Abstract
Glioblastoma multiforme (GBM) or glioblastoma is the most deadly malignant brain tumor in adults. GBM is difficult to treat mainly due to the presence of glioblastoma stem cells (GSCs). Epidermal growth factor receptor variant III (EGFRvIII) has been linked to stemness and malignancy of GSCs; however, the regulatory mechanism of EGFRvIII is largely unknown. Here, we demonstrated that Anoctamin-1 (ANO1), a Ca2+-activated Cl– channel, interacts with EGFRvIII, increases its protein stability, and supports the maintenance of stemness and tumor progression in GSCs. Specifically, shRNA-mediated knockdown and pharmacological inhibition of ANO1 suppressed the self-renewal, invasion activities, and expression of EGFRvIII and related stem cell factors, including NOTCH1, nestin, and SOX2 in GSCs. Conversely, ANO1 overexpression enhanced the above phenomena. Mechanistically, ANO1 protected EGFRvIII from proteasomal degradation by directly binding to it. ANO1 knockdown significantly increased survival in mice and strongly suppressed local invasion of GSCs in an in vivo intracranial mouse model. Collectively, these results suggest that ANO1 plays a crucial role in the maintenance of stemness and invasiveness of GSCs by regulating the expression of EGFRvIII and related signaling molecules, and can be considered a promising therapeutic target for GBM treatment.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor with a mean survival of <15 months and a 5-year survival rate of 5% [1, 2]. The poor prognosis of patients with GBM is due to its resistance to chemotherapy and radiotherapy after surgery, widespread infiltration into the surrounding normal brain tissues, and rapid growth leading to recurrence [3, 4]. One of the main reasons for malignancy and therapeutic failure in GBM patients is the presence of glioblastoma stem cells (GSCs) within the tumor bed [5, 6]. GSCs have a self-renewal ability similar to that of normal neural stem cells, and they are responsible for the development of tumors and their continued growth, therapeutic resistance, and recurrence after treatment [5]. Therefore, the elimination of GSCs could be an essential factor in increasing the efficacy of brain cancer treatment.
The amplification and rearrangement of epidermal growth factor receptor (EGFR) is a common phenomenon in GBM, and EGFR variant III (EGFRvIII) is a common mutant of EGFR that is frequently observed in GBM [7]. EGFRvIII is a deletion mutant of the EGFR gene with a deletion of exons 2–7; the product of the mutant gene is a constitutively active receptor with a truncated extracellular ligand-binding domain [8]. EGFRvIII is known to drive tumorigenesis by activating downstream signaling molecules such as Notch and STAT3 [9, 10]. As it confers resistance to chemotherapy and radiotherapy, as well as promotes tumorigenesis and the development of GBM cells, EGFRvIII is known as a tumor-specific marker of GSCs [8, 11, 12]. Indeed, it has been reported that the EGFRvIII+/CD133+ population of GBM shows high self-renewal activity and tumorigenic potential in mice [12, 13]. Therefore, targeting the EGFRvIII/STAT3 signaling pathway would be a good therapeutic strategy for eliminating GSCs and enhancing patient survival [13].
Anoctamin-1 (ANO1), also called transmembrane protein 16A (TMEM16A), is cloned as a Ca2+-activated Cl– channel (CaCC) [14,15,16]. It is widely expressed in normal cells, including secretory epithelia, smooth muscle cells, vascular endothelia, and nociceptive neurons [15,16,17,18,19]. It plays important physiological roles in cells, such as fluid secretion, muscle contraction, volume regulation, and nociception [20, 21]. In addition to its physiologically crucial role in normal cells, it has been reported that ANO1 is amplified and overexpressed in various types of cancer, including head and neck squamous cell carcinoma (HNSCC), gastric cancer, breast cancer, colorectal carcinoma, prostate cancer, and glioma. It is associated with tumor growth, invasiveness, and poor prognosis [22,23,24,25,26,27]. Although the detailed molecular mechanism underlying ANO1-induced oncogenesis is yet to be defined, several lines of evidence have suggested the critical role of ANO1 in cancer-promoting signaling. The expression level of ANO1 is correlated with the activation of two major proliferation and survival signaling molecules, mitogen-activated protein kinase (MAPK) and protein kinase B (PKB; also known as AKT), in several cancer cell lines [23,24,25]. The activation of MAPK and PKB by ANO1 is presumed to be due to the interaction of ANO1 with EGFR, which increases the expression of EGFR by a posttranslational mechanism and promotes its phosphorylation [25, 28]. The activation of EGFR signaling by ANO1 also leads to the activation of Ca2+/calmodulin-dependent kinase II signaling [24]. In addition, ANO1 promotes the proliferation, migration, and invasion of glioma cell lines by activating nuclear factor kappa B (NF-κB) signaling [27]. Our previous reports showed that ANO1 trafficking to the cell membrane is regulated by the interaction of ANO1 with 14-3-3γ or β-COP in glioma cell lines [29, 30]. The surface expression of ANO1 is crucial for the migration and invasion of glioma cell lines [29]. However, the roles of ANO1 and ANO1-related biological mechanisms in GSCs have not been investigated until now.
In the present study, we found that the suppression of ANO1 results in the inhibition of self-renewal activity, invasiveness, and tumor formation of GSCs in vitro and in vivo. Mechanistically, ANO1 directly interacted with EGFRvIII, thereby stabilizing it by protecting it from proteasomal degradation. Therefore, our data suggest that suppression of ANO1 would be a good therapeutic approach for enhancing EGFRvIII-targeted therapy for GBM.
Results
ANO1 expression is higher in GSCs than in differentiated GSCs (dGSCs) and correlates with the prognosis and grades of patients
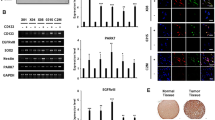
We first examined whether the expression level of ANO1 is dependent on the stemness status of GSCs. Genomic characteristics of the GSCs used were investigated by whole-genome sequencing (Fig. S1). GSCs were differentiated by culturing them in a serum-containing medium for 7 days (dGSCs). Immunoblot analysis showed that ANO1 expression was diminished in dGSCs compared to that in GSCs (Fig. 1a). Differentiation status was confirmed by a reduction in stem cell marker levels (NOTCH1 and SOX2) and enhanced levels of the differentiation marker (GFAP) in GSCs (Fig. 1a). Immunocytochemistry (ICC) analysis also revealed that the expression of ANO1 and NOTCH1 was suppressed in dGSCs (Fig. 1b). As CD133 is a cell surface marker for GSCs [31, 32], we sorted CD133+ and CD133– cells using magnetic beads conjugated with CD133 antibody. As shown in Fig. 1c, western blot analysis revealed that CD133+ cells have considerably higher levels of ANO1 when compared to that of CD133– cells. ICC analysis also showed a strong expression of ANO1 in CD133+ cells (Fig. 1d). Immunohistochemistry (IHC) of the GBM tissue array indicated the enhanced expression of ANO1 in GBM tissues compared to that in normal controls, and the expression was correlated with GBM grades (Fig. 1e), as shown in a previous report [27]. Interestingly, IHC of the GBM tissue array clearly showed a positive correlation between the expression of ANO1 and the expression of SOX2 and NOTCH1 (Fig. S1). In addition, analysis of TCGA data suggested that the expression level of ANO1 is associated with poor prognosis in patients with GBM (Fig. 1f). We also investigated the effect of the combination of ANO1 and stemness factors on the survival of GBM patients. As a result, there was a significant difference in CD133, SOX2, and OCT4 in the survival analysis of GBM patients. However, there was a marginal trend in NOTCH1 and no significant difference in nestin (Fig. S2). These data suggest that ANO1 is preferentially expressed in GSCs and its expression is correlated with grades and poor prognosis of patients with GBM.
a Western blot analysis of GSCs (X04, X08, and 0315) and differentiated GSCs (dGSCs; FBS+) using antibodies indicated in the figure. Differentiation of GSCs was achieved by treating the cells with FBS (10%) for 7 days. b ICC of ANO1 and NOTCH1 in GSCs and dGSCs. c, d Western blot (c) and ICC (d) of CD133+ and CD133– cells sorted from X08 GSCs using magnetic beads. Nuclei were counterstained with DAPI (blue). e IHC of ANO1 in the microarray of tissues of patients with GBM. Representative ANO1 staining of the tissues (left) and relative IHC scores (right). f The Kaplan–Meier survival analysis was used to compare the ANO1 expression profiles in patients with GBM. Patients with high expression of ANO1 (top 10% highest expression) were less favorable to an overall survival profile.
ANO1 regulates self-renewal capacity, tumorigenesis, and invasiveness of GSCs in vitro
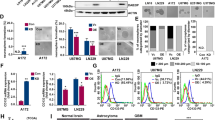
Next, we investigated the role of ANO1 in GSC biology. To suppress the expression of ANO1, we introduced lentivirus-mediated short hairpin (sh)-RNA against ANO1 into GSCs and confirmed the effect using immunoblot analysis (Fig. 2a). Knockdown of ANO1 significantly suppressed its self-renewal activity, as evidenced by sphere-forming (Fig. 2b) and limiting dilution assays (Fig. 2c). ANO1 knockdown also inhibited the colony formation of GSCs in the soft-agar clonogenic assay, a method used for measuring tumorigenic potential in vitro (Fig. 2d). In addition, suppression of ANO1 reduced the invasion of GSCs through Matrigel-coated transwells, a well-known property of GSCs (Fig. 2e). These results were similarly reproduced in the introduction of another shRNA mediated by adenovirus (Fig. S3).
a Western blot analysis of ANO1, b sphere-forming, c limiting dilution, d soft-agar colony-formation, and e invasion assays of GSCs (X08 and 0315) infected with lentiviral-control (shGFP) and lentiviral-shANO1A and D. Detailed experimental procedures are described in “Materials and methods” in the manuscript or in “Methods” in the Supplementary. *0.01 < p < 0.05; **0.005 < p < 0.01; ***p < 0.005.
To confirm the effects of ANO1 knockdown in GSCs, we employed an ANO1 inhibitor, CaCC-A01 [33]. 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT) assay showed that CaCC-A01 suppressed the growth of GSCs in a dose-dependent manner (Fig. 3a). Western blot analysis showed that treatment with the inhibitor led to a reduction in the protein levels of ANO1 in GSCs as described previously (Fig. 3b) [33]. In addition, sphere-forming and limiting dilution assays revealed a significant reduction in self-renewal activity upon CaCC-A01 treatment (Fig. 3c, d). Moreover, CaCC-A01 inhibited colony formation and invasion of GSCs in soft agar and through Matrigel, respectively (Fig. 3e, f). The activity of CaCC-A01 against GSCs was reproduced by another ANO1 inhibitor, T16Ainh-A01 (T-A01) (Fig. S4).
a Western blot analysis of ANO1, b MTT, c sphere-formation, d limiting dilution, e soft-agar colony-formation, and f invasion assays of GSCs (X08 and 0315) pretreated with or without ANO1 inhibitor (CaCC-A01) at the concentration(s) indicated in the figure. *0.01 < p < 0.05; **0.005 < p < 0.01; ***p < 0.005.
Suppression of ANO1 reduces the expression of stemness-related factors
We next examined the effect of ANO1 downregulation on the expression of stemness-associated molecules in GSCs. As shown in Fig. 4a, the suppression of ANO1 by introducing shANO1 adenovirus reduced the expression of crucial stemness factors in GSCs, including nestin, EGFRvIII, SOX2, and NOTCH1. Treatment with CaCC-A01 also led to decreased expression of these stemness factors (Fig. 4b). ICC also showed downregulation of EGFRvIII and NOTCH1 in GSCs upon shANO1 infection and CaCC-A01 treatment (Fig. 4c, d). This phenomenon was also observed when GSCs were treated with T-A01 (Fig. S5). Therefore, these data indicate that ANO1 expression is crucial for the maintenance of stemness in GSCs.
a, b Western blot analysis of GSCs probed with antibodies indicated in the figure. c, d ICC of GSCs probed with antibodies indicated in the figure. Nuclei were counterstained with DAPI (blue). GSCs (X08 and 0315) were infected with adenoviral-control (−) and adenoviral-shANO1 (+) (a, c) or pretreated with CaCC-A01 (1 μM) (b, d).
Overexpression of ANO1 enhances GSC activities
Next, we performed a gain-of-function study of ANO1 in GSCs. We transfected pDEST-GFP-ANO1 into the cells and confirmed its expression using western blot analysis (Fig. 5a). As expected, overexpression of ANO1 further enhanced the self-renewal activity of GSCs, as evidenced by the sphere-forming and limiting dilution assays (Fig. 5b, c). In addition, overexpression of ANO1 elevated colony-forming and invasion activities in these cells (Fig. 5d, e). Overall, the gain- and loss-of-function studies clearly demonstrated that ANO1 is required to maintain the stemness activity in GSCs.
ANO1 interacts with and stabilizes EGFRvIII in GSCs
Next, we investigated the molecular mechanism of ANO1-regulated stemness properties in GSCs. Since it has been reported that EGFRvIII serves as a crucial upstream stemness regulator in GSCs through p-STAT3 and NOTCH1 signaling [10], and ANO1 inhibition significantly downregulates the protein level of EGFRvIII (Fig. 4a–d), we examined whether ANO1 knockdown affects EGFRvIII transcription. As shown in Fig. 6a, the knockdown of ANO1 did not change the mRNA levels of EGFRvIII. In addition, transfection of ANO1 did not restore the mRNA levels of EGFRvIII, which were downregulated by serum differentiation (Fig. 6b). Intriguingly, however, overexpression of ANO1 in d GSCs significantly rescued the expression of EGFRvIII and related stemness factors (Fig. 6c).
a, b Semi-quantitative (sq) (left) and quantitative (q) RT-PCR (right bar graphs) of ANO1 and EGFRvIII in GSCs infected with lentiviral-control (shCon) or shRNA of ANO1 (shANO1A and D) (a) and in dGSCs (+FBS) transfected with control (GFP-Con) or ANO1 (GFP-ANO1) vectors (b). GAPDH was used as a loading control. c Western blot analysis of GSCs (parent) and dGSCs (+FBS) in (b) using antibodies described in the figure. *0.01 < p < 0.05; **0.005 < p < 0.01; ***p < 0.005.
We investigated whether the expression of EGFRvIII and/or EGFR positively correlated with ANO1 at the mRNA or protein levels in GBM tissues, glioma cell lines, and GSC lines. As a result, the mRNA expression of ANO1 was not positively correlated with that of EGFR or EGFRvIII in GBM tissues (Fig. S6a). On the other hand, IHC of GBM tissue microarray revealed that the tissues with high ANO1 expression showed a positive correlation with high expression of EGFR/EGFRvIII (Fig. S6b). Similarly, in glioma cell lines, mRNA expression levels of ANO1 and EGFR were not positively correlated (Fig. S6c). However, at the protein level, the expression patterns of ANO1 and EGFR were positively correlated (Fig. S6d). In GSC lines, no correlation was seen in the mRNA and protein levels between the two factors (Fig. S6e, f). Taken together, these results suggest that ANO1-mediated regulation of EGFRvIII and/or EGFR may not be at the mRNA level but at the protein level.
We performed immunoprecipitation (IP) experiments to determine whether ANO1 interacts with EGFRvIII and affects its downstream stemness signaling. We co-transfected Flag-ANO1 and GFP-EGFRvIII in HEK 293T cells and found that ANO1 strongly binds to EGFRvIII in the heterologous expression system (Fig. 7a). Bimolecular fluorescence complement (BiFC) analysis also indicated the direct binding of ANO1 and EGFRvIII (Fig. 7b). Endogenous IP using GSCs also demonstrated the interaction between ANO1 and EGFRvIII (Fig. 7c). In addition, ICC analysis showed the co-localization of ANO1 and EGFRvIII in these cells (Fig. 7d). To find the ANO1 domain that binds to EGFRvIII, we produced constructs expressing the C-terminal, loop, and N-terminal domain of ANO1, and then transfected them into HEK 293T cells expressing EGFRvIII and performed IP. As a result, EGFRvIII was found to bind to the C-terminal domain of ANO1 (Fig. S7a, b). We also investigated whether EGFR directly binds to ANO1 in GSCs, as reported previously in other cancer cells [24, 28], using GSC lines expressing EGFR (X02 and 528NS). As expected, the IP experiment showed that EGFR was directly bound to ANO1 in these cells (Fig. S8). Because it was reported that ANO1 suppression destabilizes EGFR in head and neck cancer cells [28], we checked whether ANO1 affects the stability of EGFRvIII. As shown in Fig. 7e, pretreatment with MG132 clearly restored the shRNA-mediated suppression of EGFRvIII expression. In addition, IP analysis showed enhanced polyubiquitination of EGFRvIII bands upon MG132 treatment in shANO1-transfected GSCs (Fig. 7f). We also confirmed the reduction in the expression of EGFR using lentiviral-shANO1 (Fig. S9a–c) and restoration of EGFR expression by pretreatment of glioma cell lines (LN229 and U251) and GSC lines (X02 and 528NS) with MG132 (Fig. S9b, c). To demonstrate whether the stabilization of EGFRvIII and/or EGFR by ANO1 is important for the stemness activity of GSCs, we transfected siEGFRvIII and siEGFR in X08 and 0315 GSCs. Interestingly, the knockdown of EGFRvIII reduced both the mRNA and protein levels of EGFR. On the other hand, knockdown of EGFR did not affect the mRNA level of EGFRvIII but caused a decrease in the protein level (Supplementary Fig. 10a, b). Knockdown of EGFRvIII or EGFR inhibited the expression of stemness factors, including nestin, NOTCH1, OCT4, and SOX2, similar to the effect of introducing shANO1 or treatment with ANO1 inhibitor (Fig. 4a, b), but did not affect ANO1 expression (Fig. S10a, b). Similarly, treatment with gefitinib, a specific EGFR/EGFRvIII inhibitor, also suppressed the expression of these stemness factors, but not that of ANO1 (Fig. S10c). siEGFRvIII and gefitinib significantly suppressed self-renewal activities as measured by sphere-forming and limiting dilution assays (Fig. S10d–g). In addition, transfection of siEGFR and/or treatment with gefitinib also suppressed self-renewal activity, expression of stemness factors, and invasion in EGFR-expressing GSC lines, X02 and 528NS (Fig. S11a–h). Conversely, overexpression of EGFRvIII in ANO1 knockdown GSCs recovered the expression of stem cell factors (Fig. S12a), and self-renewal activity (Fig. S12b). Taken together, our data strongly suggest that ANO1 maintains stemness signaling in GSCs by stabilizing EGFRvIII and/or EGFR expression by directly binding to it.
a, b IP and western blot analysis (a) and BiFC (b) of HEK 293T cells transfected with or without Flag-ANO1 and GFP-EGFRvIII (b). c Endogenous IP, western blotting, and d ICC of GSCs. e Western blot analysis of GSCs infected with adenoviral-control (shCon) or shRNA of ANO1 pretreated with or without MG132 at the concentrations indicated in (f) IP and western blot analysis of the GSCs in (e) pretreated with or without MG132 (10 nM). Antibodies used in the western blot are indicated in the figure.
ANO1 regulates the tumorigenic potential of GSCs in vivo
Finally, we examined the effect of ANO1 expression on intracranial tumor formation in GSCs and the survival of mice in vivo. We introduced lentivirus-mediated shANO1 in X08 GSCs and confirmed its effect using reverse transcriptase-polymerase chain reaction (RT-PCR) (Fig. 8a). As shown in Fig. 8b, the survival rate was greatly enhanced in shANO1-infected mice compared to that in control mice. Hematoxylin and eosin (H&E) staining of the xenograft tissues revealed that the size of the tumor was significantly reduced in the shANO1-infected GSCs compared to that in the control (Fig. 8c). In addition, ICC analysis showed that the expression of EGFRvIII was reduced in the xenograft of shANO1-infected mice (Fig. 8d). Interestingly, in contrast to the diffuse and infiltrative tumor formed by GFP control GSCs in the mouse brain, shANO1-infected GSCs formed only a demarcated tumor (Fig. 8c, d). The formation of a demarcated tumor by shANO1D-infected GSCs was maintained until the time of death due to tumor expansion (Fig. 8e). Moreover, ICC of CD31, a specific marker of endothelial cells, revealed that microvessel density was remarkably low in shANO1 tumors, indicating the reduction of tumor angiogenesis by the knockdown of ANO1 (Fig. 8f). Taken together, these data suggest that ANO1 expression is crucial for the expression of EGFRvIII, infiltrative tumor formation, and angiogenesis in GSCs in vivo.
a sqPCR and qPCR of ANO1 in GSCs infected with lentiviral-control (pGFP) or shRNA of ANO1 (shANO1A and D). GAPDH was used as the loading control. b Kaplan–Meier survival curves of mice implanted with GSCs (X08, 1 × 105 cells) transduced with lentiviral-control (pGFP) or shRNA of ANO1 (shANO1A and D). c, d Histopathology of representative mouse brain injected with GSCs. H&E staining of whole-brain (c) and images of IHC using antibodies indicated in (d). e Measurement of invasion distance of xenograft tumor. Representative images of the mouse brain (left) and quantification of invasion distance (right). f IHC of CD31 in xenograft tumor. Representative images of the IHC (left) and quantification of the vessel (right). *0.01 < p < 0.05; **0.005 < p < 0.01; ***p < 0.005.
Discussion
ANO1, a calcium-activated chloride channel, is known to play a critical role in fluid secretion, motility, muscle contraction, and nociception in a variety of normal cells [19,20,21]. Overexpression of ANO1 is frequently found in various cancers and is associated with stimulation of tumor cell proliferation, increase in tumorigenic potential, and promotion of tumor growth and metastasis [22,23,24,25]. The expression of ANO1 was also found in GBM. ANO1 has been reported to enhance tumorigenesis through NF-κB signaling in glioma cells. In addition, our group studied the cell surface localization of ANO1 upon binding to 14-3-3γ modulated the progression of glioma cells [29]. However, the effect of ANO1 expression on the biological properties of GSCs has not yet been explored. In this study, we found that ANO1 significantly affects the self-renewal activity, soft-agar colony formation, and invasion of GSCs in vitro, and is involved in tumor growth and infiltration in vivo. The role of ANO1 appears to be the stabilization of EGFRvIII through direct association with it. Therefore, ANO1 may be an effective therapeutic target for the treatment of GBM.
Many reports have shown that ANO1 is overexpressed in various cancers [22, 25,26,27]. Frequently, amplification of chromosome 11q13, where ANO1 is located, was found in many malignant cancers, including HNSCC, esophageal squamous cell cancer, and breast cancer, and is closely correlated with poor prognosis of these cancers [24, 34,35,36,37]. In addition to the amplification of chromosome 11q13, ANO1 expression can be regulated by transcriptional control via IL4 and IL13-mediated JAK/STAT6 signaling by epigenetic regulation, and by miRNAs such as miR-132 and miR-381 [38, 39]. In cell line experiments, overexpression of ANO1 promotes the proliferation and migration in many cancer cells, which are suppressed by pharmacological inhibition or knockdown of ANO1 [23, 25, 37, 40]. On the other hand, ANO1 inhibition did not affect proliferation in some cancer cells [41, 42] and even suppressed migration in HNSCC cells, indicating that the effect of ANO1 on cancer cells can be cell type-dependent [43]. However, the effect of ANO1 on stem cell-like cancer cells has not been studied yet. In this study, we examined the role of ANO1 in the biology of GSCs. Expression of ANO1 was downregulated by serum differentiation of GSCs. Magnetic sorting experiments revealed that ANO1 expression was higher in CD133+ cells compared to that in CD133– GSCs. In addition, pharmacological inhibition or shRNA-mediated knockdown of ANO1 suppressed the self-renewal and invasion activities of GSCs in vitro, as evidenced by limited dilution, sphere formation, and soft-agar colony formation assays. Furthermore, knockdown of ANO1 enhanced survival of mice, which were intracranially implanted with GSCs, suppressed the infiltration of GSCs into the normal brain in vivo. To our knowledge, this is the first report that has examined the effect of ANO1 expression on the stemness activity of GSCs.
EGFR amplification and the presence of its deletion mutant EGFRvIII have commonly observed features in malignant GBM [7]. EGFRvIII is known to be involved in therapeutic resistance, invasiveness, and tumor initiation and progression in GBM [7, 8, 11]. EGFRvIII also promotes tumor angiogenesis by enhancing Src-mediated VEGF secretion [44]. In addition, the expression of EGFRvIII confers stem cell characteristics on GBM cells by stimulating neural stem cell markers, including CD133, SOX2, and nestin, and enhancing self-renewal activity in these cells [10, 13]. Indeed, GSCs used in this study expressed high levels of EGFRvIII with a marginal expression of EGFR. Some reports have shown that ANO1 affects the activation of EGFR signaling. Bill et al. found that ANO1 interacts with EGFR and stimulates the phosphorylation of EGFR via posttranslational modifications without the modulation of its expression in HNSCC cells [28]. Britschgi et al. reported that knockdown of ANO1 suppressed EGFR signaling by reducing EGFR phosphorylation, which was caused by downregulation of the expression of EGFR ligands, EGF, and TGF-α in breast cancer cells [24]. In addition, there has been a report of a cross-activation loop in which overexpression of ANO1 activates EGFR/STAT3 signaling, and again, EGF-induced activation of EGFR/STAT3 increases ANO1 expression in breast cancer [45]. Recently, it has been shown that ANO1 expression is upregulated in pancreatic cancer by ligand-dependent EGFR signaling, is essential for EGF-induced calcium entry and migration, and is crucial for EGF-stimulated phosphoproteome remodeling in pancreatic cancer cells [46]. However, our data showed a different mechanism of ANO1 in EGFR signaling in GSCs. Our study demonstrated that ANO1 stabilizes EGFRvIII and EGFR through physical interaction. Pharmacological inhibition or knockdown of ANO1 stimulates polyubiquitination-mediated proteasomal degradation of EGFRvIII, which is blocked by pretreatment with the proteasome inhibitor, MG132. ANO1 also interacts with EGFR and is involved in the stability of EGFR in the same manner as EGFRvIII in EGFR-expressing GSCs and glioma cell lines. In addition, transfection of siRNA against EGFRvIII and EGFR or treatment with gefitinib, a specific inhibitor of both proteins, suppressed the expression of stemness factors without affecting the expression of ANO1 and inhibited self-renewal activities in GSCs. Taken together, our data strongly suggest that stabilization of EGFRvIII and EGFR by ANO1 promotes the maintenance of stemness in vitro and tumorigenesis in vivo. As far as we know, this is the first report on the regulation of stemness by ANO1 in cancer stem cells.
As overexpression of ANO1 promotes proliferation, migration, and stimulation of crucial signaling molecules in various cancer cells, the discovery of an effective ANO1 inhibitor would be a good therapeutic option for the treatment of malignant cancer. Indeed, several studies have reported the discovery of ANO1 inhibitors for putative therapeutic agents [47]. Our data indicate that the ANO1 inhibitor CaCC-A01 effectively suppresses EGFRvIII signaling in GSCs by degrading ANO1 protein, as reported previously [33]. Since it was reported that EGFRvIII-harboring GBM cells potently promote tumor growth by secreting IL6 and/or LIF to stimulate EGFR-overexpressing GBM cells [48], suppression of EGFRvIII/EGFR signaling through ANO1 targeting can be a promising therapeutic option. Therefore, the development of blood-brain barrier penetrable ANO1-degrading inhibitors would provide a good therapeutic opportunity for the treatment of malignant GBM in the future.
One of the hallmarks of GBM is its high invasiveness in adjacent normal tissue, which leads to a high incidence of recurrence even after drastic surgical resection [49]. GSCs have been recognized as the major causative cells of the invasiveness of GBM and are important factors that contribute to the poor prognosis of patients [49, 50]. Indeed, the GSCs used in this study showed highly invasive characteristics in vitro and in vivo. However, the invasiveness of GSCs transfected with shANO1 was found to be significantly suppressed in invasion assays performed in vitro, and these GSCs formed demarcated tumors with clear margins in vivo. These results suggest that ANO1 may play an important role in regulating the invasiveness of GSCs. In the near future, we plan to study the molecular mechanisms of ANO1 underlying the invasiveness of GSCs.
Materials and methods
Materials and methods used are available in Supplementary.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl J Med. 2005;352:987–96.
Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27.
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710.
Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015;22:e273–e281.
Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, et al. Stem cell-related “Self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–24.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N. Engl J Med. 2006;355:1253–61.
Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–84.
Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–9.
de la Iglesia N, Puram SV, Bonni A. STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med. 2009;9:580–90.
Yin J, Park G, Kim TH, Hong JH, Kim YJ, Jin X, et al. Pigment epithelium-derived factor (PEDF) expression induced by EGFRvIII promotes self-renewal and tumor progression of glioma stem cells. PLoS Biol. 2015;13:e1002152.
Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci USA. 2007;104:12867–72.
Del Vecchio CA, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A, et al. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene. 2013;32:2670–81.
Emlet DR, Gupta P, Holgado-Madruga M, Del Vecchio CA, Mitra SS, Han SY, et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014;74:1238–49.
Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–29.
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–4.
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5.
Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA. 2012;109:16354–9.
Ma MM, Gao M, Guo KM, Wang M, Li XY, Zeng XL, et al. TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension. 2017;69:892–901.
Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15:1015–21.
Oh U, Jung J. Cellular functions of TMEM16/anoctamin. Pflug Arch. 2016;468:443–53.
Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev. 2014;94:419–59.
Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012;72:3270–81.
Liu F, Cao QH, Lu DJ, Luo B, Lu XF, Luo RC, et al. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-beta signaling. Oncotarget. 2015;6:11585–99.
Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026–E1034.
Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, et al. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 2014;9:e115443.
Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca2+-activated Cl– channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012;326:41–51.
Liu J, Liu Y, Ren Y, Kang L, Zhang L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-kappaB signaling pathway. Mol Med Rep. 2014;9:1068–74.
Bill A, Gutierrez A, Kulkarni S, Kemp C, Bonenfant D, Voshol H, et al. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget. 2015;6:9173–88.
Lee YS, Lee JK, Bae Y, Lee BS, Kim E, Cho CH, et al. Suppression of 14-3-3gamma-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci Rep. 2016;6:26413.
Lee YS, Bae Y, Park N, Yoo JC, Cho CH, Ryoo K, et al. Surface expression of the anoctamin-1 (ANO1) channel is suppressed by protein-protein interactions with β-COP. Biochem Biophys Res Commun. 2016;475:216–22.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Bill A, Hall ML, Borawski J, Hodgson C, Jenkins J, Piechon P, et al. Small molecule-facilitated degradation of ANO1 protein: a new targeting approach for anticancer therapeutics. J Biol Chem. 2014;289:11029–41.
Huang X, Gollin SM, Raja S, Godfrey TE. High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci USA. 2002;99:11369–74.
Carles A, Millon R, Cromer A, Ganguli G, Lemaire F, Young J, et al. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene. 2006;25:1821–31.
Katoh M, Katoh M. FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 2003;22:1375–81.
Carneiro A, Isinger A, Karlsson A, Johansson J, Jonsson G, Bendahl PO, et al. Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer. 2008;8:98.
Wang H, Zou L, Ma K, Yu J, Wu H, Wei M, et al. Cell-specific mechanisms of TMEM16A Ca2+-activated chloride channel in cancer. Mol Cancer. 2017;16:1–17.
Crottes D, Jan LY. The multifaceted role of TMEM16A in cancer. Cell Calcium. 2019;82:102050.
De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharm. 2008;73:758–68.
Sauter DR, Novak I, Pedersen SF, Larsen EH, Hoffmann EK. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflug Arch. 2015;467:1495–508.
Simon S, Grabellus F, Ferrera L, Galietta L, Schwindenhammer B, Muhlenberg T, et al. DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors. Cancer Res. 2013;73:3661–70.
Shiwarski DJ, Shao C, Bill A, Kim J, Xiao D, Bertrand CA, et al. To “grow” or “go”: TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin Cancer Res. 2014;20:4673–88.
Eskilsson E, Rosland GV, Talasila KM, Knappskog S, Keunen O, Sottoriva A, et al. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro Oncol. 2016;18:1644–55.
Wang H, Yao F, Luo S, Ma K, Liu M, Bai L, et al. A mutual activation loop between the Ca2+-activated chloride channel TMEM16A and EGFR/STAT3 signaling promotes breast cancer tumorigenesis. Cancer Lett. 2019;455:48–59.
Crottès D, Lin YH, Peters CJ, Gilchrist JM, Wiita AP, Jan YN, et al. TMEM16A controls EGF-induced calcium signaling implicated in pancreatic cancer prognosis. Proc Natl Acad Sci USA. 2019;116:13026–35.
Ji Q, Guo S, Wang X, Pang C, Zhan Y, Chen Y, et al. Recent advances in TMEM16A: structure, function, and disease. J Cell Physiol. 2019;234:7856–73.
Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–45.
Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–36.
Cheng L, Wu Q, Guryanova OA, Huang Z, Huang Q, Rich JN, et al. Elevated invasive potential of glioblastoma stem cells. Biochem Biophys Res Commun. 2011;406:643–8.
Acknowledgements
This work was supported by the Bio-Synergy Research Project (NRF-2017M3A9C4092979 to JYP) of the National Research Foundation of Korea, and by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science and ICT (MSIT), the Republic of Korea (No. 50531-2019 to MJP).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, HJ., Kim, JY., Jung, CW. et al. ANO1 regulates the maintenance of stemness in glioblastoma stem cells by stabilizing EGFRvIII. Oncogene 40, 1490–1502 (2021). https://doi.org/10.1038/s41388-020-01612-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01612-5
- Springer Nature Limited