestingly, cell proliferation, migration, and chemoresistance in different cultured pancreatic cells may be favored by the soluble form of AGR2 [93]. All these data suggest AGR2 is involved in pancreas cancer proliferation and chemoresistance.
Colorectal cancer
AGR2 is one of the most overexpressed proteins in human colorectal cancer (CRC), especially in mucinous forms [94]. In CRC, the expression and prognostic role of AGR2 remain to be fully understood. Indeed, Tian et al. [74] performed a comprehensive meta-analysis using data of two CRC studies [70, 95] selected based on their high methodological quality (Newcastle–Ottawa Scale scores between 8 and 9). For the first study, blood samples of 54 stages I–IV CRC patients were collected, and AGR2 expression was analyzed by qPCR. High and low AGR2 expressers were defined based on the mean relative AGR2 mRNA expression (high expressers > mean expression level and low expressers ≤ mean expression level). Independently, this study found that AGR2 expression was correlated with stage III and high-grade tumors. In addition, progression-free survival of patients with high AGR2 expression was decreased (hazard ratio: 2.32; p = 0.037) [95]. For the second study, AGR2 expression was measured by immunohistochemistry in 432 CRC cases and a scoring system was used to make the classification based on the staining intensity: negative (0), weakly [1], moderately [2], and strongly positive [3]. Low and high expressers were defined according to their AGR2 expression as either low (score 0 or 1) or high (score 2 or 3). Low expression of AGR2 in CRC tissue samples was significantly associated with high tumor grade, left primary tumor localization as well as shorter patient survival times. A total of 78% of patients had a lower AGR2 tumor expression compared to normal colonic mucosa [70]. Interestingly, when combined together, pooled hazard ratio between the two studies, AGR2 overexpression could not predict poor outcomes in CRC: Hazard Ratio 0.80, 95% confidence interval: 0.41–1.53 [74]. CRC cell proliferation and metastatic process may be favored by the secreted form of AGR2 [71], For example, mice injected with CRC cell line HT-29 Agr2-/- developed significantly more metastases after treatment with eAGR2 than control. In different CRC cell lines, it has also been shown that eAGR2 can inhibit the canonical Wnt/b-catenin pathway (involved in proliferation) and stimulate the non-canonical Wnt/b-catenin CAMKII-JNK pathway by promoting Wnt11 overexpression, which is a regulator of cell polarity and motility, stimulating the metastatic process [71]. In induced CRC stem cells, the canonic Wnt/b-catenin pathway stimulates AGR2 expression through b-catenin binding to the AGR2 promoter [96]. AGR2 could also be involved in maintaining stem cells stemness potential since its silencing using shRNA induced the loss of their stem cell characteristics [96]. The implication of AGR2 in maintaining cancer stem cell potential has also been reported in head and neck squamous-cell carcinoma cells [97]. AGR2 might act as a tumor suppressor in CRC tumors; however, future experimental verifications in bigger sample size studies are required. The functional role of AGR3 in CRC was investigated in 8 CRC tissue samples by immunohistochemistry and 24 CRC tumor tissues by western blot. AGR3 was significantly more expressed in CRC tumor tissues than in the adjacent normal tissues for the eight patients. In addition, western blot results highlighted higher expression of AGR3 in cancer tissue in 13 out of 24 tumors compared with matched adjacent normal tissues. High expression of AGR3 was also significantly associated with poorer overall survival and AGR3 expression was identified as an independent prognostic factor for poor survival [98]. Although the results regarding the role of AGR3 in CRC are quantitatively insufficient and need further experimental validation, they suggest overexpression of AGR3 in CRC that is associated with poor prognosis.
AGR proteins in IBD and colitis-associated cancer
IBD are characterized by deficiencies in the mucus layer that is part of the intestinal epithelial barrier leading to incomplete protection against microbial aggression. Consequently, excessive immune responses are triggered which results in chronic inflammation. AGR2 has been shown to be crucial for the protection of the mucus barrier in several studies. Whole genome sequencing performed in two siblings presenting infantile IBD, revealed a new AGR2 variant which results in AGR2 loss of function. AGR2 deficiency led to increased ER stress and a decrease in MUC2 processing that impairs the proper formation of the mucus barrier and thus induces intestinal inflammation [99]. AGR2 expression was measured by qPCR in small and large bowel biopsies of 56 Crohn’s disease (CD) and 57 ulcerative colitis (UC) patients and was shown to be significantly lower in both disease groups compared to 25 healthy controls. Unfortunately, this study does not specify if the patients were undergoing treatment at the time of the study [100]. In contrast, AGR2 gene expression was significantly upregulated in rectal biopsies of active patients with UC compared to both the remission and control groups. However, AGR2 gene expression might be modulated with the use of anti-inflammatory drugs and AGR2 higher expression in active patients could be a consequence of therapeutic improvement [101]. In addition, AGR2 monomers homodimers ratio homeostasis was shown to be altered in CD. This imbalance results in AGR2 secretion leading to monocytes recruitment and inflammation [34]. Patients with IBD are two to three times more likely to develop CRC cancer than the general population. They are also at increased risk for CRC death [102, 103]. Mice genetically deficient in Muc2 developed tumors in the small and large intestines including the rectum [104]. AGR2 is essential for the production of intestinal MUC2 [61] indicating a critical role of AGR2 expression in protection against colitis-associated cancer. To our knowledge, the contribution of AGR1 and AGR3 in IBD has never been demonstrated.
Theranostic perspectives
AGR2 has a physiological role in ER and interacts with a number of cellular partners depending on the cell type considered. Fourteen AGR2-interacting proteins overlapped in three independent studies [50, 105, 106]. These proteins are involved predominantly in processes in the ER which contribute to maintaining of intracellular metabolic homeostasis and/or to responses to ER stress (UPR, protein folding, changes in cellular metabolism, and redox state). Given the importance of the AGR2 interaction network in maintaining cellular homeostasis, the specific targeting of ER-resident AGR2 by potential therapeutic tools is difficult to envisage, as demonstrated by the deleterious effects observed in mice invalidated for this gene. On the contrary, secreted or cytosolic forms of AGR2 that play pro-oncogenic and pro-inflammatory functions appear to be more relevant to consider and open new avenues from a therapeutic point of view.
Development of monoclonal antibodies and immunotherapies
Monoclonal antibodies (mAbs) directed against AGR2 have already been experimented in animal models with promising results. The main therapeutic objective is to specifically target the extracellular AGR2, without impacting the ER-resident protein. Promising results have been reported in experimental models. 18A4Hu, an anti-AGR2 mAb, reduced tumor volume and tumor weight as much as Bevacizumab, without significant side effects, in a mammary breast cancer xenograft model [107]. However, the combination with Bevacizumab was not studied, which would have been interesting as we already know that AGR2 and VEGF-A act in synergy [71, 108,109,110]. The 18A4Hu mAb is also effective on lung cancer (A549 and H460) and melanoma (B16F10) cell lines by inhibiting tumor cell growth and migration, disrupting cell morphology and junctions, and increasing p53 and p21 expression [111]. A murine xenograft model of A549 and H460 cells treated with 18A4Hu showed a three times fold reduction of tumor volume and tumor weight compared to untreated mice. Tumor analysis of treated mice showed an increase in apoptotic markers and p53 expression, and a reduction of tumor blood vessels compared to untreated mice, suggesting that 18A4Hu has antiangiogenic properties. Similarly, mice with lung metastasis of melanoma cell line B16F10, treated with 18A4Hu, exhibit ten times less metastasis in the lungs, and their survival was significantly improved (24 vs. 20 days). Again, it was well tolerated, and there was neither reduction of intestinal mucus nor occurrence of intestinal inflammation. P1G4 and P3A5 are two other anti-AGR2 humanized mAbs. P1G4 is not efficient in monotherapy on a xenograft model of pancreatic adenocarcinoma [112]. However, its association with gemcitabine decreased significantly tumor volume and increased survival compared to gemcitabine alone. P3A5 is not efficient alone or combined with gemcitabine to reduce tumor volume but it increased survival in combination with gemcitabine. A combination of mouse mAbs directed against AGR2 and C4.4A in a murine xenograft model of pancreatic cancer resulted in a 50% objective response rate, a 50% reduction in the occurrence of metastasis, and an increase in OS. Their efficacy was similar to or better than gemcitabine alone or combined with the two mAbs [93]. These preliminary results are promising as they prove that mAbs anti-AGR2 have in vivo effects in diverse cancers, and should be investigated further. Therapeutic progresses in digestive oncology remain challenging since, with the exception of immunotherapy in microsatellite instability or deficient mismatch repair CRC and oeso-gastric cancers, most of the targeted therapies currently used have been developed more than 10 years ago and no other important progresses in terms of survival have been made. In addition to the development of anti-AGR2 therapeutic antibodies, dendritic cell (DC) vaccines with AGR2 as a potent antigen could improve cancer immunotherapy. DCs were transduced with a recombinant adenovirus encoding AGR2 and these engineered DCs both increased the number of T cells secreting IFNγ and induced the lysis of AGR2-expressing CRC cell lines through the activation of potent AGR2-specific cytotoxic T cells [113]. Although limitations persist and impede the application of DCs vaccines immunotherapy, these in vitro experiments highlight the potential of AGR2 to trigger efficient immune responses against some cancers. Further research on animals is required to validate the therapeutic relevance of these findings.
AGR2 and AGR3 as biomarkers?
The identification of biomarkers using non-invasive sampling in digestive cancer is crucial to improve efficacy of diagnostic, predict prognosis, and treatment outcome (or clinical management of patients). AGR2 gene expression has been assessed in the whole blood of CRC patients before therapy by qPCR. AGR2 mRNA expression was significantly increased in the blood of patients with CRC compared to controls. In addition, the level of AGR2 gene expression was correlated with some invasive and high-grade tumor prognostic factors [95]. Thus, high AGR2 gene expression in the blood of CRC patients might be a sign of poor patient outcome. However, this would need to be confirmed with a longitudinal study, while its relevance in therapeutic strategy would remain to be defined. AGR2 protein level has been measured in the plasma of pancreatic cancer patients before treatment using ELISA and is significantly higher compared to healthy controls [114]. A diagnostic score comprising CA19.9, AGR2, and Regenerating islet-derived 1 beta (REG1B) serum levels was shown to be more efficient than CA19.9 dosage alone in differentiating PDAC from benign pancreatic tumor or healthy controls, making it a potentially interesting score to avoid systematic biopsies in the pancreatic tumor, of which are often difficult to perform with a diagnostic performance not optimal, which often necessitates to perform additional biopsies. However, this score has not been confirmed by a prospective study. In addition, AGR2 was significantly more elevated in the serum of patients with PDAC than in other tumors, while AGR2 alone was the best marker for pancreatic cancer diagnosis after CA19.9 [114]. Although these results need to be validated in larger cohorts of samples, AGR2, in combination with other biomarkers, could be used for better patient management through early disease detection. To improve cancer diagnosis (for early cancer detection), several research teams have developed highly sensitive assays for rapid and ultrasensitive detection of AGR2 protein [115, 116]. Nevertheless, the application of these highly sensitive assays to plasma samples needs to be evaluated for non-invasive early detection of cancer. However, as AGR2 is not specific to one of the digestive organs, it cannot be used alone for early cancer detection as a serum biomarker, and it needs to be integrated into diagnostic scores. Overall, very few studies have investigated AGR2 protein levels in the serum of digestive cancer patients. AGR2 might be a good candidate to predict clinical outcomes such as metastatic potential or relapse but it needs to be demonstrated with longitudinal studies in a cohort of patients. There is no data regarding AGR2 efficacy as a treatment outcome predictor in digestive cancers. Moreover, the presence of AGR3 in the serum of patients with digestive cancer has never been explored. There are also limited data on AGR3 expression in healthy and digestive cancer tissues. Only one study investigated the potential role of AGR3 as a diagnostic biomarker to distinguish between iCCA and HCC in digestive cancer (as previously described in the primary liver cancers part of this review). AGR3 expression was measured in tissues of iCCA and HCC patients by immunohistochemistry and AGR3 was found to be expressed only by intrahepatic bile duct cholangiocytes but not hepatocytes [91]. Thus, AGR3 could be used as a new biomarker for differential diagnosis between these two cancers.
Conclusion
The AGR2 protein has gained significant interest in oncology during the last few years. This holds to its multiple pro-oncogenic properties and to the particularity that its pathological roles mostly depend on its cellular or extracellular localization. AGR2 is highly expressed in the digestive system. It is overexpressed at early stages in most cancer lesions and adenocarcinoma of the digestive tube and is often associated with a poor prognosis. Less studied than in cancer, AGR2 deregulation may also play an important role in inflammation, particularly in IBD [100, 117]. However, the role of extracellular AGR2 in the tumor microenvironment, its influence on the immune infiltrate, and its autocrine and paracrine roles remain to be more clearly characterized. The development of anti-AGR2 therapies has already shown interesting results in animals and plasma/serum AGR2 could serve as a prognostic/diagnostic biomarker. In the future, their development may have prospects in diverse pathologies like cancer and inflammatory diseases and may enter into the therapeutic arsenal of the treatment of these patients.
References
Chevet E, Fessart D, Delom F, Mulot A, Vojtesek B, Hrstka R, et al. Emerging roles for the pro-oncogenic anterior gradient-2 in cancer development. Oncogene. 2013;32:2499–509.
Määttänen P, Gehring K, Bergeron JJM, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21:500–11.
Kemmink J, Darby NJ, Dijkstra K, Nilges M, Creighton TE. The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr Biol. 1997;7:239–45.
Matsusaki M, Kanemura S, Kinoshita M, Lee YH, Inaba K, Okumura M. The protein disulfide isomerase family: from proteostasis to pathogenesis. Biochim Biophys Acta Gen Subj. 2020;1864:129338.
Lemaire SD, Miginiac-Maslow M. The thioredoxin superfamily in Chlamydomonas reinhardtii. Photosynth Res. 2004;82:203–20.
Matsuo Y. Introducing thioredoxin-related transmembrane proteins: emerging roles of human TMX and clinical implications. Antioxid Redox Signal. 2022;36:984–1000.
Kozlov G, Määttänen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–36.
Ivanova AS, Tereshina MB, Ermakova GV, Belousov VV, Zaraisky AG. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci Rep. 2013;3:1279.
Fessart D, Mahouche I, Brouste V, Velasco V, Soubeyran I, Soubeyran P, et al. Anterior gradient-2 (AGR2) overexpression in colon cancer: a potential prognostic biomarker. bioRxiv: 2021.09.07.459258 [Preprint]. 2021 [cited 2022 Jun 5]. Available from: https://www.biorxiv.org/content/, https://doi.org/10.1101/2021.09.07.459258v1.
Higa A, Mulot A, Delom F, Bouchecareilh M, Nguyên DT, Boismenu D, et al. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J Biol Chem. 2011;286:44855–68.
Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11:2807–50.
Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–51.
Fessart D, Villamor I, Chevet E, Delom F, Robert J. Integrative analysis of genomic and transcriptomic alterations of AGR2 and AGR3 in cancer. Open Biol. 2022;12:220068.
Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–30.
Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–5.
Shih LJ, Lu YF, Chen YH, Lin CC, Chen JA, Hwang SPL. Characterization of the agr2 gene, a homologue of X. laevis anterior gradient 2, from the zebrafish, Danio rerio. Gene Expr Patterns. 2007;7:452–60.
Bradley L, Wainstock D, Sive H. Positive and negative signals modulate formation of the Xenopus cement gland. Dev Camb Engl. 1996;122:2739–50.
Jach D, Cheng Y, Prica F, Dumartin L, Crnogorac-Jurcevic T. From development to cancer – an ever-increasing role of AGR2. Am J Cancer Res. 2021;11:5249–62.
Petek E, Windpassinger C, Egger H, Kroisel PM, Wagner K. Localization1 of the human anterior gradient-2 gene (AGR2) to chromosome band 7p21.3 by radiation hybrid mapping and fluorescencein situ hybridisation. Cytogenet Genome Res. 2000;89:141–2.
cBioPortal for Cancer Genomics [cited 2021 Jun 14]. Available from: https://www.cbioportal.org/.
Knoblach B, Keller BO, Groenendyk J, Aldred S, Zheng J, Lemire BD, et al. ERp19 and ERp46, new members of the thioredoxin family of endoplasmic reticulum proteins. Mol Cell Proteom. 2003;2:1104–19.
Williamson RA, Howard MJ, Jäntti HP, Rautio SM, Kellokumpu S, Alanen HI, et al. Functional characterization of ERp18, a new endoplasmic reticulum-located thioredoxin superfamily member. J Biol Chem. 2003;278:28912–20.
Ivanova AS, Shandarin IN, Ermakova GV, Minin AA, Tereshina MB, Zaraisky AG. The secreted factor Ag1 missing in higher vertebrates regulates fins regeneration in Danio rerio. Sci Rep. 2015;5:8123.
Alanen HI, Williamson RA, Howard MJ, Lappi AK, Jäntti HP, Rautio SM, et al. Functional characterization of ERp18, a new endoplasmic reticulum-located thioredoxin superfamily member. J Biol Chem. 2003;278:28912–20.
Jeong W, Lee DY, Park S, Rhee SG. ERp16, an endoplasmic reticulum-resident thiol-disulfide oxidoreductase: biochemical properties and role in apoptosis induced by endoplasmic reticulum stress. J Biol Chem. 2008;283:25557–66.
TXNDC12 protein expression summary – The Human Protein Atlas [cited 2022 Jul 25]. Available from: https://www.proteinatlas.org/ENSG00000117862-TXNDC12.
Raykhel I, Alanen H, Salo K, Jurvansuu J, Nguyen VD, Latva-Ranta M, et al. A molecular specificity code for the three mammalian KDEL receptors. J Cell Biol. 2007;179:1193–204.
Obacz J, Takacova M, Brychtova V, Dobes P, Pastorekova S, Vojtesek B, et al. The role of AGR2 and AGR3 in cancer: similar but not identical. Eur J Cell Biol. 2015;94:139–47.
AGR3 protein expression summary – The Human Protein Atlas [cited 2022 Jul 25]. Available from: https://www.proteinatlas.org/ENSG00000173467-AGR3.
Adam PJ, Boyd R, Tyson KL, Fletcher GC, Stamps A, Hudson L, et al. Comprehensive proteomic analysis of breast cancer cell membranes reveals unique proteins with potential roles in clinical cancer. J Biol Chem. 2003;278:6482–9.
Fletcher GC, Patel S, Tyson K, Adam PJ, Schenker M, Loader JA, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–85.
Bonser LR, Schroeder BW, Ostrin LA, Baumlin N, Olson JL, Salathe M, et al. The endoplasmic reticulum resident protein AGR3. Required for regulation of ciliary beat frequency in the airway. Am J Respir Cell Mol Biol. 2015;53:536–43.
Nguyen VD, Biterova E, Salin M, Wierenga RK, Ruddock LW. Crystal structure of human anterior gradient protein 3. Acta Crystallogr Sect F Struct Biol Commun. 2018;74:425–30.
Maurel M, Obacz J, Avril T, Ding YP, Papadodima O, Treton X, et al. Control of anterior gradient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med. 2019;11:e10120.
Patel P, Clarke C, Barraclough DL, Jowitt TA, Rudland PS, Barraclough R, et al. Metastasis-promoting anterior gradient 2 protein has a dimeric thioredoxin fold structure and a role in cell adhesion. J Mol Biol. 2013;425:929–43.
Delom F, Mohtar MA, Hupp T, Fessart D. The anterior gradient-2 interactome. Am J Physiol Cell Physiol. 2020;318:C40–7.
Clarke DJ, Murray E, Faktor J, Mohtar A, Vojtesek B, MacKay CL, et al. Mass spectrometry analysis of the oxidation states of the pro-oncogenic protein anterior gradient-2 reveals covalent dimerization via an intermolecular disulphide bond. Biochim Biophys Acta. 2016;1864:551–61.
Černocká H, Vonka P, Kasalová V, Sommerova L, Vandova V, Hrstka R, et al. AGR2-AGR3 hetero-oligomeric complexes: Identification and characterization. Bioelectrochemistry Amst Neth. 2021;140:107808.
Fomenko DE, Gladyshev VN. CxxS: fold-independent redox motif revealed by genome-wide searches for thiol/disulfide oxidoreductase function. Protein Sci Publ Protein Soc. 2002;11:2285–96.
Gray TA, MacLaine NJ, Michie CO, Bouchalova P, Murray E, Howie J, et al. Anterior gradient-3: a novel biomarker for ovarian cancer that mediates cisplatin resistance in xenograft models. J Immunol Methods. 2012;378:20–32.
Maslon MM, Hrstka R, Vojtesek B, Hupp TR. A divergent substrate-binding loop within the pro-oncogenic protein anterior gradient-2 forms a docking site for reptin. J Mol Biol. 2010;404:418–38.
Oka OBV, Pierre AS, Pringle MA, Tungkum W, Cao Z, Fleming B, et al. Activation of the UPR sensor ATF6α is regulated by its redox-dependent dimerization and ER retention by ERp18. Proc Natl Acad Sci USA. 2022;119:e2122657119.
Oka OB, van Lith M, Rudolf J, Tungkum W, Pringle MA, Bulleid NJ. ERp18 regulates activation of ATF6α during unfolded protein response. EMBO J. 2019;38:e100990.
Sicari D, Centonze FG, Pineau R, Le Reste PJ, Negroni L, Chat S, et al. Reflux of endoplasmic reticulum proteins to the cytosol inactivates tumor suppressors. EMBO Rep. 2021;22:e51412.
Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA. et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–102.
CALU calumenin [Homo sapiens (human)] – Gene – NCBI [cited 2021 Jan 22]. Available from: https://www.ncbi.nlm.nih.gov/gene/813.
RCN1 reticulocalbin 1 [Homo sapiens (human)] – Gene – NCBI [cited 2021 Jan 22]. Available from: https://www.ncbi.nlm.nih.gov/gene/5954.
Honoré B, Vorum H. The CREC family, a novel family of multiple EF-hand, low-affinity Ca2+-binding proteins localised to the secretory pathway of mammalian cells. FEBS Lett. 2000;466:11–8.
Mohtar MA, Hernychova L, O’Neill JR, Lawrence ML, Murray E, Vojtesek B, et al. The sequence-specific peptide-binding activity of the protein sulfide isomerase AGR2 directs its stable binding to the oncogenic receptor EpCAM. Mol Cell Proteom. 2018;17:737–63.
Bouchalova P, Sommerova L, Potesil D, Martisova A, Lapcik P, Koci V, et al. Characterization of the AGR2 interactome uncovers new players of protein disulfide isomerase network in cancer cells. Mol Cell Proteom. 2022;21:100188.
Bergström JH, Berg KA, Rodríguez-Piñeiro AM, Stecher B, Johansson MEV, Hansson GC. AGR2, an endoplasmic reticulum protein, is secreted into the gastrointestinal mucus. PLoS ONE. 2014;9:e104186.
Moidu NA, Rahman NSA, Syafruddin SE, Low TY, Mohtar MA. Secretion of pro-oncogenic AGR2 protein in cancer. Heliyon. 2020;6:e05000.
Gray TA, Alsamman K, Murray E, Sims AH, Hupp TR. Engineering a synthetic cell panel to identify signalling components reprogrammed by the cell growth regulator anterior gradient-2. Mol Biosyst. 2014;10:1409–25.
Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 2006;34:D535–9.
Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteom. 2004;3:534–47.
Hrstka R, Bouchalova P, Michalova E, Matoulkova E, Muller P, Coates PJ, et al. AGR2 oncoprotein inhibits p38 MAPK and p53 activation through a DUSP10-mediated regulatory pathway. Mol Oncol. 2016;10:652–62.
AGR2 protein expression summary – The Human Protein Atlas [cited 2022 Jul 25]. Available from: https://www.proteinatlas.org/ENSG00000106541-AGR2.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–9.
Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, et al. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–85.
Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–5.
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20.
Norris AM, Gore A, Balboni A, Young A, Longnecker DS, Korc M. AGR2 is a SMAD4-suppressible gene that modulates MUC1 levels and promotes the initiation and progression of pancreatic intraepithelial neoplasia. Oncogene. 2013;32:3867–76.
Gupta A, Wodziak D, Tun M, Bouley DM, Lowe AW. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J Biol Chem. 2013;288:4321–33.
Li J, Hu J, Luo Z, Zhou C, Huang L, Zhang H, et al. AGR2 is controlled by DNMT3a-centered signaling module and mediates tumor resistance to 5-Aza in colorectal cancer. Exp Cell Res. 2019;385:111644.
Dumartin L, Alrawashdeh W, Trabulo SM, Radon TP, Steiger K, Feakins RM, et al. ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene. 2017;36:3094–103.
Lepreux S, Bioulac-Sage P, Chevet E. Differential expression of the anterior gradient protein-2 is a conserved feature during morphogenesis and carcinogenesis of the biliary tree. Liver Int. 2011;31:322–8.
Nancarrow DJ, Clouston AD, Smithers BM, Gotley DC, Drew PA, Watson DI, et al. Whole genome expression array profiling highlights differences in mucosal defense genes in Barrett’s esophagus and esophageal adenocarcinoma. PLoS ONE. 2011;6:e22513.
Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, Wang Y. et al. AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett. 2016;11:2075–83.
Riener MO, Thiesler T, Hellerbrand C, Amann T, Cathomas G, Fritzsche FR, et al. Loss of anterior gradient-2 expression is an independent prognostic factor in colorectal carcinomas. Eur J Cancer. 2014;50:1722–30.
Tian S, Hu J, Tao K, Wang J, Chu Y, Li J, et al. Secreted AGR2 promotes invasion of colorectal cancer cells via Wnt11-mediated non-canonical Wnt signaling. Exp Cell Res. 2018;364:198–207.
cBioPortal for Cancer Genomics: AGR2, AGR3 and 1 other gene in Pan-cancer analysis of whole genomes (ICGC/TCGA, Nature 2020) [cited 2022 Jul 16]. Available from: https://www.cbioportal.org/results?cancer_study_list=pancan_pcawg_2020&tab_index=tab_visualize&case_set_id=pancan_pcawg_2020_all&Action=Submit&gene_list=AGR2%250AAGR3%250ATXNDC12.
Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, West CR, et al. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. 2009;175:1848–57.
Tian SB, Tao KX, Hu J, Liu ZB, Ding XL, Chu YN, et al. The prognostic value of AGR2 expression in solid tumours: a systematic review and meta-analysis. Sci Rep. 2017;7:15500.
Pizzi M, Fassan M, Realdon S, Balistreri M, Battaglia G, Giacometti C, et al. Anterior gradient 2 profiling in Barrett columnar epithelia and adenocarcinoma. Hum Pathol. 2012;43:1839–44.
DiMaio MA, Kwok S, Montgomery KD, Lowe AW, Pai RK. Immunohistochemical panel for distinguishing esophageal adenocarcinoma from squamous cell carcinoma: a combination of p63, cytokeratin 5/6, MUC5AC, and anterior gradient homolog 2 allows optimal subtyping. Hum Pathol. 2012;43:1799–807.
DepMap Data Explorer [cited 2021 Feb 12]. Available from: https://depmap.org/portal/interactive/.
Lee DH, Lee Y, Ryu J, Park SG, Cho S, Lee JJ, et al. Identification of proteins differentially expressed in gastric cancer cells with high metastatic potential for invasion to lymph nodes. Mol Cells. 2011;31:563–71.
Tsuji T, Satoyoshi R, Aiba N, Kubo T, Yanagihara K, Maeda D, et al. Agr2 mediates paracrine effects on stromal fibroblasts that promote invasion by gastric signet-ring carcinoma cells. Cancer Res. 2015;75:356–66.
Chen S, Dong H, Yang S, Guo H. Cathepsins in digestive cancers. Oncotarget. 2017;8:41690–700.
Bai Z, Ye Y, Liang B, Xu F, Zhang H, Zhang Y, et al. Proteomics-based identification of a group of apoptosis-related proteins and biomarkers in gastric cancer. Int J Oncol. 2011;38:375–83.
Wu J, Chen XH, Wang XQ, Yu Y, Ren JM, Xiao Y, et al. ERp19 contributes to tumorigenicity in human gastric cancer by promoting cell growth, migration and invasion. Oncotarget. 2015;6:11794–805.
Jeon Y, Kwon SM, Rhee H, Yoo JE, Chung T, Woo HG, et al. Molecular and radiopathologic spectrum between HCC and intrahepatic cholangiocarcinoma. Hepatology. 2022; https://doi.org/10.1002/hep.32397.
Yosudjai J, Inpad C, Chomwong S, Dana P, Sawanyawisuth K, Phimsen S, et al. An aberrantly spliced isoform of anterior gradient-2, AGR2vH promotes migration and invasion of cholangiocarcinoma cell. Biomed Pharmacother Biomedecine Pharmacother. 2018;107:109–16.
Brychtova V, Zampachova V, Hrstka R, Fabian P, Novak J, Hermanova M, et al. Differential expression of anterior gradient protein 3 in intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Exp Mol Pathol. 2014;96:375–81.
Simon EP, Freije CA, Farber BA, Lalazar G, Darcy DG, Honeyman JN, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci USA. 2015;112:E5916–25.
Vivekanandan P, Micchelli STL, Torbenson M. Anterior gradient-2 is overexpressed by fibrolamellar carcinomas. Hum Pathol. 2009;40:293–9.
Yuan K, Xie K, Lan T, Xu L, Chen X, Li X, et al. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ. 2020;27:1355–68.
Mizuuchi Y, Aishima S, Ohuchida K, Shindo K, Fujino M, Hattori M, et al. Anterior gradient 2 downregulation in a subset of pancreatic ductal adenocarcinoma is a prognostic factor indicative of epithelial–mesenchymal transition. Lab Invest. 2015;95:193–206.
Riener MO, Pilarsky C, Gerhardt J, Grützmann R, Fritzsche FR, Bahra M, et al. Prognostic significance of AGR2 in pancreatic ductal adenocarcinoma. Histol Histopathol. 2009;24:1121–8.
Brychtova V, Hermanova M, Karasek P, Lenz J, Selingerova I, Vojtesek B, et al. Anterior gradient 2 and mucin 4 expression mirrors tumor cell differentiation in pancreatic adenocarcinomas, but aberrant anterior gradient 2 expression predicts worse patient outcome in poorly differentiated tumors. Pancreas. 2014;43:75–81.
Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008;68:7811–8.
Arumugam T, Deng D, Bover L, Wang H, Logsdon CD, Ramachandran V. New blocking antibodies against novel AGR2-C4.4A pathway reduce growth and metastasis of pancreatic tumors and increase survival in mice. Mol Cancer Ther. 2015;14:941–51.
Kim HS, Kang SH, Park CH, Yang WI, Jeung HC, Chung HC, et al. Genome-wide molecular characterization of mucinous colorectal adenocarcinoma using cDNA microarray analysis. Oncol Rep. 2011;25:717–27.
Valladares-Ayerbes M, Blanco-Calvo M, Reboredo M, Lorenzo-Patiño MJ, Iglesias-Díaz P, Haz M, et al. Evaluation of the adenocarcinoma-associated gene AGR2 and the intestinal stem cell marker LGR5 as biomarkers in colorectal cancer. Int J Mol Sci. 2012;13:4367–87.
Dahal Lamichane B, Jung SY, Yun J, Kang S, Kim DY, Lamichane S, et al. AGR2 is a target of canonical Wnt/β-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem Biophys Res Commun. 2019;515:600–6.
Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6:8807–21.
Chi J, Zhang H, Hu J, Song Y, Li J, Wang L, et al. AGR3 promotes the stemness of colorectal cancer via modulating Wnt/β-catenin signalling. Cell Signal. 2020;65:109419.
Al-Shaibi AA, Abdel-Motal UM, Hubrack SZ, Bullock AN, Al-Marri AA, Agrebi N, et al. Human AGR2 deficiency causes mucus barrier dysfunction and infantile inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2021;12:1809–30.
Zheng W, Rosenstiel P, Huse K, Sina C, Valentonyte R, Mah N, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–8.
Camarillo GF, Goyon EI, Zuñiga RB, Salas LAS, Escárcega AEP, Yamamoto-Furusho JK. Gene expression profiling of mediators associated with the inflammatory pathways in the intestinal tissue from patients with ulcerative colitis. Mediators Inflamm. 2020;2020:9238970.
Jess T, Frisch M, Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin Gastroenterol Hepatol. 2013;11:43–8.
Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in Crohn’s disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5:475–84.
Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9.
Tiemann K, Garri C, Lee SB, Malihi PD, Park M, Alvarez RM, et al. Loss of ER retention motif of AGR2 can impact mTORC signaling and promote cancer metastasis. Oncogene. 2019;38:3003–18.
Worfolk JC, Bell S, Simpson LD, Carne NA, Francis SL, Engelbertsen V, et al. Elucidation of the AGR2 interactome in esophageal adenocarcinoma cells identifies a redox-sensitive chaperone hub for the quality control of MUC-5AC. Antioxid Redox Signal. 2019;31:1117–32.
Guo H, Chen H, Zhu Q, Yu X, Rong R, Merugu SB, et al. A humanized monoclonal antibody targeting secreted anterior gradient 2 effectively inhibits the xenograft tumor growth. Biochem Biophys Res Commun. 2016;475:57–63.
Vanderlaag KE, Hudak S, Bald L, Fayadat-Dilman L, Sathe M, Grein J, et al. Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-α and survivin. Breast Cancer Res. 2010;12:R32.
Guo H, Zhu Q, Yu X, Merugu SB, Mangukiya HB, Smith N, et al. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene. 2017;36:5098–109.
Jia M, Guo Y, Zhu D, Zhang N, Li L, Jiang J, et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim Biophys Acta Mol Basis Dis 2018;1864:1622–33.
Negi H, Merugu SB, Mangukiya HB, Li Z, Zhou B, Sehar Q, et al. Anterior gradient-2 monoclonal antibody inhibits lung cancer growth and metastasis by upregulating p53 pathway and without exerting any toxicological effects: a preclinical study. Cancer Lett. 2019;449:125–34.
Liu Z, Zhang Y, Niu Y, Li K, Liu X, Chen H, et al. A systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of colorectal cancer. PLoS ONE. 2014;9:e103910.
Lee HJ, Hong CY, Kim MH, Lee YK, Nguyen-Pham TN, Park BC, et al. In vitro induction of anterior gradient-2-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses as a potential therapy for colorectal cancer. Exp Mol Med. 2012;44:60–7.
Makawita S, Dimitromanolakis A, Soosaipillai A, Soleas I, Chan A, Gallinger S, et al. Validation of four candidate pancreatic cancer serological biomarkers that improve the performance of CA19.9. BMC Cancer. 2013;13:404.
Białobrzeska W, Dziąbowska K, Lisowska M, Mohtar MA, Muller P, Vojtesek B, et al. An ultrasensitive biosensor for detection of femtogram levels of the cancer antigen AGR2 using monoclonal antibody modified screen-printed gold electrodes. Biosensors. 2021;11:184.
Arshavsky-Graham S, Ward SJ, Massad-Ivanir N, Scheper T, Weiss SM, Segal E. Porous silicon-based aptasensors: toward cancer protein biomarker detection. ACS Meas Sci Au. 2021;1:82–94.
Ye X, Wu J, Li J, Wang H. Anterior gradient protein 2 promotes mucosal repair in pediatric ulcerative colitis. BioMed Res Int. 2021;2021:6483860.
Vesiclepedia: Gene summary [cited 2022 Jul 26]. Available from: http://microvesicles.org/gene_summary?gene_id=51060.
Fessart D, Domblides C, Avril T, Eriksson LA, Begueret H, Pineau R, et al. Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. eLife. 2016;5:e13887.
Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–10.
Jessop CE, Watkins RH, Simmons JJ, Tasab M, Bulleid NJ. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J Cell Sci. 2009;122:4287–95.
Gupta A, Dong A, Lowe AW. AGR2 gene function requires a unique endoplasmic reticulum localization motif. J Biol Chem. 2012;287:4773–82.
Acknowledgements
We thank the Immuno-histopathology platform H2P2 for their expertise and work (https://histopathologie.univ-rennes1.fr/) and Raphael Pineau for his technical help. This work was funded by grants from INCa (PRT-K20-136) to CC and JE, INCa (PLBIO) to EC, La Ligue Contre le Cancer to EO-D and AL and from La Ligue Contre le Cancer Gironde and from the Site de recherche intégrée sur le cancer de Bordeaux (SIRIC Brio) to FD. LAE acknowledges funding from the Swedish research council (grant no 2019-3684) and the Swedish Cancer Foundation (grant no 21-1447 Pj). EB was funded by an “année-recherche” grant from the ministry of health.
Author information
Authors and Affiliations
Contributions
EB, EC, EO-D, and CP wrote the first draft of the manuscript, made the figures, and finalized the document. LAE carried out the structural analyses presented in Fig. 1. CP, FDM, JE, RH, AM, CC, FD, XT, LAE, and AL critically read the manuscript and worked on it.
Corresponding authors
Ethics declarations
Competing interests
EO-D, XT, and EC are founders of Thabor Therapeutics.
Ethics approval and consent to participate
According to the French regulation, patients did not oppose to the analysis, and the study was approved by the Rennes Ethics Committee (Avis no. 21.122).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boisteau, E., Posseme, C., Di Modugno, F. et al. Anterior gradient proteins in gastrointestinal cancers: from cell biology to pathophysiology. Oncogene 41, 4673–4685 (2022). https://doi.org/10.1038/s41388-022-02452-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02452-1
- Springer Nature Limited
This article is cited by
-
AGR2-mediated unconventional secretion of 14-3-3ε and α-actinin-4, responsive to ER stress and autophagy, drives chemotaxis in canine mammary tumor cells
Cellular & Molecular Biology Letters (2024)