Abstract
Osimertinib (AZD9291) is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), used for treating patients with advanced non-small-cell lung cancer (NSCLC) harboring EGFR-activating mutations or the resistant T790M mutation. However, acquired resistance to osimertinib is inevitable in EGFR-mutant NSCLC. By employing a global mass spectrometry-based phosphoproteomics approach, we identified that the activated p21-activated kinase 2 (PAK2)/β-catenin axis acts as a driver of osimertinib resistance. We found that PAK2 directly phosphorylates β-catenin and increases the nuclear localization of β-catenin, leading to the increased expression and transcriptional activity of β-catenin, which in turn enhances cancer stem-like properties and osimertinib resistance. Moreover, we revealed that HER3 as an upstream regulator of PAK2, drives the activation of PAK2/β-catenin pathways in osimertinib-resistant cells. The clinical relevance of these findings was further confirmed by examining tissue specimens from patients with EGFR-mutant NSCLC. The results demonstrated that the levels of HER3, phospho-PAK2 (p-PAK2) and β-catenin in the tissues from patients with EGFR-mutant NSCLC, that had relapsed after treatment with osimertinib, were elevated compared to those of the corresponding untreated tissues. Additionally, the high levels of HER3, p-PAK2 and β-catenin correlated with shorter progression-free survival (PFS) in patients with EGFR-TKI-treated NSCLC. We additionally observed that the suppression of PAK2 via knockdown or pharmacological targeting with PAK inhibitors markedly restored the response of osimertinib-resistant NSCLC cells to osimertinib both in vitro and in vivo. In conclusion, these results indicated that the PAK2-mediated activation of β-catenin is important for osimertinib resistance and targeting the HER3/PAK2/β-catenin pathway has potential therapeutic value in NSCLCs with acquired resistance to osimertinib.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with non-small-cell lung cancer (NSCLC) comprising 85% of lung cancers [1]. Activating mutations of epidermal growth factor receptor (EGFR), including the deletion of exon 19 and the L858R mutation, contribute to the progression of NSCLC [2]. EGFR-tyrosine kinase inhibitors (EGFR-TKIs) are the recommended first-line treatment for EGFR mutation-positive advanced NSCLCs. The majority of NSCLCs with activating EGFR mutations respond dramatically to first- and second-generation EGFR-TKIs, including gefitinib, erlotinib, and afatinib [3,4,5]. However, the occurrence of the secondary EGFR T790M mutation confers acquired resistance to first- and second-generation TKIs, and is detected in approximately 50% of patients after disease progression [6, 7].
Osimertinib, a third-generation EGFR-TKI, selectively inhibits EGFR with activating mutations and/or the resistant T790M mutation [8, 9]. Osimertinib is effective in patients with T790M-positive EGFR-mutated NSCLC, refractory to classical EGFR-TKIs, including gefitinib and erlotinib [10]. Furthermore, osimertinib has a superior clinical response and outcome than gefitinib and erlotinib, when administered as a first-line therapy in EGFR-mutated NSCLCs [11, 12]. Although osimertinib is successful as a first-line and second-line therapy, acquired resistance to osimertinib is inevitable, similar to that observed for other EGFR-TKIs [13]. It is therefore necessary to elucidate the mechanism underlying osimertinib resistance in NSCLCs for improving the efficacy of clinical treatment, and for developing novel therapeutic strategies.
Studies on acquired osimertinib resistance have revealed that several mechanisms, including the C797S mutation in EGFR, amplification of MET and HER2, emergence of other driver oncogenes (mutations in RAS, BRAF, and PIK3CA), and histological and phenotypic transformation, have been reported in patients with EGFR-mutated lung cancer, with or without the T790M mutation in EGFR [14,15,16]. Recent studies have highlighted that aberrant downstream alterations in EGFR signaling are associated with osimertinib resistance [17]. Several kinases with altered expression and phosphorylation have been identified in osimertinib-resistant cells, and the combination of osimertinib with a targeted therapy against these kinases has emerged as a promising therapeutic strategy [18,19,20]. However, an optimal therapeutic strategy remains to be developed for patients with NSCLC with acquired resistance to osimertinib.
In this study, we performed iTRAQ-based quantitative phosphoproteomics analyses for identifying the global dynamic modifications that occur in osimertinib-resistant NSCLC cells. We identified a novel signaling pathway, involving HER3, PAK2 and β-catenin, that is associated with resistance to osimertinib. We observed that the HER3/PAK2/β-catenin signaling pathway is activated in osimertinib-resistant NSCLC cells and clinical samples, and that the activation of the HER3/PAK2/β-catenin signaling pathway confers stemness properties and osimertinib resistance. Moreover, we demonstrated that the suppression of PAK2 restored the response of osimertinib-resistant cells to osimertinib, and could serve as a potential therapeutic strategy for overcoming acquired resistance to osimertinib.
Results
Quantitative phosphoproteomic analysis of osimertinib resistance in NSCLC cells
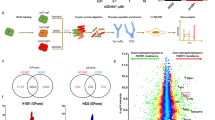
Osimertinib-resistant H1975/OR cells were derived from H1975 cells, an EGFR-L858R/T790M-mutant third-generation EGFR-TKI-sensitive cell line, by long-term dose escalating treatment with osimertinib over a period of 10 days followed by maintenance in 2 μM of osimertinib over 12 weeks (Fig. 1A–C, Fig. S1A). The resistant phenotype was subsequently confirmed by tumor xenograft assays (Fig. 1D). In order to investigate the molecular alterations associated with osimertinib resistance, we analyzed the phosphoproteomic profiles of parental H1975 cells and H1975/OR cells by iTRAQ-based quantitative proteomics (Fig. 1E). Altogether, we identified 5525 unique phosphorylation sites on 1735 proteins from H1975/OR cells. Of these, 4610, 706, and 209 proteins were phosphorylated at serine, threonine, and tyrosine residues, respectively (Fig. 1F). Using a 2-fold cut off, we observed that, relative to the parental H1975 cells, 267 phosphorylation sites in 213 proteins were hyperphosphorylated, while 76 phosphorylation sites on 67 proteins were downregulated in H1975/OR cells (Fig. 1G). Gene Ontology (GO) analysis revealed that these upregulated phosphoproteins were significantly enriched in the GO terms ‘cell adhesion molecule binding’, ‘cadherin binding’, ‘catenin binding’, ‘mRNA 3′-UTR binding’, and ‘structural constituent of ribosome’. (Fig. 1H). Analyses using the STRING database revealed that a subset of proteins related to cell adhesion molecule binding showed a well-annotated protein-protein interaction network (PPIN) centered around ITGB1 (S785), CTNNB1 (S552), PAK2 (S141), VCL (T442), and FLNA (T2336) (Fig. 1I, J). We further verified the differential protein phosphorylation levels in osimertinib-resistant NSCLC cells by western blotting. In agreement with the data obtained from phosphoproteomics, we observed that the phosphorylation levels of PAK2 (S141) and β-catenin (S552) were significantly increased in H1975/OR cells, compared to those of the parental H1975 cells (Fig. 1K). These findings were further validated using T790M-negative PC9 and HCC827 cell line models of osimertinib resistance. To this end, PC9 and HCC827 cells were cultured in osimertinib until the emergence of resistant clones (Fig. S1B). Similar to the earlier results, the phosphorylations of PAK2 (S141) and β-catenin (S552) were increased in PC9/OR and HCC827/OR cells (Fig. 1L). Altogether the results of proteomics analysis suggested that the increased phosphorylation of proteins related to cell adhesion molecule binding in H1975/OR cells may play a role in maintaining the resistant phenotype, and that these phosphorylation sites might be served as potential biomarkers of osimertinib resistance.
A–C Osimertinib-resistant H1975/OR cells were derived from H1975 cells by long-term dose escalating treatment with osimertinib. A Morphology of H1975 and H1975/OR cells. B H1975 and H1975/OR cells were treated with osimertinib at the indicated concentrations for 72 h, and cell viability was evaluated using the MTS assay. C IC50 values of osimertinib for H1975 and H1975/OR cells. D H1975 or H1975/OR cells were subcutaneously implanted into nude mice, which were subsequently administered osimertinib (10 mg/kg/day, og) or DMSO (0.01% DMSO in PBS). Tumor growth was measured at the indicated times. E Experimental workflow of iTRAQ-based quantitative phosphoproteomics analysis for H1975/OR and H1975 cells. F Number of phosphorylation sites at serine, threonine, and tyrosine residues in the proteins from H1975/OR cells. G Volcano plot showing the log2 fold-change and significance (−log10 p-value) of the proteome dataset. Red dots represent significantly upregulated proteins, while the blue dots represent the significantly downregulated proteins. H GO analysis of the upregulated phosphoproteins. I PPIN of the upregulated phosphoproteins. J Proteins that exhibited an increased phosphorylation in H1975/OR cells. K The expression of p-PAK2, p-β-catenin, PAK2, and β-catenin in H1975/OR and H1975 cells was analyzed by western blotting. L The expression of p-PAK2, p-β-catenin, PAK2, and β-catenin in osimertinib-resistant PC9/OR and HCC827/OR cells, and osimertinib-sensitivity PC9 and HCC827 cells was analyzed by western blotting (**p < 0.01).
Activation of β-catenin is associated with osimertinib resistance and cancer stemness
Previous studies have suggested that the phosphorylation of β-catenin at S552 increases the stabilization and nuclear localization of β-catenin [21]. We subsequently evaluated the abundance of β-catenin in osimertinib-resistant NSCLC cells. Western blot analysis revealed that the protein levels of β-catenin were apparently increased in H1975/OR cells, compared to those of the parental H1975 cells (Fig. 2A). The cellular localization of β-catenin was further analyzed by Immunofluorescence. The results demonstrated that the nuclear localization of β-catenin was increased in H1975/OR cells compared to that in the parental H1975 cells (Fig. 2B). Moreover, TOPFlash/FOPFlash reporter assays revealed that the transcriptional activity of β-catenin in H1975/OR cells was markedly increased in comparison to that in the parental H1975 cells (Fig. 2C). The activation of TOPFlash was probably mediated by endogenous TCF and β-catenin, as indicated by the increased coimmunoprecipitation of TCF4 and β-catenin in H1975/OR cells (Fig. S2A). Similarly, the total expression of β-catenin was increased in PC9/OR and HCC827/OR cells (Fig. S2B), and the transcriptional activity of β-catenin was also increased (Fig. S2C). In order to confirm the functional role of β-catenin in the induction of osimertinib resistance, stable β-catenin-depleted H1975/OR cells were constructed (Fig. 2D), and we observed that knockdown of β-catenin significantly increased osimertinib sensitivity (Fig. 2E) and osimertinib-induced cell apoptosis (Fig. 2F, G). In contrast, overexpression of β-catenin in H1975 cells (Fig. S3A) induced in vitro resistance to osimertinib (Fig. 2H), and decreased the population of cells undergoing osimertinib-mediated apoptosis (Fig. S3B). Furthermore, overexpression of β-catenin increased osimertinib resistance in PC9 and HCC827 cells (Fig. S3C, D), whereas knockdown of β-catenin increased osimertinib sensitivity in PC9/OR and HCC827/OR cells (Fig. S3E, F). Previous studies have highlighted that cancer stem cells (CSCs) play a critical role in promoting resistance to EGFR-TKI [22], and β-catenin has been implicated in maintaining a stem-like phenotype [23]. In this study, we observed that the expression of the CSC marker SOX2 (Fig. 2I), number of CD133+ cells (Fig. 2J, Fig. S3G), and sphere formation (Fig. 2K, L) were significantly increased in H1975/OR cells compared to those of the H1975 parental cells. We also observed that the properties of the CSCs were diminished in β-catenin-knockdown H1975/OR cells (Fig. 2I–L, Fig. S3G). These data suggested that β-catenin plays a role in the osimertinib resistance and the CSC characteristics of NSCLC cells.
A Expression of β-catenin in H1975 and H1975/OR cells was evaluated by western blotting. B Cellular localization of β-catenin in H1975 and H1975/OR cells was analyzed by IF studies. C Transcriptional activity of β-catenin in H1975 and H1975/OR cells was assessed by a TOPFlash/FOPFlash reporter assay. D H1975/OR cells were transfected with β-catenin shRNA or control shRNA, and the expression of β-catenin was measured by western blotting. E The H1975/OR cells transfected with β-catenin shRNA or control shRNA were treated with osimertinib at the indicated concentrations for 72 h, following which cell viability was evaluated using the MTS assay. F, G H1975/OR cells transfected with β-catenin shRNA or control shRNA were treated with 2 μM osimertinib for 48 h, following which cellular apoptosis was detected by flow cytometry. H H1975 cells transfected with pCMV-β-catenin or pCMV were treated with 0.5 μM osimertinib for 72 h, and cell viability was evaluated using the MTS assay. I–L H1975/OR cells were transfected with β-catenin shRNA or control shRNA. I The expression of β-catenin and SOX2 was detected by western blotting. J The population of CD133+ cells was evaluated by flow cytometry. K, L Measurement of sphere formation (*p < 0.05, **p < 0.01).
PAK2 interacts with β-catenin and regulates the phosphorylation and transcriptional activity of β-catenin in osimertinib-resistant NSCLC cells
Previous studies have demonstrated that PAKs regulate the phosphorylation of β-catenin [24]. As the osimertinib-resistant NSCLC cells expressed high levels of phospho-PAK2 (p-PAK2) (Fig. 1), we assessed the possibility that PAK2 phosphorylates β-catenin in osimertinib-resistant NSCLC cells. To this end, we first measured the signaling dynamics between PAK2 and β-catenin across a time course of 10 d in H1975 cells treated with osimertinib, and compared with those of H1975/OR cells exposed to osimertinib for over 12 weeks (Fig. 3A). While the inhibitory effect of osimertinib on EGFR was observed throughout the duration of treatment, the inhibitory effect of osimertinib on p-PAK2, β-catenin, and phospho-β-catenin (p-β-catenin) (S552) was only evident at 48 h. However, we observed a gradual increase in the levels of β-catenin and p-β-catenin (S552) following PAK2 activation during the establishment of tolerance and maintained acquired resistance (Fig. 3B). This suggested that activation of PAK2-β-catenin signaling occurs after constant EGFR inhibition and is maintained in drug-tolerant cells and cells with acquired resistance. We next evaluated the effect of PAK2 on the expression and phosphorylation of β-catenin. To this end, we treated H1975/OR cells with a short hairpin RNA (shRNA) targeting PAK2 and observed that the PAK2 shRNA significantly decreased the expression and phosphorylation of β-catenin (Fig. 3C). Similar results were observed in PC9/OR and HCC827/OR cells (Fig. 3D). Conversely, the overexpression of PAK2 significantly increased the expression and phosphorylation levels of β-catenin in H1975, PC9, and HCC827 cells (Fig. 3E, Fig. S4A). Moreover, we found that PAK2 had no effect on the activation of AKT and ERK, which have been implicated in the regulation of β-catenin phosphorylation [25, 26] (Fig. S4B). Previous studies have demonstrated that PAKs can interact with β-catenin [27]. As expected, we identified an association between PAK2 and β-catenin in H1975/OR cells by coimmunoprecipitation studies (Fig. 3F). To this end, H975 cells were transiently transfected with Flag-PAK2 and subjected to an immunoprecipitation (IP) assay using a Flag-tagged antibody. The results demonstrated that β-catenin co-precipitated with Flag (Fig. 3G), suggesting that PAK2 phosphorylates β-catenin by directly interacting with β-catenin. We further investigated the role of PAK2 in the nuclear localization of β-catenin, and found that knockdown of PAK2 decreased the nuclear localization of β-catenin (Fig. 3H). We employed the TOPFlash/FOPFlash reporter assay to ascertain whether PAK2 affects the transcriptional activity of β-catenin-TCF/LEF. Notably, knockdown of PAK2 strongly decreased the activity of the TOPFlash reporter, and consequently reduced the transcriptional activity of β-catenin-TCF (Fig. 3I, Fig. S4C). Taken together, these findings demonstrated that activation of PAK2 increases the phosphorylation and nuclear localization of β-catenin, and in turn promotes the transcriptional activity of β-catenin in osimertinib-resistant NSCLC cells.
A H1975 cells were treated with 1 μM osimertinib at the indicated times, and the levels of the indicated proteins were evaluated by western blotting. B Intensity curve of protein expression. C–E H1975/OR cells were transfected with PAK2 shRNA or control shRNA (C); PC9/OR and HCC827/OR cells were transfected with PAK2 shRNA or control shRNA (D); H1975 cells were transfected with PAK2-expressing vector or control vector (E); the expression of PAK2, p-PAK2, β-catenin and p-β-catenin was evaluated by western blotting. F The total protein extracts of H1975/OR cells were subjected to IP studies using a PAK2 antibody, followed by IB with a β-catenin antibody. Reciprocal IP was performed using β-catenin, followed by IB with a PAK2 antibody. G The total protein extracts of H1975 cells transfected with Flag-PAK2 were subjected to IP studies using a Flag antibody, followed by IB with a β-catenin antibody. H, I H1975/OR cells were transfected with PAK2 shRNA or control shRNA, H The cellular localization of β-catenin was analyzed by IF. I The transcriptional activity of β-catenin was assessed by a TOPFlash/FOPFlash reporter assay (**p < 0.01).
PAK2 induces osimertinib resistance by promoting β-catenin-dependent stemness in NSCLCs
After establishing that PAK2 activity increases in osimertinib-resistant NSCLC cells, and that PAK2 induces the phosphorylation and transcriptional activity of β-catenin, we proceeded to examine the effect of PAK2 on CSC properties. We observed that knockdown of PAK2 effectively suppressed tumorsphere formation in H1975/OR cells (Fig. 4A, B), whereas overexpression of PAK2 markedly increased tumorsphere formation in H1975 cells (Fig. 4A, B). The ability of PAK2 in promoting the population of CSCs was also confirmed by the increase in the population of CD133+ in PAK2-overexpressing H1975 cells (Fig. 4C, D). In contrast, knockdown of PAK2 significantly decreased the population of CD133+ H1975/OR cells (Fig. 4C, D). Moreover, knockdown of PAK2 significantly decreased the expression of SOX2 (Fig. 4E). We established a β-catenin-expressing construct and transfected it into PAK2-knockdown H1975/OR cells. Transfection with the β-catenin-expressing vector successfully restored the expression of SOX2 that had been suppressed by PAK2 shRNA (Fig. 4E). Additionally, the suppressive effects of PAK2 shRNA on CSC properties were reversed by the overexpression of β-catenin in H1975/OR cells (Fig. 4F, G). These results suggested that PAK2 induced β-catenin-dependent stemness in NSCLC cells. We further investigated the effects of PAK2 on osimertinib resistance. We observed that knockdown of PAK2 significantly increased osimertinib sensitivity (Fig. 4H). Moreover, knockdown of PAK2 significantly increased osimertinib-induced cell apoptosis in H1975/OR cells (Fig. 4I, Fig. S5A). However, the overexpression of β-catenin attenuated this effect and reduced osimertinib sensitivity (Fig. 4H, I, Fig. S5A). Similar results were observed in PC9/OR and HCC827/OR cells (Fig. S5B). In order to verify the role of PAK2 in mediating osimertinib resistance in vivo, H1975/OR cells stably transfected with control shRNA or PAK2 shRNA were subcutaneously injected into nude mice for establishing tumor xenografts, following which the mice were administered either DMSO or osimertinib (10 mg/kg/day, og). Consistent with our in vitro results, we observed that although the H1975/OR tumors were resistant to osimertinib, the knockdown of PAK2 significantly increased the inhibitory effects of osimertinib in vivo, as indicated by the decrease in tumor size and weight (Fig. 4J–L). Residual tumors from H1975/OR xenografts were subjected to IHC staining for determining stemness. We observed a marked decrease in the staining intensity of β-catenin and SOX2 in PAK2-knockdown H1975/OR xenograft tumors, which was consistent with the results obtained from the in vitro experiments (Fig. S6). Previously studies suggested that PAKs have both overlapping and distinct functions [28]. We then investigated the functional role of PAK1 and PAK3 in osimertinib resistance of NSCLC cells. We found that the phosphorylation of PAK1 was increased in PC9/OR cells and HCC827/OR cells but not in H1975/OR cells (Fig. S7A). However, the phosphorylation of PAK3 showed no obvious change in osimertinib-resistant cells (Fig. S7A). We further investigated the effects of PAK1 siRNA on osimertinib resistance, and observed that knockdown of PAK1 was not sufficient to increase osimertinib sensitivity in osimertinib-resistant cells (Fig. S7B, C). Moreover, we found that knockdown of PAK1 had no effect on the phosphorylation and expression levels of β-catenin (Fig. S7D). Taken together, these results suggested that the activity of PAK2 is responsible for generating osimertinib resistance and CSC properties via the activation of β-catenin in NSCLC cells.
A, B H1975/OR cells were transfected with PAK2 shRNA or control shRNA; A H1975 cells were transfected with PAK2-expressing vector or control vector. B Measurement of sphere formation. C, D H1975/OR cells were transfected with PAK2 shRNA or control shRNA, and H1975 cells were transfected with PAK2-expressing vector or control vector, and the population of CD133+ cells was evaluated by flow cytometry. E–G H1975/OR cells were transfected with PAK2 shRNA, or PAK2 shRNA combined with pCMV-β-catenin, E The expression of PAK2, β-catenin, and SOX2 was detected by western blotting. F Measurement of sphere formation. G The population of CD133+ cells was evaluated by flow cytometry. H, I H1975/OR cells transfected with PAK2 shRNA alone, or with PAK2 shRNA combined with pCMV-β-catenin, were treated with 2 μM osimertinib for 48 h; H Evaluation of cell viability using the MTS assay; I Detection of cellular apoptosis by flow cytometry. J–L H1975/ORshControl or H1975/ORshPAK2 cells (5 × 106) were subcutaneously inoculated into nude mice for generating xenograft tumors. When the size of the tumors reached ~60 mm3, the mice were randomly allocated into two groups: vehicle control group (0.01% DMSO in PBS), and osimertinib group (10 mg/kg/day, og), that received the respective treatments via oral gavage for 21 days. J The volume of the tumors was measured on the indicated days. K At the end of the experiment, the tumors were dissected and imaged as indicated. L The weights of the tumors were measured (*p < 0.05, **p < 0.01).
Increased HER3 contributes to osimertinib resistance and regulates PAK2 phosphorylation
Acquired osimertinib resistance is highly heterogeneous, encompassing EGFR-dependent as well as EGFR-independent mechanisms [29]. However, we did not detect EGFR-C797S, S768I, L861Q, G719X and 20-Ins mutants in osimertinib-resistant cells (Fig. S8), which suggested that PAK2/β-catenin-induced osimertinib resistance was primarily through EGFR-independent mechanisms. To evaluate potential mechanisms underlying the activation of PAK2/β-catenin in osimertinib-resistant cells, H1975/OR cells were treated with different receptor tyrosine kinase (RTK) ligands, including HGF, IGF1, EGF, and NRG1. We found that treatment with NRG1 significantly increased the phosphorylation of PAK2 in H1975/OR cells (Fig. S9). We further confirmed that NRG1 increased the phosphorylation of PAK2 and β-catenin in HCC827/OR and PC9/OR cells (Fig. 5A). NRG1 is a specific ligand for HER3 receptor [30]. Western blotting revealed increased HER3 in osimertinib-resistant cells compared to parental cells (Fig. 5B). Based on the involvement of HER3 in EGFR-TKI resistance [31], we speculated that this receptor mediates the activation of PAK2/β-catenin in osimertinib-resistant cells. To test this hypothesis, we used RNAi to specifically silence HER3 expression. As expected, we found that silencing HER3 resulted in significantly reduced PAK2 and β-catenin phosphorylation in 1975/OR and PC9/OR cells (Fig. 5C). PAKs were originally identified as protein kinases that function downstream of the Rho GTPases Cdc42 and Rac [28]. Indeed, we found that Rac/Cdc42 was activated in osimertinib-resistant cells (Fig. 5B), whereas activation of Rac/Cdc42 was also impaired upon HER3 silencing (Fig. 5C), implying a main role for HER3 in driving the activation of these pathways in osimertinib-resistant cells. Moreover, knockdown of HER3 resulted in increased osimertinib-induced cell apoptosis (Fig. 5D, E) and increased sensitivity to osimertinib (Fig. 5F). Taken together, these data indicated that HER3 as an upstream regulator of PAK2, drives resistance to osimertinib in NSCLC cells.
A HCC827/OR and PC9/OR cells were treated with NRG1 at indicated concentrations for 24 h, the expression of p-PAK2, p-β-catenin, PAK2, and β-catenin was analyzed by western blotting. B The expression of HER3, p-Rac1/Cdc42, and Rac1/Cdc42 in osimertinib-resistant cells and osimertinib-sensitivity cells was analyzed by western blotting. C H1975/OR and PC9/OR cells were transfected with HER3 shRNA or control shRNA, the expression of PAK2, p-PAK2, β-catenin, p-β-catenin, HER3, p-Rac1/Cdc42, and Rac1/Cdc42 was evaluated by western blotting. D–F H1975/OR cells transfected with HER3 shRNA were treated with 2 μM osimertinib for 48 h, cellular apoptosis was detected by flow cytometry (D, E); IC50 values were evaluation by MTS assay (F) (*p < 0.05, **p < 0.01).
HER3/PAK2/β-catenin signaling is activated in clinical samples of osimertinib-resistant NSCLCs and is associated with poor clinical outcomes in patients with EGFR-mutant NSCLC
We proceeded to establish the clinical relevance of the increased HER3/PAK2/β-catenin signaling in mediating the acquired resistance to osimertinib in NSCLC. Staining and automated quantification of the levels of HER3, p-PAK2 and β-catenin in matched diagnosis and relapse samples from seven patients with advanced-stage T790M EGFR-mutant NSCLC, who underwent treatment with osimertinib, revealed that the levels of HER3, p-PAK2 and β-catenin were significantly increased after acquired resistance compared to those before treatment (Fig. 6A, B). We also observed that the levels of SOX2 were increased in these patients with acquired osimertinib resistance (Fig. 6A, B). Furthermore, we retrospectively collected and analyzed specimens obtained from a cohort of 50 patients with EGFR-mutant NSCLC, who had received treatment with an EGFR-TKI (erlotinib or gefitinib). We observed that a higher proportion of patients who were sensitive to EGFR-TKIs expressed lower levels of p-PAK2 than those of the group with EGFR-TKI resistance (Fig. 6C, D). We further observed that the high expression of HER3, β-catenin and SOX2 was significantly positively correlated with resistance to EGFR-TKIs (Fig. 6E–G), and positively correlated with p-PAK2 expression (Fig. 6H–J). Notably, patients with low levels of p-PAK2 lived for over a year without tumor growth (PFS of 11.6 months), versus those with high levels of p-PAK2 (PFS of 8.5 months), following treatment with EGFR-TKIs (Fig. 6K). Consistently, the high levels of HER3, β-catenin and SOX2 also correlated with shorter PFS in patients with NSCLC who had received treatment with EGFR-TKI (Fig. 6L–N). Altogether, these data suggested that activation of HER3/PAK2/β-catenin signaling is a feature of acquired EGFR-TKI resistance, and could be used as biomarkers of EGFR TKI-resistant NSCLCs.
A Images of p-PAK2, β-catenin, SOX2, and HER3 staining in T790M mutant NSCLC tissues from patient 2 and patient 7 before and after osimertinib resistance. B IHC staining scores for p-PAK2, β-catenin, SOX2 and HER3 in T790M mutant NSCLC tissues from 7 patients before and after osimertinib resistance. C Representative images of p-PAK2 staining in tissues of EGFR-mutant NSCLC. (Upper) p-PAK2low (−/ + ), (Lower) p-PAK2high ( + + / + + + ). D–F Percentage histogram depicting the correlation between the expression of p-PAK2 (D), β-catenin (E), SOX2 (F), or HER3 (G), and sensitivity to EGFR-TKIs in patients with EGFR-mutant NSCLC. H–J Percentage histogram depicting the correlation between the expression of p-PAK2 and β-catenin (H), p-PAK2 and SOX2 (I), p-PAK2 and HER3 (J) in EGFR-mutant NSCLC tissues. K–N Scatter diagram depicting the correlation between the PFS and the expression of p-PAK2 (K), β-catenin (L), SOX2 (M), or HER3 (N) in patients with EGFR-mutant NSCLC.
Targeting PAK2 synergizes with osimertinib in suppressing the growth of osimertinib-resistant NSCLC
Based on the above findings, we subsequently evaluated the effects of the pharmacological inhibition of PAK2 using the PAK2 inhibitors, G5555 [32] and FRAX597 [33], on osimertinib resistance. We found that treatment with G5555 or FRAX597 effectively inhibited PAK2 phosphorylation in a dose-dependent manner (Fig. 7A). In agreement with the results of PAK2 knockdown experiments, inhibition of PAK2 by G5555 or FRAX597 significantly diminished pS552-β-catenin and reduced total β-catenin in H1975/OR cells (Fig. 7A). Notably, we found that the combination of G5555 or FRAX597 with osimertinib synergistically inhibited the growth of H1975/OR cells (Fig. 7B, Fig. S10A). Flow cytometric analyses revealed that in combination with osimertinib, G5555 or FRAX597 markedly increased the percentage of apoptotic H1975/OR cells to 37.6% and 43.1%, respectively, whereas osimertinib alone slightly altered the population of apoptotic H1975/OR cells (Fig. 7C). Similar results were observed in PC9/OR cells cotreated with osimertinib and G5555 or FRAX597 (Fig. S10B–D). In order to further investigate the effect of PAK2 inhibitors in vivo, H1975/OR cells were subcutaneously injected into nude mice for establishing tumor xenografts. After the formation of tumors, the mice were randomly divided into four groups and treated with osimertinib (10 mg/kg/day), FRAX597 (10 mg/kg/day), or a combination of the agents. Consistent with our in vitro results, treatment with osimertinib or FRAX597 alone slightly reduced tumor growth; however, combined treatment with osimertinib plus FRAX597 significantly inhibited tumor growth (Fig. 7D–F). Similarly, we observed a significant decrease in tumor growth in mice treated with the combination of osimertinib and G5555, compared to that of mice treated with osimertinib or G5555 alone (Fig. S11A–C). The body weights of the mice in all the groups remained stable, indicating that treatment with osimertinib, FRAX597, or G5555 was not associated with significant toxicity (Fig. S11D). The stemness of H1975/OR xenografts decreased following co-treatment with osimertinib and FRAX597, as indicated by the reduced expression of SOX2 (Fig. 7G) and CD133 (Fig. S11E). Taken together, these data indicated that targeting PAK2 might serve as a potential therapeutic strategy for overcoming osimertinib resistance in NSCLC.
A H1975/OR cells were treated with PAK2 inhibitors, G5555 or FRAX597, at the indicated concentrations for 48 h, and the expression of p-PAK2, PAK2, p-β-catenin, and β-catenin was measured by western blotting. B H1975/OR cells were co-treated with osimertinib and a PAK2 inhibitor, G5555 or FRAX597, at the indicated concentrations for 72 h, cell viability were measured by MTS assay. C H1975/OR cells were co-treated with 2 μM osimertinib and 5 μM G5555 or 5 μM FRAX597 for 48 h, following which cellular apoptosis was detected by flow cytometry. D–F H1975/OR cells (5 × 106) were subcutaneously inoculated into nude mice for generating xenograft tumors. When the size of the tumors reached ~60 mm3, the mice were randomly allocated into four groups and treated with the vehicle control (0.01% DMSO in PBS), osimertinib (10 mg/kg/day, og), FRAX597 (10 mg/kg/day, og), or a combination of osimertinib and FRAX597 for 36 days. D Volume of the tumors was measured on the indicated days; E The tumors were dissected at the end of the experiment. F Measurement of tumor weights. G The tumor tissues were resected, fixed, sectioned, and mounted on slides. Specimens of tumor tissue were subjected to IHC staining using antibodies specific to p-PAK2, p-β-catenin, and SOX2. H Mechanism of PAK2/β-catenin signaling in promoting osimertinib resistance in NSCLCs (**p < 0.01).
Discussion
Understanding and identifying the mechanisms underlying resistance to osimertinib is critical for improving the therapeutic efficacy and survival rate of patients with NSCLC. In this study, we identified a novel signaling pathway, involving HER3, PAK2 and β-catenin, associated with osimertinib resistance. We found that HER3/PAK2/β-catenin signaling was activated in osimertinib-resistant NSCLC cells and clinical samples, and that the activation of HER3/PAK2/β-catenin signaling conferred stemness and osimertinib resistance. We additionally demonstrated that the suppression of PAK2 via gene knockdown or pharmacological inhibition restored the response of osimertinib-resistant cells to osimertinib, and this could serve as a potential therapeutic strategy for overcoming acquired resistance to osimertinib (Fig. 7H).
Recently studies demonstrated that the increased expression and activation of β-catenin play an important role in acquired resistance to first- and second-generation EGFR-TKIs [34]. β-catenin is stabilized and activated via phosphorylation by mutant EGFRs [35], and inhibition of β-catenin enhances the anticancer effect of irreversible EGFR-TKIs in EGFR-mutant NSCLCs with a T790M mutation [36]. The present study is the first to demonstrate the principal role of β-catenin in osimertinib resistance in NSCLC. We observed that the expression of β-catenin was upregulated in both T790M-positive and T790M-negative osimertinib-resistant cells, while the depletion of β-catenin increased osimertinib sensitivity. Furthermore, the expression of β-catenin was increased in a population of osimertinib-resistant clinical samples of NSCLC, indicating that the activation of β-catenin is a clinically relevant mechanism underlying the acquired resistance to osimertinib. Apart from promoting cancer progression via the transcriptional induction of proliferation-related genes [37], β-catenin-activating signal cascades also promote CSC properties [38, 39]. Increasing the expression of CSC markers has been previously considered to be associated with acquired resistance to EGFR-TKIs in NSCLCs [22, 40]. SOX2 is an important transcription factor that regulates the self-renewal of cancer stem cells and the therapeutic response to EGFR-TKIs in lung cancer [41, 42]. Consistent with these observations, the results of our study demonstrated that osimertinib resistance was associated with CSC properties and an increased abundance of SOX2. We also observed that the expression of SOX2 was positively correlated with the expression of β-catenin in osimertinib-resistant NSCLC tissues. Moreover, the knockdown of β-catenin decreased the expression of SOX2 and reduced stemness, indicating that β-catenin plays a crucial role in CSC properties and osimertinib resistance in NSCLCs.
PAK2 is a member of the PAK family of serine/threonine kinases that play critical roles in cancer progression, including cellular proliferation, and regulation of cellular apoptosis [28, 43]. PAK2 is activated by binding with upstream GTPases (Rac1 or Cdc42), which leads to phosphorylation at multiple Ser/Thr sites [44, 45]. In this study, we identified that the phosphorylation of Ser141 was significantly increased in osimertinib-resistant cells, indicating that PAK2 exists in the active state in resistant cells. Recent studies clarify the critical role of HER3 in EGFR-TKI resistance and highlight a rationale for combination therapy with HER3-DXd and EGFR-TKI in EGFR-mutated NSCLC [31]. Enhanced HRG/ErbB3 signaling has also been implicated in Rac1 Activation[46]. Consistent with these observations, we revealed that HER3 as an upstream regulator of PAK2 drives the activation of Rac/PAK2 pathways in osimertinib-resistant NSCLC cells. Moreover, consistent with the reports that PAKs are essential for the activation of β-catenin [47], we first demonstrated that PAK2 increased the phosphorylation levels of β-catenin at Ser552, that prevents the ubiquitination and proteasomal degradation of β-catenin [25]. Second, we observed that PAK2 promoted the phosphorylation of β-catenin by directly interacting with β-catenin, and enhanced its transcriptional activity in osimertinib-resistant NSCLC cells. Third, we observed that the expression of p-PAK2 and β-catenin was significantly positively correlated in osimertinib-resistant NSCLC tissues. Therefore, these results elucidated a critical role of PAK2 in regulating the expression and transcriptional activity of β-catenin, and suggested that a novel PAK2–β-catenin axis is responsible for osimertinib resistance in NSCLC.
Furthermore, current studies revealed that PAK2 might serve as a possible therapeutic target for NSCLC. We demonstrated that the depletion of PAK2 reduced resistance to osimertinib in osimertinib-resistant NSCLC cells. A previous study similarly demonstrated that the depletion of PAK2 reduced chemotherapeutic resistance in head and neck cancer cells [48]. Notably, we observed that depletion of PAK2 induced a marked reduction in the expression of β-catenin, and thereby reduced CSC properties. These results strengthened our hypothesis that the activation of the PAK2-β-catenin axis plays an essential role in osimertinib resistance in NSCLC cells. Recently studies demonstrated that PAK inhibitors have antitumor activities against various types of human cancers [43, 49, 50]. Several studies have also demonstrated that PAK2 inhibitors sensitize cancer cells to growth inhibition by chemotherapeutic agents [51,52,53]. In this study, we demonstrated that the PAK inhibitors, FRAX597 [33] and G5555 [32], effectively inhibited the phosphorylation of PAK2, which reduced β-catenin phosphorylation and expression in osimertinib-resistant NSCLC cells. Notably, FRAX597 or G5555, in combination with osimertinib, synergistically inhibited cell growth and enhanced apoptosis in osimertinib-resistant NSCLC cells both in vitro and in vivo. This suggested that inhibition of PAK2 might serve as an effective strategy for overcoming acquired osimertinib resistance. It was evident that PAK inhibitors used herein were effective against all Group I PAKs, but did not exclusively target PAK2. As FRAX597 or G5555 effectively decreased the phosphorylation levels of PAK2 in osimertinib-resistant cells, and as the results of this study demonstrated that PAK2 knockdown restored the responses of osimertinib-resistant cells and tumors to osimertinib, it is fair to assume that the suppression of PAK2, but not PAK1 or PAK3, contributes to the effects of FRAX597 and G5555 in overcoming osimertinib resistance.
In conclusion, this study revealed a novel mechanism underlying the functional interactions between PAK2 and β-catenin, and elucidated a critical role of the HER3/PAK2/β-catenin axis in patients with EGFR-mutant NSCLC with acquired resistance to osimertinib. Based on this important finding, we suggest that targeting PAK2 might serve as an effective strategy for overcoming acquired resistance to osimertinib.
Materials and methods
Cell culture
H1975 (EGFR L858R + T790M), PC9 (EGFR E746-A750del), and HCC827 (EGFR E746-A750del) cells were obtained from ATCC. The osimertinib-resistant NSCLC H1975/OR, PC9/OR, and HCC827/OR were successfully established by continually exposing H1975, PC9, and HCC827 cells, respectively, to a gradually increasing concentration of osimertinib for more than 12 weeks. All the cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). All the cell lines were incubated at 37 °C in a humidified atmosphere containing 5% CO2. The cells were authenticated by DNA profiling by short tandem repeat (STR) analysis.
Patients and specimens
This study was approved by the Ethics Committee of Guangzhou Medical University. All samples were collected with informed consent from the patients and all examining procedures were performed with the approval of the internal review and ethics boards of the hospital. Seven pairs of osimertinib pretreatment and resistance tumor specimens were obtained from seven patients diagnosed with advanced-stage EGFR-T790M mutant NSCLC in the Affiliated Tumor Hospital of Guangzhou Medical University between 2018 and 2021. Tumor specimens from 50 NSCLC patients with EGFR-mutations who had received EGFR-TKIs (erlotinib or gefitinib) treatment were acquired from Affiliated Tumor Hospital of Guangzhou Medical University between 2013 and 2018. Follow-up information was from patients’ medical record.
Statistical analyses
All the data were presented as the mean ± SD. All the statistical analyses were performed using SPSS version 16.0 and GraphPad Prism version 6. A chi-square test was used to analyze the relationships among the gene expression levels. Student’s t-tests were performed to calculate the p-value, and p < 0.05 was considered to be statistically significant.
RNA interference and plasmid transfection, Cell viability assay, Cell apoptosis assay, Western blotting, Co-immunoprecipitation, Flow cytometric analysis, Mammosphere assay, Immunofluorescence, TOPFlash/FOPFlash Luciferase Reporter Assay, Animal experiments, IHC staining, and iTRAQ-based proteomics are described in the Supplementary Experimental Procedure.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl J Med. 2010;362:2380–8.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–89.
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl J Med. 2005;352:786–92.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Disco. 2014;4:1046–61.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl J Med. 2017;376:629–40.
Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31:1536–44.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl J Med. 2018;378:113–25.
Ramalingam SS, Yang JCH, Lee CK, Kurata T, Kim DW, John T, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:841–9.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl J Med. 2020;382:41–50.
Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1:982–4.
Ortiz-Cuaran S, Scheffler M, Plenker D, Dahmen L, Scheel AH, Fernandez-Cuesta L, et al. Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors. Clin Cancer Res. 2016;22:4837–47.
Zeng Y, Yu D, Tian W, Wu F. Resistance mechanisms to osimertinib and emerging therapeutic strategies in nonsmall cell lung cancer. Curr Opin Oncol. 2022;34:54–65.
Eberlein CA, Stetson D, Markovets AA, Al-Kadhimi KJ, Lai ZW, Fisher PR, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 2015;75:2489–500.
Taniguchi H, Yamada T, Wang R, Tanimura K, Adachi Y, Nishiyama A, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10:259.
Zhang X, Maity TK, Ross KE, Qi Y, Cultraro CM, Bahta M, et al. Alterations in the global proteome and phosphoproteome in third generation EGFR TKI resistance reveal drug targets to circumvent resistance. Cancer Res. 2021;81:3051–66.
Shah KN, Bhatt R, Rotow J, Rohrberg J, Olivas V, Wang VE, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med. 2019;25:111–8.
Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–36.
Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–61.
Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–64.
Lu H, Liu S, Zhang G, Bin W, Zhu Y, Frederick DT, et al. PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature 2017;550:133–6.
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9.
Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–70.
Sun J, Khalid S, Rozakis-Adcock M, Fantus IG, Jin T. P-21-activated protein kinase-1 functions as a linker between insulin and Wnt signaling pathways in the intestine. Oncogene 2009;28:3132–44.
Rane CK, Minden A. P21 activated kinase signaling in cancer. Semin Cancer Biol. 2019;54:40–9.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–37.
Carraway KL 3rd, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, et al. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994;269:14303–6.
Yonesaka K, Tanizaki J, Maenishi O, Haratani K, Kawakami H, Tanaka K, et al. HER3 augmentation via blockade of EGFR/AKT signaling enhances anticancer activity of HER3-targeting patritumab deruxtecan in EGFR-mutated non-small cell lung cancer. Clin Cancer Res. 2022;28:390–403.
Rudolph J, Murray LJ, Ndubaku CO, O’Brien T, Blackwood E, Wang W, et al. Chemically diverse group I p21-Activated Kinase (PAK) inhibitors impart acute cardiovascular toxicity with a narrow therapeutic window. J Med Chem. 2016;59:5520–41.
Licciulli S, Maksimoska J, Zhou C, Troutman S, Kota S, Liu Q, et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J Biol Chem. 2013;288:29105–14.
Arasada RR, Shilo K, Yamada T, Zhang J, Yano S, Ghanem R, et al. Notch3-dependent beta-catenin signaling mediates EGFR TKI drug persistence in EGFR mutant NSCLC. Nat Commun. 2018;9:3198.
Nakayama S, Sng N, Carretero J, Welner R, Hayashi Y, Yamamoto M, et al. beta-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res. 2014;74:5891–902.
Togashi Y, Hayashi H, Terashima M, de Velasco MA, Sakai K, Fujita Y, et al. Inhibition of beta-Catenin enhances the anticancer effect of irreversible EGFR-TKI in EGFR-mutated non-small-cell lung cancer with a T790M mutation. J Thorac Oncol. 2015;10:93–101.
Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356.
Zhang F, Li P, Liu S, Yang MQ, Zeng SS, Deng JJ, et al. beta-Catenin-CCL2 feedback loop mediates crosstalk between cancer cells and macrophages that regulates breast cancer stem cells. Oncogene 2021;40:5854–65.
Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165.
Del ReM, Arrigoni E, Restante G, Passaro A, Rofi E, Crucitta S, et al. Concise review: resistance to tyrosine kinase inhibitors in non-small cell lung cancer: the role of cancer stem cells. Stem Cells. 2018;36:633–40.
Kuo MH, Lee AC, Hsiao SH, Lin SE, Chiu YF, Yang LH, et al. Cross-talk between SOX2 and TGFbeta signaling regulates EGFR-TKI tolerance and lung cancer dissemination. Cancer Res. 2020;80:4426–38.
Chiu CF, Chang YW, Kuo KT, Shen YS, Liu CY, Yu YH, et al. NF-kappa B-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc Natl Acad Sci USA. 2016;113:E2526–E35.
Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25.
Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–72.
Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene 2017;605:20–31.
Lopez-Haber C, Barrio-Real L, Casado-Medrano V, Kazanietz MG. Heregulin/ErbB3 signaling enhances CXCR4-Driven Rac1 activation and breast cancer cell motility via hypoxia-inducible factor 1alpha. Mol Cell Biol. 2016;36:2011–26.
Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–U17.
Gupta A, Ajith A, Singh S, Panday RK, Samaiya A, Shukla S. PAK2-c-Myc-PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis. 2018;9:825.
Kumar R, Li DQ. PAKs in human cancer progression: from inception to cancer therapeutic to future oncobiology. Adv Cancer Res. 2016;130:137–209.
Araiza-Olivera D, Feng Y, Semenova G, Prudnikova TY, Rhodes J, Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene 2018;37:944–52.
Yeo D, He H, Patel O, Lowy AM, Baldwin GS, Nikfarjam M. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. Bmc Cancer. 2016;16:24.
Huynh N, Shulkes A, Baldwin G, He H. Up-regulation of stem cell markers by P21-activated kinase 1 contributes to 5-fluorouracil resistance of colorectal cancer. Cancer Biol Ther. 2016;17:813–23.
Wang K, Huynh N, Wang X, Pajic M, Parkin A, Man J, et al. PAK inhibition by PF-3758309 enhanced the sensitivity of multiple chemotherapeutic reagents in patient-derived pancreatic cancer cell lines. Am J Transl Res. 2019;11:3353–64.
Funding
This work was supported by the National Natural Science Foundation of China (81972194, 82172810, 81772825); Guangdong Natural Science Funds (2019A1515010113); The Science and Technology Program of Guangzhou (202201010839); Medical Scientific Research Foundation of Guangdong (B2020165); Discipline construction project of Guangdong Medical University (4SG22005G).
Author information
Authors and Affiliations
Contributions
HL, ZH, KL, and JD designed the experiments, analyzed the data, and drafted the manuscript and figures. YY, PL, YH, DC, SF, JW, MY, SZ, and XL detected the cells’ biological function, performed IHC analyses and animal experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, Y., Li, P., Huang, Y. et al. P21-activated kinase 2-mediated β-catenin signaling promotes cancer stemness and osimertinib resistance in EGFR-mutant non-small-cell lung cancer. Oncogene 41, 4318–4329 (2022). https://doi.org/10.1038/s41388-022-02438-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02438-z
- Springer Nature Limited