Abstract
Lung cancer is one of the major causes of cancer death and clarification of its molecular pathology is highly prioritized. The physiological importance of mRNA degradation through the CCR4-NOT deadenylase has recently been highlighted. For example, mutation in CNOT3, a gene coding for CNOT3 subunit of the CCR4-NOT complex, is found to be associated with T-cell acute lymphoblastic leukemia, T-ALL, though its contribution to other cancers has not been reported. Here, we provide evidence suggesting that CNOT3 is required for the growth of non-small cell lung cancer. Depletion of CNOT3 suppresses proliferation of A549 human non-small cell lung cancer cells with enhanced mRNA stability and subsequent elevated expression of p21. In addition, we identified the mRNA for Krüppel-like factor 2 transcription factor, an inducer of p21, as a novel mRNA degradation target of CNOT3 in non-small cell lung cancer cells. Aberrant up-regulation of Krüppel-like factor 2 by CNOT3 depletion leads to impairment in the proliferation of A549 cells. Consistent with these findings, elevated mRNA expression of CNOT3 in non-small cell lung cancer in comparison with the paired normal lung epithelium was confirmed through scrutinization of the RNA-sequencing datasets from The Cancer Genome Atlas. Moreover, we found an inverse correlation between CNOT3 and CDKN1A (encoding p21) mRNA expression using the combined datasets of normal lung epithelium and non-small cell lung cancer. Thus, we propose that the up-regulation of CNOT3 facilitates the development of non-small cell lung cancer through down-regulation of Krüppel-like factor 2 and p21, contrary to tumor suppressive functions of CNOT3 in T-ALL.
Similar content being viewed by others
Introduction
Lung cancer is the top cause of cancer deaths for both men and women in the US [1]. Hence, clarification of molecular pathology of lung cancer is highly prioritized. Lung cancer is classified into two major pathological types: small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [2]. NSCLC accounts for 85% of all lung cancer cases and is considered as a typical type of lung cancer. Further, NSCLC is comprised of three main subtypes: lung adenocarcinoma (LADC), lung squamous cell carcinoma (LSqCC), and large-cell lung carcinoma, and LADC and LSqCC account for most of NSCLC [2, 3].
Cancer can be developed through the mutation or loss of genes regulating mRNA homeostasis because its deviation leads to aberrant protein expression that could cause unregulated cell proliferation. MicroRNA is one of the most essential factors for determining the fate of its target mRNA expression through mRNA degradation. In fact, a variety of microRNAs were identified as oncogenic factors (oncomiRs) or tumor suppressors by intense studies [4]. In addition, mRNA decay after shortening poly(A) tail by deadenylases is also a key factor in determining mRNA fate [5]. The CCR4-NOT complex is a major deadenylase in mammals [6]. Naturally, it is assumed that the CCR4-NOT also exerts similar functions as microRNAs that are relevant to lung carcinogenesis [7].
Mammalian CCR4-NOT complex consists of eight subunits: CNOT1, CNOT2, CNOT3, CNOT6 or 6L, CNOT7 or 8, CNOT9, CNOT10, and CNOT11 [8]. Of these subunits, CNOT6/6L/7/8 possess deadenylase activities [6]. CNOT1 is considered as a scaffold protein for this complex [9] and CNOT3 is also reported to be necessary for the integrity of the complex and deadenylase activities [10, 11]. Recent studies reveal that the depletion of CNOT3 results in various abnormalities and diseases including impairment in embryonic development, leanness, and osteoporosis in mice [11,12,13]. Importantly, frameshift and missense mutations of the CNOT3 gene have been identified in T-cell acute lymphoblastic leukemia (T-ALL) patients by exosome-sequencing [14]. Moreover, knockdown of CNOT3 induces tumor development using sensitized drosophila eye cancer model [14]. These findings suggest that CNOT3 functions as a tumor suppressor in T-ALL development. However, the role of CNOT3 in NSCLC development remains unknown.
In this study, we investigated the functions and target mRNAs of CNOT3 using human LADC and LSqCC cell lines. We have addressed the status of CNOT3 expression through scrutinization of RNA-sequencing (RNA-seq) datasets of LADC and LSqCC patients from The Cancer Genome Atlas (TCGA). Importantly, we identified the mRNA for KLF2 transcription factor as a novel target of CNOT3 in lungs. Our data suggest the possibility that the up-regulation of CNOT3 is required for NSCLC development by controlling the stability of CDKN1A and KLF2 mRNAs, contrary to the supposed roles in T-ALL. Our findings would give novel insights into a relationship between cancer development and deadenylase-initiated mRNA decay.
Results
CNOT3 mRNA expression is up-regulated in NSCLC
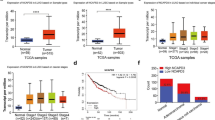
We first analyzed the RNA-seq datasets of TCGA database to examine if the expression of CNOT3 is altered in human NSCLC compared to normal lung epithelium. Since frequent frameshift and missense mutations of CNOT3 were reported in T-ALL, and since knockdown of CNOT3 with enhanced Notch signaling resulted in the development of eye cancer in Drosophila [14], CNOT3 was thought to be anti-oncogenic. However, the RNA-seq analysis revealed that mRNA expression of CNOT3 is up-regulated in NSCLC, both LADC and LSqCC (Fig. 1a, b). Because this up-regulation was less obvious in LADC than LSqCC, we also examined mRNA expression change of CNOT3 in LADC using the microarray datasets downloaded from ONCOMINE database [15]. The up-regulation of CNOT3 mRNA in LADC was commonly observed in all the datasets [16,17,18,19,20], with statistical significance except for one dataset using the minimum sample size in total [20]. (Supplementary Figure S1). Moreover, we examined CNOT3 promoter activities in primary normal lung epithelial cells and NSCLC cell lines using the cap analysis of gene expression (CAGE)-seq [21] from the Functional ANnoTation Of the Mammalian genome 5 (FANTOM5) database [22]. Higher number of CAGE tag counts were detected at p1 promoter of CNOT3 in NSCLC cell lines compared to human primary normal lung epithelium, indicating that NSCLC cells have higher CNOT3 promoter activity (Fig. 1c). These findings indicate that CNOT3 expression is elevated in NSCLC compared to normal lung epithelium.
CNOT3 mRNA expression is up-regulated in NSCLC. a, b Expression of CNOT3 using the RNA-seq data from TCGA for LADC (a, N = 57) and LSqCC (b, N = 51). The red bar indicates NSCLC sample and blue bar indicates normal epithelium sample in the same patient (paired normal). RPKM reads per kilobase of exon model per million mapped reads. c Normalized CAGE tag counts for CNOT3 p1 promoter from FANTOM5 database. Primary normal lung epithelium (N = 16) and NSCLC cell lines (N = 16) (Left). The averages of two groups were compared (Right). **p < 0.01 by two-sided Student’s t-test. Data are presented as mean ± SD
Proliferation of human NSCLC cells is attenuated by CNOT3 depletion
According to the CAGE-seq data shown in Fig. 1c, CNOT3 promotor activity in A549 LADC cell line was about 2-fold higher than its average in normal lung epithelium (average: 35.4 and A549 cells: 68.5). Hence, in order to investigate the role of CNOT3 in NSCLC cells, we established A549 cells stably expressing tetracycline-inducible shRNA against CNOT3 targeting two different sequences within exons of CNOT3 using lentivirus (A549-T-shCNOT3-1 and -2 cells). About 70–80% knockdown of CNOT3 mRNA expression was achieved by their induction (Fig. 2a). We found that CNOT3 depletion reduces the protein expression of some other CCR4-NOT subunits (CNOTs) without decreasing mRNA expression (Fig. 2b and Supplementary Figure S2) as previously reported in murine embryonic fibroblasts (MEFs) [10]. CNOT3 overexpression restored this reduction in the expression of CNOTs (Fig. 2c), indicating that CNOT3 is essential for the integrity of the CCR4-NOT complex in human NSCLC cells as well as MEFs. We also examined the subcellular localization of CNOT3 in A549 cells since CNOT3 was also reported to be involved in transcriptional activities [23] or chromatin modification [24]. In contrast with the finding that CNOT3 is expressed in the nucleus to the same extent with cytoplasm in colorectal cancer cells [25], CNOT3 expression in the cytoplasm was much higher than nucleus in A549 cells (Fig. 2d), suggesting that CNOT3 is mainly involved in the regulation of mRNA degradation. Next, we checked the effects of CNOT3 knockdown on cell viability or proliferation because necroptosis or mitotic arrest was induced in CNOT3-depleted MEFs [10] or HeLa cells [26], respectively. As shown in Fig. 2e, increase in dead cells or mitotic cells was hardly observed in CNOT3-depleted A549 cells. However, cell proliferation rate was reduced by about 50% by the induction of two different CNOT3 shRNAs (Fig. 2f). Although exogenous CNOT3 overexpression did not accelerate the proliferation of A549 cells (Fig. 2g), this growth inhibition of A549-T-shCNOT3-1 cells was rescued by CNOT3 overexpression (Fig. 2h). These findings indicate CNOT3 is necessary for the proper growth of A549 NSCLC cells.
Depletion of CNOT3 attenuates the proliferation of human NSCLC cells. a qRT-PCR for CNOT3 in A549 cells expressing tetracycline-inducible shRNA against non-target control (A549-T-shNTC), A549-T-shCNOT3-1, and -2 cells with or without DOX. Cells were treated with DOX for 3 days. b Cell lysate of each A549 stable with or without 3 days of DOX treatment was subjected to immunoblotting with antibodies, as indicated under Materials and methods. c A549-T-shCNOT3-1 cells were infected with lentivirus expressing GFP or CNOT3. Immunoblotting was performed as described in (b). d Subcellular fractionation of each A549 stable with or without 3 days of DOX treatment was performed as described under Materials and methods. Soluble nuclear extracts and cytoplasmic extracts were subjected to immunoblotting. e Representative pictures of A549-T-ShCNOT3-1 cells with or without 4 days of DOX treatment. f–h Cell proliferation assay for each A549 stable. For (f) and (h), each A549 stable was treated with or without 4 days of DOX treatment. NS not significant, ***p < 0.005 compared to each (−) or GFP sample by two-sided Student’s t-test or Welch’s t-test. Data are presented as mean ± SD from three technical replicates, or two or three biological replicates
CNOT3 depletion induces p21 expression and inhibits the cell cycle progression
To know the mechanism by which CNOT3 depletion attenuates the proliferation of A549 cells, we examined the status of retinoblastoma (RB) protein, a master regulator of cell cycle progression, when CNOT3 was knocked down. We found that the ratio of unphosphorylated form of RB (pRB) to hyperphosphorylated form (ppRB) was increased by CNOT3 depletion, while cleavage of poly(ADP-ribose) polymerase (PARP) which is essential for caspase-dependent apoptosis was not enhanced (Fig. 3a). We further found the increase in the cell population in G0/G1 phase and no alteration in the subG1 phase of A549-T-shCNOT3-1 cells by CNOT3 knockdown (Fig. 3b), suggesting that CNOT3 depletion does not induce apoptosis but induces cell cycle arrest of A549 cells.
Depletion of CNOT3 induces the expression of p21 in human NSCLC cells. a, d Cell lysate of each A549 stable with or without 2 or 3 days of DOX treatment was subjected to immunoblotting with antibodies, as indicated under Materials and methods. ppRB, pRB, and full-length form of PARP are shown with arrows. Quantification of pRB/ppRB, p21 or p27/α-tubulin or β-actin was performed using Image J. The values were normalized to that of T-shCNOT3-1 without DOX. Average of the values obtained from four (p21 and p27) or three (pRB) independent sets of samples are shown. b Cell cycle analysis for A549-T-shCNOT3-1 cells with or without 3 days of DOX treatment. c qRT-PCR for CDKN1A, CDKN1B, CDKN1C, CDKN2C, and CDKN2D using the cDNA from the same samples with Fig. 2a. e A549-T-shCNOT3-1 cells with or without 3 days of DOX treatment were treated with DMSO or ActD for 3 or 6 h. qRT-PCR for CDKN1A, CDKN1B, and CDKN2D using the cDNA from the cells with indicated time of treatment. NS not significant, *p < 0.05, **p < 0.01, ***p < 0.005 compared to each (−) sample by two-sided Student’s t-test, paired t-test, or Welch’s t-test. Data are presented as mean ± SD from three technical replicates or individual samples
During G1-to-S transition of cell cycle, cyclin-dependent kinase (CDK) inhibitors, CIP/KIP proteins (p21, p27, and p57), function as a brake for cell cycle progression through inactivation of Cyclin D-CDK4, Cyclin D-CDK6, and Cyclin E-CDK2 complex [27]. We hypothesized that CNOT3 depletion aberrantly up-regulates the expression of CIP/KIP proteins via impairment of mRNA decay. Among the CIP/KIP proteins, the expression of p21 was clearly elevated by CNOT3 depletion at both mRNA and protein level (Fig. 3c, d). Less up-regulation of p27 compared to p21 at mRNA and protein level, especially in A549-T-shCNOT3-1 cells, was observed, and the up-regulation of CDKN1C (coding p57) was not observed (Fig. 3c, d). Further, to rule out a possible involvement of the other CDK inhibitors, the INK4 family, we also checked expression change of CDKN2C (coding p18) and CDKN2D (coding p19) by CNOT3 depletion and their up-regulation was not observed in common with induction of shCNOT3-1 and -2 (Fig. 3c). CDKN2A (coding p16) and CDKN2B (coding p15) alleles are deficient in A549 cells [28, 29].
We next evaluated mRNA stability of CDKN1A (coding p21), CDKN1B (coding p27), and CDKN2D using Actinomycin D (ActD; Wako, Osaka, Japan), a transcription inhibitor. Quantitative real-time RT-PCR (qRT-PCR) revealed that mRNAs of CDKN1A and CDKN1B, but not CDKN2D, were stabilized by CNOT3 depletion in A549 cells (Fig. 3e). These results suggest that CNOT3 depletion directly up-regulates the expression of CDKN1A and CDKN1B through their enhanced mRNA stabilization.
Identification of KLF2 transcription factor as a target of CNOT3
Because the expression of p21 was highly up-regulated, we assumed that the up-regulation of p21 was most likely to be responsible for the induction of cell cycle arrest by CNOT3 depletion. However, the stabilization of CDKN1B mRNA was more obvious than CDKN1A mRNA, contrary to the results of expression change (Fig. 3c, d). Then, we hypothesized that CNOT3 also suppresses the expression of p21 through Factor X which induces p21 expression. In other words, we assumed that p21 expression is regulated by direct and indirect ways, the latter being via Factor X (Fig. 4a).
Identification of KLF2 transcription factor as a target of CNOT3. a Schematic model of regulation of p21 by CNOT3. We hypothesized that p21 expression is suppressed by a Factor X which is a target of CNOT3, in addition with direct mRNA decay by CNOT3. We narrowed down the candidate X to up-regulated 110 probes with microarray based on the criteria as follows: (1) More than 2-fold increase by both shCNOT3-1 and -2 induction. (2) Less than 1.5-fold increase by shNTC induction (to exclude non-specific increase). (3) More than 1.5-fold higher expression both in A549-T-shCNOT3-1 and -2 induced cells than A549-T-shNTC cells without DOX (to confirm the higher expression against A549-T-shNTC stable). We identified KLF2 as a Factor X. b qRT-PCR for KLF2 using the cDNA form the same samples with Fig. 1a. c qRT-PCR for KLF2 using the cDNA form A549-T-shCNOT3-1 cells expressing GFP or CNOT3 with or without 3 days of DOX treatment. d A549-T-shCNOT3-1 cells with or without 3 days of DOX treatment were treated with DMSO or ActD for 1 or 2 h. qRT-PCR for KLF2 and KLF6 using the cDNA from the cells with indicated time of treatment is shown. qRT-PCR for HPRT1 using the cDNA from the same samples with Fig. 2c is also shown. e Poly(A) tail assay for HPRT1 and KLF2 using the RNA from A549-T-shCNOT3-1 cells with or without 3 days of DOX treatment. Synthesized cDNA by reverse transcription was subjected to electrophoresis. NS not significant, ***p < 0.005 compared to each (−) or indicated sample by two-sided Student’s t-test or Welch’s t-test. Data are presented as mean ± SD from three technical replicates
To identify Factor X, we performed microarray analysis and highlighted on up-regulated genes commonly observed in two types of shCNOT3-induced A549 cells. We narrowed down the candidate gene to 110 up-regulated probes based on our criteria and we found some transcription-related genes (Fig. 4a and Supplementary Table S1, green color; transcription-related genes). Transcription factors directly regulate the expressions of target genes and are potent candidates. We found the only one transcription factor, Krüppel-like factor 2 (KLF2) among the 110 probes, which is also called as lung Krüppel-like factor (LKLF) [30] since its expression is abundant in lungs and necessary for lung development [31]. KLF2 is known to induce p21 expression in Jurkat T-ALL cells via direct promoter regulation [30]. In addition, KLF2 was also reported to induce less preferentially p27 expression than p21 expression in mouse pre-B cells [32]. Thus, we hypothesized that the up-regulation of KLF2 is responsible, at least partly, for p21 induction by CNOT3 depletion.
We confirmed the up-regulation of KLF2 by CNOT3 depletion by qRT-PCR and this up-regulation was suppressed by CNOT3 overexpression (Fig. 4b, c). We also found that KLF2 mRNA was drastically stabilized by CNOT3 depletion while mRNA of KLF6, another inducer of p21 and p27 in the same KLF family [33], was not up-regulated and was much less stabilized than KLF2 (Supplementary Figure S3 and Fig. 4d). Furthermore, the poly(A) tail length of KLF2 mRNA was elongated in CNOT3-knocked down A549 cells while that of very stable HPRT1 mRNA was not changed (Fig. 4d, e). These findings indicate that KLF2 transcription factor is a bona fide direct target of CNOT3.
KLF2 is at least in part responsible for the growth inhibition by CNOT3 depletion
To confirm the involvement of KLF2 in the growth inhibition by CNOT3 depletion, we established A549-T-shCNOT3-1 cells stably expressing shRNA against KLF2 using lentivirus (Fig. 5a). The mRNA expression level of CDKN1A under CNOT3 knockdown was lowered by knockdown of KLF2, in accord with the previous findings that KLF2 transcriptionally induces CDKN1A expression [34] (Fig. 5b). In addition, cell proliferation assay revealed that the growth inhibition by CNOT3 depletion was significantly attenuated by knockdown of KLF2 (Fig. 5c). These findings indicate that the up-regulation of KLF2 mediates the induction of p21 and growth inhibition by CNOT3 depletion, at least partly.
The growth inhibition by CNOT3 depletion is mediated by the up-regulation of KLF2. a Schematic model of establishment of A549 cells with stable double knockdown of both CNOT3 and KLF2 to examine the involvement of KLF2 in the growth inhibition by CNOT3 depletion. b qRT-PCR for KLF2 and CDKN1A using the cDNA form A549-T-shCNOT3-1 cells expressing shNTC or shKLF2 with or without 3 days of DOX treatment. c Cell proliferation assay for each A549 cells with or without 5 days of DOX treatment. The cell number of each A549 stable without DOX was standardized to 100%. *p < 0.05, **p < 0.01, or ***p < 0.005 by two-sided Student’s t-test or Welch’s t-test. Data are presented as mean ± SD from three technical or biological replicates
p21 and KLF2 are common targets of CNOT3 in human NSCLC
To determine whether the regulation of KLF2 expression by CNOT3 is commonly observed in NSCLC cells or only in A549 cells which harbor KRAS mutation [35], we examined the expression of KLF2 using siRNA against CNOT3 (siCNOT3) in other human LADC and LSqCC cell lines with different mutation or amplification profiles of KRAS, TTF1, and EGFR; NCI-H441: KRAS mutation and TTF1 amplification [35], NCI-H520: wild-type, and NCI-H1975: EGFR mutation [36]. In all the NSCLC cell lines we examined, the up-regulation of KLF2 mRNA was commonly observed by two different siCNOT3 in good agreement with knockdown efficiency of CNOT3 (Fig. 6a and Supplementary Figure 4), suggesting that KLF2 is a common target of CNOT3 in NSCLC, and that this regulation of KLF2 expression by CNOT3 is not limited to the NSCLC with some major specific mutations. Importantly, impairment of the proliferation by CNOT3 depletion was also observed in all these cell lines (Supplementary Figure S5).
KLF2 and p21 are common targets of CNOT3 in human NSCLC. a qRT-PCR for CNOT3 in NCI-H441, NCI-H520, or NCI-H1975 cells with siNTC, siCNOT3#1, or siCNOT3#2 transfection. **p < 0.01, ***p < 0.005 versus siNTC by Tukey–Kramer post-hoc test. Data are presented as mean ± SD from three technical replicates. b, c Scatter plot of CNOT3 and CDKN1A (Left), CNOT3 and KLF2 (Middle), or KLF2 and CDKN1A (Right) for LADC (b) or LSqCC (c) using RNA-seq datasets from TCGA database. These datasets include the data used in Fig. 1. b Normal lung epithelium (Red, N = 58) and LADC (Blue, N = 518). c Normal lung epithelium (Red, N = 51) and LSqCC (Blue, N = 502). The r values indicate Pearson’s correlation. The p values were calculated based on Student’s t-distribution. RPKM reads per kilobase of exon model per million mapped reads
Furthermore, we examined the correlation of CNOT3 and p21 or KLF2 using the TCGA datasets for both LADC and LSqCC in order to confirm if the expressions of these genes are regulated by CNOT3 in clinical samples in vivo. Since the expressions of p21 and KLF2 are supposed to be altered during carcinogenesis based on their tumor suppressive functions, we used combined datasets of NSCLC and paired normal lung epithelium to get a more clear correlation, although the paired normal corresponded to just some of the cancer samples. In accord with the fact that KLF2 is a direct inducer of p21, we confirmed a positive correlation between KLF2 and p21 using these combined datasets (Fig. 6b, c). Importantly, we found a negative correlation of CNOT3 and p21, with the comparable extent of Pearson’s correlation value with that of KLF2 and p21 (Fig. 6b, c), suggesting that CNOT3 negatively regulates the expression of p21 in human lung epithelium. Furthermore, a negative correlation between CNOT3 and KLF2 was found in the LSqCC datasets (Fig. 6c). These findings indicate that KLF2 and p21 are common targets of CNOT3 in NSCLC.
Discussion
The mammalian CCR4-NOT complex is a multi-functional protein assembly that regulates transcription, translational inhibition, and mRNA degradation [37]. Increasing reports have demonstrated that individual subunit possesses a unique role in cancer development and progression. For instance, CNOT2 was reported to inhibit metastasis of mouse breast cancer cells, while CNOT7 promotes it [38, 39]. In the study using MCF7 breast cancer cell lines, cell cycle arrest was induced by knockdown of CNOT1, CNOT3 [40], CNOT7, or CNOT8 [41], while impaired cell survival was observed by the knockdown of CNOT6 or CNOT6L in addition with cell cycle arrest [40]. Hence, the role of the CCR4-NOT in cancer needs to be clarified by focusing on each subunit, not a whole complex, to avoid complexity. Importantly, frequent frameshift and missense mutations of CNOT3 were reported in T-ALL recently and CNOT3 was found to be a tumor suppressor using sensitized drosophila eye cancer model [14]. These findings propelled us to examine the function of the CCR4-NOT, by focusing on CNOT3, in another major cancer, lung cancer.
CNOT3 is known to be essential for the modulation of transcription activities [37]. Importantly, CNOT3 hetero-deficient mice developed impaired heart function through dysregulation of chromatin modification [24]. However, we found that CNOT3 is predominantly located in the cytoplasm in A549 cells, not the nucleus (Fig. 2d), different from colorectal cancer cells [25]. Thus, in the present study, we focused on mRNA degradation mediated by CNOT3 which is supposed to take place in the cytoplasm, and found stabilization of CDKN1A and CDKN1B mRNA by CNOT3 depletion (Fig. 3e).
We also identified KLF2 as a novel common target of CNOT3 in NSCLC (Figs. 4a and 6a). KLF2 is reported to induce p21 expression in NSCLC cells including A549 cells and its expression is associated with lung cancer progression or prognosis [34, 42]. It should be noted that KLF2 is included among the up-regulated genes by combined knockdown of CNOT7 and CNOT8 in MCF7 cells [41]. These indicate that the CCR4-NOT also participates in the regulation of tumor suppressor KLF2 as well as microRNAs [43] and long non-coding RNAs [44]. It is also worth mentioning that some important mRNAs such as KLF2 are universally regulated by the CCR4-NOT complex in the various organs.
Tumor suppressor, p53 is another major regulator of p21 and its mRNA was reported to be directly regulated by CNOT3 in developing mouse B lymphocytes [45]. The up-regulation of TP53 mRNA (encoding p53) by CNOT3 knockdown was not commonly observed in A549 cells (Supplementary Figure S6a). In addition, TP53 mRNA was strikingly stable in lung cancer cells and was not stabilized by CNOT3 depletion (Supplementary Figure S6b), contrary to developing mouse B lymphosites [45]. These suggest that some target mRNAs of the CNOT3 are fairly cell- or tissue-type dependent, contrary to the finding regarding KLF2. However, we found that greater increase of p53 at protein level (average: 2.20 fold) than mRNA level (1.32 fold) by CNOT3 depletion in A549-T-shCNOT3-1 cells (Supplementary Figures S6c and d), suggesting the possibility that CNOT3 is also involved in the translation inhibition of p53. This increase in p53 protein might partly account for the growth inhibition by CNOT3, of which knockdown of KLF2 showed only modest restoration.
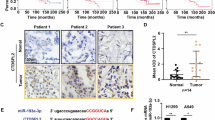
A recent report showed that nuclear CNOT3 is important for colorectal cancer progression and highly linked to the prognosis [25]. We examined the prognosis of NSCLC patients by Kaplan–Meier plots with classifications based on the CNOT3 expression (top half versus bottom half, top third versus bottom third, and top quartile versus bottom quartile) using the Oncolnc (www.oncolnc.org) [46]. Unfortunately, however, we were not able to find a significant difference in survival between the two groups (data not shown). This finding suggests that CNOT3 is most likely to be involved in the carcinogenesis step of NSCLC, rather than progression, through down-regulation of tumor suppressors.
We found the up-regulation of CNOT3 in NSCLC (Fig. 1 and Supplementary Figures S1), however, how CNOT3 expression is regulated in the body has been scarcely investigated. We examined if CNOT3 gene is frequently amplified in NSCLC by analyzing the dataset from Campbell et al. [47]. Gene alteration frequency of CNOT3 (0.96%, amplification) is much less than that of MYC (8.74%, amplification) or CDKN2A (21.07%, deletion) which is one of the most frequently altered genes in terms of copy number in this dataset [47] (Supplementary Figure S7), suggesting that gene amplification is not the cause of the up-regulation of CNOT3 in NSCLC. Therefore, it is speculated that CNOT3 expression level is elevated through alteration of other genes or cancer microenvironment. Elucidation of the mechanism of CNOT3 up-regulation during lung carcinogenesis awaits further studies.
We also found the expression change of the other subunits commonly observed in the LADC and LSqCC: up-regulation of CNOT1, CNOT2, CNOT7, CNOT9, and CNOT11 and down-regulation of CNOT6L (Supplementary Figures S8 and 9). We further found that the depletion of CNOT2 or both CNOT7 and CNOT8 using siRNA inhibits the cell proliferation of A549 cells, however, depletion of CNOT1 did not affect it unexpectedly (Supplementary Figures S10 and 11). Further studies are necessary for the elucidation of how CNOT1 and CNOT3 regulate the functions of the CCR4-NOT complex or other interacting molecules in a specific way and cause this discrepancy in the future.
In conclusion, we demonstrated that CNOT3 depletion attenuates the growth of NSCLC through direct and indirect regulation of p21 via mRNA degradation (Fig. 7). The findings in this study suggest that CNOT3 facilitates the development of NSCLC through modulation of mRNA decay machinery.
Schematic representation of the role of CNOT3 in NSCLC cells. CNOT3, one key subunit of the CCR4-NOT complex, is highly expressed in NSCLC cells and is required for the proper expression of some other subunits including CNOT1, CNOT2, CNOT7, and CNOT9. CNOT3 regulates the expression of p21 through mRNA degradation. CNOT3 also specifically degrades the mRNA of KLF2, which regulates the expression of p21 through transcription. When CNOT3 is depleted in NSCLC cells, the expression levels of KLF2 and p21 are elevated, resulting in the impairment of cell proliferation
Materials and methods
TCGA and CBio cancer genomics portal data
Level 3 RNA-seq data containing gene expression and clinical information for LADC and LSqCC were downloaded from TCGA data portal (https://tcga-data.nci.nih.gov/docs/publications/tcga/) by November 2015. The graphs for the mutation and copy number analysis of NSCLC were generated by the CBio Cancer Genomics Portal (http://www.cbioportal.org) [48, 49] using the dataset from Campbell et al. [47] which consists of the highest number of NSCLC patients (TCGA data together with the data from Imielinski et al. [50]: 660 LADC and 484 LSqCC) available on this website as of October 2016.
ONCOMINE data
Microarray datasets for LADC (condition; sample number ≥20) were downloaded from ONCOMINE database (www.oncomine.org) [15] by December 2014. There are three probes for CNOT3 mRNA expression (203239_s_at, 211141_s_at, and 229143_at) for Affymetrix (CA, USA) microarrays. 203239_s_at and 211141_s_at probes were used in common and the former one was used as a representative probe because of higher sensitivity. A probe for CNOT3 mRNA for Illumina (CA, USA) microarray was ILMN_2207393.
CAGE tag count analysis
CAGE data with raw read counts were obtained from FANTOM5 database and analyzed as previously reported [51, 52]. The data used in this study is composed of 16 primary normal lung epithelial cell samples (tracheal (TEC1–3), bronchial (BEC1–7), small airway (SAEC1–3), and alveolar (AEC1–3) epithelial cells) and 16 NSCLC cell lines (LADC (A549, PC-14, NCI-H441, NCI-H358, SW1573, and NCI-H650), LSqCC (EBC-1, LC-1F, REPF-LC-AI, and KNS-62), lung large cell carcinoma (IA-LM, NCI-H460, LU65, and Lu99B), and unclassified NSCLC (ChaGo-K-1 and NCI-H1385)). The CAGE tag counts between samples were normalized using edgeR package as described previously [53, 54].
Cell culture and reagents
A549 cells were obtained from RIKEN Cell Bank. NCI-H441, NCI-H520, and NCI-H1975 cells were purchased from ATCC. Cells were cultured in DMEM medium (Thermo Fisher Scientific, MA, USA) for A549 cell line or RPMI-1640 medium (Thermo Fisher Scientific) for NCI-H441, NCI-H520, and NCI-H1975 cell lines. Both of the media contained 10% fetal bovine serum (FBS), penicillin (50 U/mL), and streptomycin (50 U/mL). All the cells were grown up in a 5% CO2 atmosphere at 37 °C. Doxycycline (DOX; TaKaRa, Shiga, Japan) and ActD were used at a concentration of 1 or 3 µg/mL and 5 µg/mL, respectively.
Cell proliferation assay
A549-T-shNTC, CNOT3-1, and CNOT3-2 cells, or GFP or CNOT3-overexpressing cells (1–3 × 104 cells) were seeded in triplicate in 12-well plates (defined as Day 0) and the cells were treated with DOX on the next day when necessary. Regarding the growth assay using siRNA (5–25 × 104 cells), cells were seeded in duplicate or triplicate in 6-well or 12-well plates. The number of the cells were counted with TC20™ Automated Cell Counter (BIO-RAD, CA, USA) or hemocytometer (some results using triplicates were obtained from duplicated wells because of technical error). Experiments were performed at least twice under similar conditions and representative results are shown in figures.
Cell cycle analysis
A549-T-shCNOT3-1 cells were seeded in 10 cm dishes and the cells were treated with DOX on the next day. The cells were collected 3 days after DOX treatment, washed with PBS, and fixed in an ice-cold 70% EtOH and stored at −30 °C until use. Cells were washed in 1× PBS twice, dissociated in staining buffer (PBS, 0.1% Triton X-100, 2% FBS) and labeled with 7-aminoactinomycin D (7-AAD; BD Biosciences, NJ, USA). FACS ARIA III (BD Biosciences) was used for the acquisition of at least 30.000 events. Data was analyzed with FlowJo 10.3 software (Tree Star, OR, USA) using Dean–Jett–Fox algorithm. SubG1 fraction was calculated as 100 − (G0/G1 + S + G2/M). Experiments were performed twice under similar conditions and representative results (36 × 104 cells seeded) are shown in figures.
Lentivirus production
We used a lentiviral vector system to establish A549 cells stably expressing coding protein, shRNA or tetracycline-inducible shRNA, without sorting after infection. Entry vectors for shRNA and tetracycline-inducible shRNA (pENTR4-H1 and pENTR4-H1tetOx1), destination vectors (CSII-EF-RfA, CS-RfA-EG, and CS-RfA-ETV), GFP-expressing lentiviral vector (CS-CDF-EG-PRE), packaging vector (pCAV-HIVgp), and VSV-G and Rev-expressing vector (pCMV-VSV-G-RSV-Rev) were provided by Dr. Hiroyuki Miyoshi (RIKEN, Tsukuba, Japan). Lentivirus was produced basically according to the protocol made by Dr. Miyoshi (http://cfm.brc.riken.jp/lentiviral-vectors/protocols/).
RNA analysis
Total RNAs were extracted using RNeasy Mini Kit or RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). First-strand cDNA synthesis was performed as described previously [55]. qRT-PCR was performed using FastStart Universal SYBR Green Master (ROX; Roche, Basel, Switzerland) or TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus; TaKaRa) and the ABI PRISM 7900HT Sequence Detection System (Thermo Fisher Scientific). All samples were run in triplicate and the value for GAPDH mRNA was used for normalization. This GAPDH value was regarded as a fixed value without errors when calculating the standard deviation of each objective mRNA expression. Regarding the measurement of remaining RNA, the control expression was also regarded as a fixed value for statistics. Comparison of poly(A) tail length was performed as previously described [10]. Primers used in this study are listed in Supplementary Table S2. Experiments were performed at least twice under similar conditions and representative results are shown in figures.
Microarray analysis
Microarray analysis was performed using total RNAs extracted as described above and Gene Chip Human Genome U133 Plus 2.0 Array (Affymetrix) as described previously [10]. We used GeneSpring 12.6 (Agilent Technologies) to analyze the data using the MAS5 algorithm. We excluded the probes which showed Absent expression in all the six samples from the analysis. The complete dataset has been submitted to the NCBI Gene Expression Omnibus [56] and can be accessible through GEO Series accession number GSE114694.
RNA interference and oligonucleotides
A549, NCI-H441, NCI-H520, and NCI-H1975 cells were transfected with siRNA for 3 days using RNAiMAX (Thermo Fisher Scientific) according to the manufacturer’s protocol. Targeting sequences of siRNA for NTC, CNOT3#1, CNOT3#2 [26], CNOT1 (the same with CNOT1#1 in Ito et al. [9]), CNOT2, and CNOT7 [9] were previously described and the sequence for CNOT8 is as follows: 5′-GACCCUUCUCGAGGACAUUUG-3′. shRNA constructs were designed as previously reported [57] and targeting sequences are as follows: 5′-GCGCGCTTTGTAGGATTCG-3′ (NTC), 5′-GGACCAGTTTGAGAGTGAAGT-3′ (CNOT3-1), 5′-GCCACATGGAGGATGAGATCT-3′ (CNOT3-2), 5′- ACCACGATCCTCCTTGACGAG-3′ (KLF2).
Immunoblotting
Cells were lysed with TNE lysis buffer containing 0.1% SDS, 50 mM Tris-HCl, 120 mM NaCl, 5 mM EDTA, 1% NP-40, and protease inhibitor (Nakalai Tesque, Kyoto, Japan or Wako). Western blotting was basically performed as previously reported [58]. TBS buffer containing 5% skim milk or BSA fraction V and 0.1% Tween-20 was used for blocking. For some blots, Can Get Signal® Immunoreaction Enhancer Solution (TOYOBO, Osaka, Japan) was used for enhancement of detection. We used an antibody against CNOT3 which is commercially available (H00004849-M01, Abnova, Taipei, Taiwan). Antibodies against CNOT1, CNOT2, CNOT6L, CNOT7 were described previously [10]. Rabbit CNOT9 antibody was obtained as described previously [59]. We purchased RB (554136) antibody from BD Biosciences and PARP antibody (#9542) from Cell Signaling Technology, MA, USA. Goat Lamin B antibody (sc-6217), mouse p53 (sc-126), and β-actin (sc-69879) antibodies were from Santa Cruz, TX, USA. p21 antibodies were from BD Biosciences (556430) and Santa Cruz (sc-6246), and p27 antibodies were from Santa Cruz (sc-528) and Abcam, Cambridge, England (ab32034). α-tubulin (T9026) was purchased from Sigma-Aldrich, MO, USA. Quantification of the bands was performed using ImageJ in a vertical way. When it was impossible to measure in a vertical way because of the continuous band, it was performed in a horizontal way.
Subcellular fractionation
Fractionation of cytoplasmic and soluble nuclear proteins of GFP or CNOT3-overexpressing A549-T-shCNOT3-1 cells with or without DOX treatment was performed using Subcellular Protein Fractionation Kit for Cultured Cells (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Statistical analysis
Two-tailed Student’s t-tests, paired t-tests, or Welch’s t-tests were used for the comparison of two samples. Tukey–Kramer post-hoc tests were performed for the comparison of the multiple samples in Fig. 6a and Supplementary Figures S4 and 10b using the R program (https://www.r-project.org/). p Values in Fig. 6d, e were calculated using the Pearson’s correlation value based on Student’s t-distribution. p Values in Supplementary Figure S1 was derived from ONCOMINE [15]. Results were considered to be statistically significant at p < 0.05.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–44.
Petersen I, Warth A. Lung cancer: developments, concepts, and specific aspects of the new WHO classification. J Cancer Res Clin Oncol. 2016;142:895–904.
Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–33.
Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol. 2008;9:337–44.
Bartlam M, Yamamoto T. The structural basis for deadenylation by the CCR4-NOT complex. Protein Cell. 2010;1:443–52.
Castro D, Moreira M, Gouveia AM, Pozza DH, De Mello RA. MicroRNAs in lung cancer. Oncotarget. 2017;8:81679–85.
Lau NC, Kolkman A, van Schaik FM, Mulder KW, Pijnappel WW, Heck AJ, et al. Human Ccr4-Not complexes contain variable deadenylase subunits. Biochem J. 2009;422:443–53.
Ito K, Takahashi A, Morita M, Suzuki T, Yamamoto T. The role of the CNOT1 subunit of the CCR4-NOT complex in mRNA deadenylation and cell viability. Protein Cell. 2011;2:755–63.
Suzuki T, Kikuguchi C, Sharma S, Sasaki T, Tokumasu M, Adachi S, et al. CNOT3 suppression promotes necroptosis by stabilizing mRNAs for cell death-inducing proteins. Sci Rep. 2015;5:14779.
Morita M, Oike Y, Nagashima T, Kadomatsu T, Tabata M, Suzuki T, et al. Obesity resistance and increased hepatic expression of catabolism-related mRNAs in Cnot3+/− mice. EMBO J. 2011;30:4678–91.
Zheng X, Yang P, Lackford B, Bennett BD, Wang L, Li H, et al. CNOT3-dependent mRNA deadenylation safeguards the pluripotent state. Stem Cell Reports. 2016;7:897–910.
Watanabe C, Morita M, Hayata T, Nakamoto T, Kikuguchi C, Li X, et al. Stability of mRNA influences osteoporotic bone mass via CNOT3. Proc Natl Acad Sci USA. 2014;111:2692–7.
De Keersmaecker K, Atak ZK, Li N, Vicente C, Patchett S, Girardi T, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet. 2013;45:186–90.
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6.
Selamat SA, Chung BS, Girard L, Zhang W, Zhang Y, Campan M, et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res. 2012;22:1197–211.
Hou J, Aerts J, den Hamer B, van Ijcken W, den Bakker M, Riegman P, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 2010;5:e10312.
Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72:100–11.
Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, Dasgupta A, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS One. 2008;3:e1651.
Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ, Liang SC, et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genomics. 2007;8:140.
Kanamori-Katayama M, Itoh M, Kawaji H, Lassmann T, Katayama S, Kojima M, et al. Unamplified cap analysis of gene expression on a single-molecule sequencer. Genome Res. 2011;21:1150–9.
Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon MJ, Haberle V, et al. A promoter-level mammalian expression atlas. Nature. 2014;507:462–70.
Zheng X, Dumitru R, Lackford BL, Freudenberg JM, Singh AP, Archer TK, et al. Cnot1, Cnot2, and Cnot3 maintain mouse and human ESC identity and inhibit extraembryonic differentiation. Stem Cells. 2012;30:910–22.
Neely GG, Kuba K, Cammarato A, Isobe K, Amann S, Zhang L, et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–53.
Cejas P, Cavazza A, Yandava CN, Moreno V, Horst D, Moreno-Rubio J, et al. Transcriptional regulator CNOT3 defines an aggressive colorectal cancer subtype. Cancer Res. 2017;77:766–79.
Takahashi A, Kikuguchi C, Morita M, Shimodaira T, Tokai-Nishizumi N, Yokoyama K, et al. Involvement of CNOT3 in mitotic progression through inhibition of MAD1 expression. Biochem Biophys Res Commun. 2012;419:268–73.
Nabel EG. CDKs and CKIs: molecular targets for tissue remodelling. Nat Rev Drug Discov. 2002;1:587–98.
Kawabe S, Roth JA, Wilson DR, Meyn RE. Adenovirus-mediated p16INK4a gene expression radiosensitizes non-small cell lung cancer cells in a p53-dependent manner. Oncogene. 2000;19:5359–66.
Schuster K, Venkateswaran N, Rabellino A, Girard L, Pena-Llopis S, Scaglioni PP. Nullifying the CDKN2AB locus promotes mutant K-ras lung tumorigenesis. Mol Cancer Res. 2014;12:912–23.
Wu J, Lingrel JB. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene. 2004;23:8088–96.
Wani MA, Wert SE, Lingrel JB. Lung Kruppel-like factor, a zinc finger transcription factor, is essential for normal lung development. J Biol Chem. 1999;274:21180–5.
Winkelmann R, Sandrock L, Kirberg J, Jack HM, Schuh W. KLF2—a negative regulator of pre-B cell clonal expansion and B cell activation. PLoS One. 2014;9:e97953.
Tetreault MP, Yang Y, Katz JP. Kruppel-like factors in cancer. Nat Rev Cancer. 2013;13:701–13.
Yin L, Wang JP, Xu TP, Chen WM, Huang MD, Xia R, et al. Downregulation of Kruppel-like factor 2 is associated with poor prognosis for nonsmall-cell lung cancer. Tumour Biol. 2015;36:3075–84.
Harris T, Pan Q, Sironi J, Lutz D, Tian J, Sapkar J, et al. Both gene amplification and allelic loss occur at 14q13.3 in lung cancer. Clin Cancer Res. 2011;17:690–9.
Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7.
Shirai YT, Suzuki T, Morita M, Takahashi A, Yamamoto T. Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front Genet. 2014;5:286.
Faraji F, Hu Y, Wu G, Goldberger NE, Walker RC, Zhang J, et al. An integrated systems genetics screen reveals the transcriptional structure of inherited predisposition to metastatic disease. Genome Res. 2014;24:227–40.
Faraji F, Hu Y, Yang HH, Lee MP, Winkler GS, Hafner M, et al. Post-transcriptional control of tumor cell autonomous metastatic potential by CCR4-NOT deadenylase CNOT7. PLoS Genet. 2016;12:e1005820.
Mittal S, Aslam A, Doidge R, Medica R, Winkler GS. The Ccr4a (CNOT6) and Ccr4b (CNOT6L) deadenylase subunits of the human Ccr4-Not complex contribute to the prevention of cell death and senescence. Mol Biol Cell. 2011;22:748–58.
Aslam A, Mittal S, Koch F, Andrau JC, Winkler GS. The Ccr4-NOT deadenylase subunits CNOT7 and CNOT8 have overlapping roles and modulate cell proliferation. Mol Biol Cell. 2009;20:3840–50.
Jiang W, Xu X, Deng S, Luo J, Xu H, Wang C, et al. Methylation of Kruppel-like factor 2 (KLF2) associates with its expression and non-small cell lung cancer progression. Am J Transl Res. 2017;9:2024–37.
Wehrkamp CJ, Natarajan SK, Mohr AM, Phillippi MA, Mott JL. miR-106b-responsive gene landscape identifies regulation of Kruppel-like factor family. RNA Biol. 2017;15:391–403.
Li W, Sun M, Zang C, Ma P, He J, Zhang M, et al. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225.
Inoue T, Morita M, Hijikata A, Fukuda-Yuzawa Y, Adachi S, Isono K, et al. CNOT3 contributes to early B cell development by controlling Igh rearrangement and p53 mRNA stability. J Exp Med. 2015;212:1465–79.
Anaya J. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci. 2016;2:e67.
Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–16.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–20.
Horie M, Yamaguchi Y, Saito A, Nagase T, Lizio M, Itoh M, et al. Transcriptome analysis of periodontitis-associated fibroblasts by CAGE sequencing identified DLX5 and RUNX2 long variant as novel regulators involved in periodontitis. Sci Rep. 2016;6:33666.
Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–61.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Horie M, Kaczkowski B, Ohshima M, Matsuzaki H, Noguchi S, Mikami Y, et al. Integrative CAGE and DNA methylation profiling identify epigenetically regulated genes in NSCLC. Mol Cancer Res. 2017;15:1354–65.
Hoshino Y, Katsuno Y, Ehata S, Miyazono K. Autocrine TGF-beta protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem. 2011;149:55–65.
Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10.
Shirai YT, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Bone morphogenetic protein-2 and -4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol. 2011;179:2920–30.
Hoshina N, Tanimura A, Yamasaki M, Inoue T, Fukabori R, Kuroda T, et al. Protocadherin 17 regulates presynaptic assembly in topographic corticobasal Ganglia circuits. Neuron. 2013;78:839–54.
Morita M, Suzuki T, Nakamura T, Yokoyama K, Miyasaka T, Yamamoto T. Depletion of mammalian CCR4b deadenylase triggers elevation of the p27Kip1 mRNA level and impairs cell growth. Mol Cell Biol. 2007;27:4980–90.
Acknowledgements
The authors would like to thank Dr. Hiroyuki Miyoshi (RIKEN) for lentivirus vector system. The authors also thank all the members of Cell Signal Unit at the Okinawa Institute of Science and Technology Graduate University (OIST) for discussion and advice. This research was supported by subsidiary of OIST and KAKENHI Grants-in-Aid for scientific research (S) (21229006 to TY), for Scientific Research on Innovative Areas (25121734 to TY), for Young Scientists (B) (26830066 to AM and 16K18437 to MH), and for Research Activity Start-up (24890289 to Y-TS and 26893050 to MH) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Shirai, YT., Mizutani, A., Nishijima, S. et al. CNOT3 targets negative cell cycle regulators in non-small cell lung cancer development. Oncogene 38, 2580–2594 (2019). https://doi.org/10.1038/s41388-018-0603-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0603-7
- Springer Nature Limited
This article is cited by
-
Gene regulatory networks reveal sex difference in lung adenocarcinoma
Biology of Sex Differences (2024)
-
Targeting the up-regulated CNOT3 reverses therapeutic resistance and metastatic progression of EGFR-mutant non-small cell lung cancer
Cell Death Discovery (2023)
-
Identification of a miRNA multi-targeting therapeutic strategy in glioblastoma
Cell Death & Disease (2023)
-
Translation factor eIF5a is essential for IFNγ production and cell cycle regulation in primary CD8+ T lymphocytes
Nature Communications (2022)
-
RHBDD1 silencing inhibited cell growth and invasion of non-small cell lung cancer by mediating ZEB1/PI3K/AKT signaling pathway
Journal of Molecular Histology (2021)