Abstract
The adenomatous polyposis coli (APC) protein has a tumor-suppressor function by acting as a negative regulator of the Wnt signaling pathway. While its role as a tumor suppressor is well-defined, the post-translational modifications that regulate APC stability are not fully understood. Here we showed that MKRN1, an E3 ligase, could directly interact with and ubiquitylate APC, promoting its proteasomal degradation. In contrast, an E3 ligase-defective MKRN1 mutant was no longer capable of regulating APC, indicating that its E3 ligase activity is required for APC regulation by MKRN1. Strengthening these results, MKRN1 ablation resulted in reduced β-catenin activity and decreased expression of Wnt target genes. The ability of the Wnt-dependent pathway to induce cancer cell proliferation, migration, and invasion was impaired by MKRN1 depletion, but restored by simultaneous APC knockdown. Taken together, these results demonstrate that MKRN1 functions as a novel E3 ligase of APC that positively regulates Wnt/β-catenin-mediated biological processes.
Similar content being viewed by others
Introduction
Wnt proteins, secretory proteins that are highly conserved across many species, were first identified for their oncogenic function and later shown to be involved in embryonic development by regulating body patterning, cellular proliferation, and migration [1,2,3,4,5,6]. The Wnt signaling pathway is mediated via ligation of the frizzled (Fz) receptor, a G-protein coupled receptor, and its co-receptors such as lipoprotein receptor-related protein (LRP)-5/6 [7]. Activation of Wnt receptors is next recognized by Disheveled (Dsh), which branches off the canonical and noncanonical Wnt pathways [8].
Under normal conditions, β-catenin is tightly maintained at a low level by the destruction complex consisting of Axin1, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α) in the cytosol [8,9,10]. Activation of the canonical pathways leads to the accumulation of β-catenin in the cytoplasm and the nucleus by abrogation of the destruction complex [11]. Accumulated β-catenin coactivates transcription factors in the TCF/LEF family and controls diverse biological processes, including development, differentiation, and proliferation, in various tissues, adult stem cells, and progenitor cells [6, 12,13,14,15,16]. In contrast, hyperactivation of Wnt pathways is associated with many human diseases, including major sporadic and hereditary colorectal cancers [10, 17,18,19,20,21].
The APC gene encodes one of the main components of the destruction complex, which places a major inhibitory role in Wnt signaling by instigating the ubiquitin-mediated degradation of β-catenin. APC interacts with numerous components of the destruction complex, including AXIN1, GSK3β, CK1, PP2A, and β-catenin, and these interactions are critical for the phosphorylation and degradation of β-catenin [7, 22,23,24].
APC is currently recognized as one of the most frequently mutated genes in human cancers [25]. Most of these mutations result from frameshifts that produce truncated proteins lacking the serine/alanine/methionine/proline repeats around codon 1580, or the 15 and 20 amino-acid repeats, both of which are critical for binding to Axin or regulating β-catenin stability, respectively [17]. Therefore, cancer cells expressing truncated APC have high levels of β-catenin in the cytoplasm and nucleus, which results in the deregulated expression of Wnt target genes. Approximately 15% of colorectal tumors with wild-type (WT) APC harbor mutations in the β-catenin phosphodegron, which result in β-catenin stabilization and, thus, constitutive activation of Wnt signaling [26]. Interestingly, mutations in APC and β-catenin are mutually exclusive, suggesting that these proteins play roles in the same axis of the Wnt functional pathway [26,27,28].
While truncating APC mutations are closely related to cancer development, recent findings suggest that many cancers harbor WT APC with abnormal activation of the Wnt pathway due to other causes [9]. These observations suggest the existence of a WT APC destabilization pathway for inducing tumorigenesis. Despite the important roles of APC in Wnt/β-catenin signaling, only a handful of mechanisms that regulate APC protein levels have been reported. For example, APC activity is known to correlate with its phosphorylation mediated by CK1 and GSK3β kinases [29,30,31]. APC phosphorylation by this method dramatically increases its affinity for β-catenin, leading to suppression of Wnt signaling [32, 33]. APC is itself the target of K63 ubiquitylation by the E3 ligase HECTD1 in the absence of Wnt signaling, and these modifications are also required for its interaction with other components of the destruction complex [33,34,35,36,37].
While ubiquitin-dependent APC regulation can alter Wnt signaling activity [34, 35, 37,38,39], the direct activity of an E3 ligase leading to proteasome-dependent APC proteolysis has not yet been described [40, 41]. Here we introduce an E3 ligase, MKRN1, that negatively regulates APC. MKRN1 has been previously shown to regulate various targets, such as hTERT, p53, FADD, p14ARF, PTEN, and PPARγ, among others [42,43,44,45,46,47,48]. MKRN1 interacts with the Arm repeat domain of APC to promote its ubiquitylation and proteasomal degradation. Overall, MKRN1 acts as a positive regulator of the Wnt/β-catenin signaling pathway by suppressing APC. Thus, Wnt/β-catenin-dependent biological processes, such as cellular proliferation, migration, and invasion events, were repressed upon MKRN1 ablation, which was reversed by the co-depletion of APC. These results implicate MKRN1 as a plausible target to suppress Wnt-related pathways.
Results
MKRN1 ablation downregulates the Wnt/β-catenin signaling pathway
Based on the ability of MKRN1 to inhibit various tumor suppressors, the possible oncogenic properties of MKRN1 in the Wnt/β-catenin signaling pathway were evaluated. Upon MKRN1 ablation, β-catenin transcriptional activity was impaired in the HEK293-TOP-FLASH stable cell line (STF293) treated with control medium (L929 cell-conditioned medium; L-CM), Wnt3a-conditioned medium (Wnt3a-CM), or recombinant Wnt3a (Fig. 1a; Supplementary Figure S1b). For these experiments, we employed MKRN1 siRNAs and antibodies, which had been previously validated (Supplementary Figure S1a) [42, 44]. Conversely, when MKRN1 was overexpressed, the stability of β-catenin was increased by increasing amount of MKRN1. Supporting these findings, TOP-FLASH activities also escalated under this condition, suggesting a positive role of MKRN1 in the Wnt pathway (Supplementary Figure S2). Furthermore, overexpression of siRNA-resistant MKRN1 (siR-MKRN1) into MKRN1-depleted cells reactivated Wnt signaling, suggesting that MKRN1 is indeed an activator of Wnt pathways (Supplementary Figures S2c and d). Consistent with these results, expression of Wnt/β-catenin target genes was suppressed in human embryonic kidney (HEK)-293T cells depleted of MKRN1 upon Wnt3a-CM treatment (Fig. 1b). Overexpression of siR-MKRN1 under this condition reactivated Wnt/β-catenin target genes, including Axin2 and Lef1, which were reduced in MKRN1-depleted cells, suggesting that MKRN1 siRNA could specifically target MKRN (Supplementary Figure S2e). Because β-catenin targets were suppressed upon MKRN1 depletion, the link between β-catenin protein levels and target gene expression was further investigated by transiently silencing MKRN1 in HEK293FT cells. Interestingly, the levels of both nuclear and cytosolic β-catenin were decreased in MKRN1-depleted cells (Fig. 1c). Furthermore, immunofluorescence analysis showed that β-catenin accumulation both in cytoplasmic and nuclear fractions during Wnt stimulation was suppressed by MKRN1 knockdown (Fig. 1d; Supplementary Figure S1f). Consistently, the intensity of β-catenin was restored to normal levels when MKRN1 was overexpressed in MKRN1-depleted H1299 cells (Supplementary Figure S2f). Overall, these results suggest that MKRN1 might function as a positive regulator of the Wnt/β-catenin signaling pathway, possibly by modulating β-catenin levels.
MKRN1 ablation suppresses Wnt/β-catenin signaling. a Effects of MKRN1 depletion on β-catenin transcriptional activity. STF293 were transfected with control siRNA (siCtrl) or siRNAs targeting MKRN1 (siMKRN1#5 and siMKRN1#7). TOP-FLASH activities were measured after treating the transfected cells with conditioned medium from L-CM or Wnt3a-CM (left graph), PBS, or 200 ng/mL of recombinant human Wnt3a ligand (rhWnt3a) (right graph) for 18 h. The data represent the average values from three independent experiments. The error bars represent the mean ± SD of triplicates. b Effects of MKRN1 ablation on β-catenin target gene expression. Quantitative real-time PCR (qRT-PCR) was performed to evaluate the expression of the indicated Wnt target genes after MKRN1 knockdown by siRNAs in HEK293T cells treated with L-CM or Wnt3a-CM. The quantities of the indicated mRNAs were normalized to GAPDH. The data represent the average values from three independent experiments. The error bars represent the mean ± SD of triplicates. c Effects of MKRN1 ablation on β-catenin levels. HEK293T cells were transfected with siCtrl or siMKRN1, followed by treatment with L-CM or Wnt3a-CM for 2 h. The nuclear and cytoplasmic fractions of these cells were analyzed by WB with antibodies against β-catenin, MKRN1, GAPDH, and PARP. PARP and GAPDH were used for nuclear and cytosolic markers, respectively. The MKRN1 bands are indicated by arrowheads. The asterisk indicates nonspecific bands above MKRN1. d β-catenin immunostaining upon MKRN1 knockdown. H1299 cells were transfected with siCtrl or siMKRN1, followed by L-CM or Wnt3a-CM treatment for 24 h. Endogenous β-catenin localization was detected using α-β-catenin mouse antibodies followed by secondary FITC mouse antibodies for immunofluorescence analysis. The nuclei were stained with DAPI. The cells were analyzed using fluorescence microscopy. All images were captured under the same exposure conditions. Representative images of β-catenin immunostaining are shown (scale bar, 10 μm). ns=non-significant, *P < 0.05, **P < 0.01, ***P < 0.001
Cells depleted of MKRN1 display suppressed Wnt responses
The Wnt/β-catenin signaling pathway controls various physiological processes, including cellular proliferation, migration, and invasion [49]. To elucidate the role of MKRN1 in the Wnt pathway, cell proliferation assays were conducted with HEK293FT cells stimulated with L-CM or Wnt3a-CM. Cells grown in Wnt3a-CM showed accelerated proliferation compared with cells grown in L-CM. In contrast, MKRN1 depletion resulted in severe proliferation defects even during Wnt3a treatment (Fig. 2a; Supplementary Figure S3a). Additionally, the long-term clonogenic ability of cells stimulated with Wnt3a-CM was also suppressed by MKRN1 depletion (Fig. 2b). Next, Wnt-dependent cell migration was tested using scratch wound-healing assays with or without MKRN1 depletion (Fig. 2c). Similar to the results of the proliferation assays, Wnt3a-CM enhanced wound healing in control cells but not in MKRN1-depleted cells. Because wound-healing analysis does not fully represent cellular migration, cells were allowed to migrate overnight in response to either L-CM or Wnt3a-CM using transwell migration assays. We observed that Wnt3a treatment stimulated cellular migration by approximately threefold over a 12-h period. In contrast, MKRN1 ablation attenuated Wnt3a-induced cellular migration (Fig. 2d). Similarly, while cell invasion ability was increased by Wnt3a-CM, cells depleted of MKRN1 failed to respond in this manner (Fig. 2e). We next tested whether the reduction in Wnt activity observed in MKRN1-depleted cells could be rescued by reintroducing siR-MKRN1. Upon expression of siR-MKRN1, cellular proliferation, scratch wound-healing ability, cell migration, or invasion abilities were restored to levels similar to those of the positive control (Supplementary Figure S4). In summary, MKRN1 increased Wnt activity, which was reduced in response to MKRN1 deficiency. Thus, MKRN1 depletion hinders cell responses toward Wnt3a-dependent cellular phenomena, implying that MKRN1 might act as a stimulatory protein for Wnt pathways.
MKRN1 ablation suppresses Wnt/β-catenin-mediated cell proliferation, migration, and invasion. a Effects of MKRN1 depletion on β-catenin-mediated proliferation. HEK293FT cells were transfected with siMKRN1 followed by incubation with L-CM or Wnt3a-CM. The cells were fixed in formaldehyde and stained with crystal violet solution. Representative images of triplicate experiments are shown. b Clonogenic assay upon MKRN1 knockdown. HEK293FT cells were transfected with the indicated siRNAs. After 24 h, cells were seeded in six-well dishes. Colonies were fixed and stained with crystal violet after 1 week. The experiments were repeated three times. c Scratch wound-healing assay during MKRN1 depletion. HEK293FT cell monolayers transfected with siCtrl or siMKRN1#7 were scratched and incubated in L-CM or Wnt3a-CM. Wound closure was measured 24, 48, and 72 h after the scratch. Dashed lines represent the initial scratch wounds. d, e Transwell migration or invasion assays under MKRN1 ablation. HEK293FT cells were transfected with the indicated siRNAs. Cells were then seeded in collagen (d)- or Matrigel (e)-coated transwell chambers and incubated with L-CM or Wnt3a-CM, followed by crystal violet staining. The migratory or invasive cells were imaged and quantified by measuring intensity using ImageJ software (right panel). Representative images are shown (left panel, magnification, ×40; scale bar, 250 μm). The data represent the average values from three independent experiments. The error bars represent the mean ± SD of triplicates. ***P < 0.001
APC is a target of MKRN1
To identify targets affected by MKRN1, the protein levels of factors associated with the Wnt pathway were examined upon MKRN1 depletion. Notably, elevated levels of APC protein, a component of the destruction complex, were observed in MKRN1-depleted cells, which might contribute to reduced levels of β-catenin (Fig. 3a). APC stabilization upon MKRN1 depletion was also observed in other cell lines, including the human lung cancer cell line NCI-H1299, the human colon cancer cell line HCT116, and the colon carcinoma cell line RKO (Supplementary Figure S5c). Quantitative reverse transcriptase PCR (qRT-PCR) result suggested that APC mRNA levels were not influenced by MKRN1 ablation, indicating that APC is regulated by MKRN1 via post-translational modification (Fig. 3b). Furthermore, stabilized APC protein induced by MKRN1 depletion was reduced by the reintroduction of siR-MKRN1 without affecting the APC mRNA levels in 293FT cells (Supplementary Figures S4b, S5a and b).
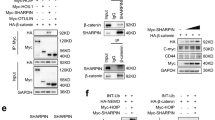
Ablation of MKRN1 induces APC stabilization. a WB analysis of Wnt-associated proteins upon MKRN1 knockdown. HEK293FT cells transfected with siMKRN1#5, #7, or siCtrl were treated with L-CM or Wnt3a-CM for 2 h. The indicated endogenous proteins were detected by WB analyses using specific antibodies. b qRT-PCR analyses for APC and MKRN1 mRNA. Total RNAs were purified from HEK293FT cells transfected with siMKRNs or siCtrl, and qRT-PCR was performed. GAPDH was used as an internal control. The data represent the average values from three independent experiments. The error bars represent the mean ± SD of triplicates. c–f Interaction between MKRN1 and APC. c, d HEK293FT cells were transfected with plasmids expressing HA-MKRN1 and MYC-APC or empty vector. Cell lysates were subjected to immunoprecipitation using α-MYC (c) or α-HA antibodies (d) followed by WB using antibodies against MYC and HA. e, f Endogenous interaction between MKRN1 and APC. HEK293FT cell lysates were subjected to immunoprecipitation using α-APC antibodies as in e or α-MKRN1 antibodies as in f, followed by WB using antibodies against APC and MKRN1. The asterisk (*) indicates IgG heavy chain. g–i Domain mapping of APC regions interacting with MKRN1. HEK293FT cells were transfected with plasmids expressing MYC-APC and its deletion mutants in the presence of FLAG-MKRN1. The cell lysates were immunoprecipitated using antibodies against MYC followed by WB analysis. The asterisk (*) indicates the antibody heavy chains (g, h). Schematics showing MYC-APC and its deletion mutants (i)
To further assess whether MKRN1 suppresses APC stabilization, we adopted CRISPR/Cas9 technology to knock out the MKRN1 gene in 293FT cells [50]. The targeted locus of MKRN1 is shown in Supplementary Figure S7a with the sequence of the guided RNAs. We established three MKRN1 KO cell lines named sg#1, sg#2, and sg#3, and one control cell line with empty vector (Supplementary Figure S7). Consistent with our MKRN1 knockdown experiments using siMKRN1s, sg#1, sg#2, and sg#3 exhibited stabilization of APC protein with a concomitant reduction of β-catenin (Supplementary Figure S7a and b). In addition, the cellular proliferation of MKRN1 KO cell lines was significantly stifled with or without Wnt treatment compared with the control, supporting the siMKRN1 data (Supplementary Figure S7c and d; Fig. 2). These data indicate that the specific ablation of MRKN could lead to the stabilization of APC, possibly resulting in the suppression of cell growth. These results led us to test for an interaction between MKRN1 and APC. Immunoprecipitation assays performed using overexpressed MKRN1 and APC confirmed that the two proteins can interact with each other (Fig. 3c, d). The same analyses under endogenous conditions further showed that Axin1, an essential member of the destruction complex, may be involved in the MKRN1 and APC interaction (Fig. 3e, f) [15, 51]. However, Axin1 depletion had no effect on the interaction between the two proteins or on APC levels during MKRN1 depletion, suggesting that Axin1 might not be an essential component for the association between MKRN1 and APC (Supplementary Figures S6c and d). Next, we investigated the binding regions responsible for the MKRN1 and APC interaction. The data suggested that MKRN1 could bind to the N-terminal domain of APC, amino acids 1–733, which contain the Armadillo (Arm) repeat domain (Fig. 3g). When further analyzed, MKRN1 was capable of binding the Arm domain alone, indicating that this region is likely responsible for the APC-MKRN1 interaction (Fig. 3h, i). Furthermore, the C terminus of MKRN1 was required for its interaction with APC (Supplementary Figures S6c and d). Taken together, the observation that MKRN1 binds to and destabilizes APC suggests that MKRN1 might function as an E3 ligase for APC.
MKRN1 induces APC degradation
We next investigated the mechanism by which MKRN1 depletion stabilizes APC protein. Treatment with MG132, a proteasome inhibitor, abrogated cycloheximide (CHX)-induced APC destabilization as previously reported (Supplementary Figure S8a) [52]. Knockdown of MKRN1 also markedly prolonged the half-life of APC, suggesting that MKRN1 might induce APC degradation (Supplementary Figure S8b). Furthermore, MG132 treatment had no additional effect on APC stabilization under MKRN1 depletion (Supplementary Figure S8c). These results suggest that APC stability is regulated in a proteasome-dependent manner, with MKRN1 being involved in this process. In agreement with these observations, endogenous and exogenous APC were dose-dependently destabilized by MKRN1 overexpression (Fig. 4a, b). Additionally, the half-life of APC under CHX treatment was shortened when ectopic MKRN1 was present (Fig. 4c). MG132 treatment inhibited MKRN1-mediated destabilization of APC, suggesting the involvement of the 26S proteasome-dependent proteolysis (Fig. 4d). Because MKRN1 is an E3 ligase, the necessity for MKRN1 enzymatic activity was further evaluated. MKRN1 H307E, an E3 ligase-defective mutant, was incapable of inducing APC degradation and reducing its half-life, but it could still interact with APC (Fig. 4e, f) [42]. In summary, MKRN1-mediated APC destabilization requires MKRN1 E3 ligase activity and is dependent on proteasomal degradation processes.
MKRN1 induces the proteasomal degradation of APC. a, b APC destabilization during MKRN1 overexpression. a HEK293FT cells were transfected with varying amounts of MKRN1-expressing vectors. The protein levels of endogenous APC and overexpressed MKRN1 were determined by WB using antibodies against APC and MKRN1. b HEK293FT cells were transfected with plasmids expressing MYC-APC and increasing amounts of MKRN1. Protein levels of APC and MKRN1 were determined by WB using antibodies against MYC, MKRN1, and GFP. pEGFP-C2 expressing enhanced green fluorescent protein (EGFP) was used as a transfection control. c HEK293FT cells were treated with 100 µg/mL cycloheximide (CHX) for the indicated times after transfection with plasmids expressing MYC-APC and HA-MKRN1 or HA only. Proteins were detected using antibodies against MYC, HA, and β-actin. Relative amounts of APC were calculated by normalization to β-actin levels (right panel). d Effect of MG132 on APC destabilization. HEK293FT cells transfected as described above were treated with MG132 for the indicated times. Proteins were detected as described above. e Effect of H307E on APC stabilization. e HEK293FT cells transfected with plasmids expressing FLAG-MKRN1 or FLAG-H307E with MYC-APC were lysed and subjected to WB using antibodies against MYC, FLAG, and GFP. f Interaction between APC and H307E. HEK293FT cells transfected with plasmids expressing HA-MKRN1 or HA-H307E with MYC-APC were lysed followed by immunoprecipitation with α-HA antibodies. Proteins were detected using antibodies against MYC and HA
MKRN1 is an E3 ubiquitin-ligase of APC
Because MKRN1 E3 ligase activity is required for promoting APC degradation, ubiquitylation analyses were performed under denaturing conditions. APC ubiquitylation was increased by MKRN1 and further enhanced during MG132 treatment (Fig. 5a). APC ubiquitylation was not increased when MKRN1 H307E was employed, indicating that MKRN1 E3 ligase activity is required for APC ubiquitylation (Fig. 5b). A similar result was obtained for MKRN1 WT or H307E when the ubiquitylation of APC was detected by immunoprecipitating for MYC-APC followed by western blot (WB) detection of HA-Ub under denaturing conditions (Fig. 5c). Supporting these results, purified recombinant MKRN1 was able to ubiquitylate APC in vitro, whereas H307E was not (Fig. 5d). Finally, MKRN1 depletion reduced the endogenous ubiquitylation of APC, suggesting that MKRN1 might continually destabilize APC under baseline conditions. Furthermore, reconstitution of siR-MKRN1 in MKRN1-depleted cells reversed these processes, indicating the specific role of MKRN1 in APC ubiquitylation (Fig. 5e). To identify the region of APC required for MKRN1-mediated degradation, we utilized three APC deletion mutants, hAPC 1–1337, hAPC 1–733, and hAPC 407–775 (Arm domain), which were able to bind MKRN1 (Figures S3g-i). All deletion mutants except hAPC 407–775 were degraded by MKRN1 (Supplementary Figure S9a). Because hAPC 1–733 was the minimal deletion mutant that could be degraded by MKRN1 in our assay, we further analyzed whether this mutant was degraded by MKRN1 in a fashion similar to the WT APC. Binding analyses employing purified recombinant proteins showed that MKRN1 was able to bind to hAPC 1–733 directly (Supplementary Figure S9b). While WT MKRN1 or H307E was able to bind hAPC 1–733, only WT MKRN1 could reduce the half-life of hAPC 1–733 and mediate its poly-ubiquitylation (Supplementary Figures S9c-g).
MKRN1 promotes the poly-ubiquitylation of APC. a The ubiquitylation of APC by MKRN1. HEK293FT cells were transfected with plasmids expressing MYC-APC, His-Ub, and HA-MKRN1 followed by MG132 treatment. Cell extracts prepared in 6 M guanidine-HCl were pulled down using Ni2+-NTA beads. The total extracts (input) and His-purified proteins were detected by WB analysis as indicated. b, c The effect of H307E on APC ubiquitylation. b Ubiquitylation assays using HEK293FT cells transfected with plasmids expressing MYC-APC, His-Ub, FLAG-MKRN1, and FLAG-H307E were performed as described above. c HEK293FT cells were transfected with plasmids expressing MYC-APC, HA-Ub, or FLAG-MKRN1 and FLAG-H307E followed by MG132 treatment. The cells were lysed in 1% SDS buffer, and the lysates were immunoprecipitated with α-MYC antibodies, followed by WB analysis. d The in vitro ubiquitylation of APC by MKRN1. An in vitro Ub assay was performed using purified WT APC from HEK293FT cells and GST-MKRN1 or GST-H307E from bacteria. APC ubiquitylation status was analyzed by WB as indicated. e Endogenous APC ubiquitylation upon MKRN1 ablation. HEK293FT cells transfected with siMKRN1#5 with or without plasmid expressing FLAG-siR-MKRN1 were lysed in 1% SDS buffer followed by immunoprecipitation with α-APC or IgG (negative control) antibodies. The ubiquitylation of endogenous APC was detected using an HRP-conjugated α-UB FK2 antibody
The implication of MKRN1 specifically targeting the N-terminal region of APC for its degradation led us to investigate the role of MKRN1 in the modulation of APC variants in actual cancer cells. It is well known that the N-terminal truncated variant of APC serves as a dominant-negative regulator of WT APC, leading to constant activation of β-catenin and thus initiating colorectal cancer cell (CRC) progression. Thus, we employed three CRC cell lines, including RKO with WT APC and DLD1, SW480, and SW620, which harbor N-terminal APC mutants as shown in Supplementary Figure 10a. Following MKRN1 depletion, all three cell lines displayed increased levels of WT or mutant APC as expected (Supplementary Figure 10b and c). Of note, RKO cell proliferation was significantly retarded under MKRN1 depletion, while there was no change in DLD1, SW480, and SW620 cells. Taken together, MKRN1 appeared to target amino acids 1–733 of APC for direct ubiquitylation and proteasomal degradation. Importantly, while MKRN1 depletion negatively affected the proliferation of cancer cell lines with WT APC, its ablation did not greatly affect the growth of CRC cell lines with mutant APC. These findings indicated that MKRN1 might have an oncogenic influence over certain type of cancer cells with WT APC regulating the β-catenin pathway.
MKRN1 ablation downregulates Wnt/β-catenin signaling, which is reversed by APC depletion
Because MKRN1 ablation suppressed Wnt/β-catenin signaling, we next questioned whether this process could be rescued by concurrent APC depletion. First, β-catenin destabilization during MKRN1 depletion was reversed when APC was depleted by siRNA (Fig. 6a). Confirming these results, the suppression of β-catenin transcriptional activity during MKRN1 depletion was recovered by the simultaneous removal of APC (Fig. 6b). Suppression of Wnt3a-CM-mediated cell proliferation and clonogenic expansion under MKRN1 depletion was also reversed by APC depletion (Fig. 6c, d). Similar effects between MKRN1 and APC were observed with scratch wound-healing, migration, and invasion analyses (Fig. 6e–g). These data imply that MKRN1 depletion leads to a repression of Wnt/β-catenin through APC stabilization.
APC depletion rescues Wnt/β-catenin activity during MKRN1 knockdown. a The effect of depleting APC and MKRN1 on β-catenin levels. HEK293 cells were transfected with the indicated combinations of siMKRN1#5 and siAPC. Protein levels were determined by WB using antibodies against APC, β-catenin, MKRN1, and β-actin. Relative β-catenin protein levels normalized to β-actin are shown. b The effect of APC and MKRN1 depletion on β-catenin transcriptional activity. HEK293-TOP-FLASH cells were transfected with the indicated combinations of siMKRN1#5 and siAPC. Experiments were performed as described in Fig. 1a. c–g The effect of depleting APC and MKRN1 on cellular proliferation and motility. HEK293FT cells were transfected with the indicated combination of siMKRN1s and siAPC followed by incubation with L-CM or Wnt3a-CM. The experiments were performed as described in Fig. 2a–e. The data represent the average values from three independent experiments. The error bars represent the mean ± SD of triplicates. Representative images are shown (scale bar, 250 μm). ns=non-significant, **P < 0.01, ***P < 0.001
Finally, to examine the physiological significance of MKRN1, MKRN1 expression was examined in silico. Among various cancers, MKRN1 mRNA levels were increased meaningfully in cervical cancer. These findings corroborate our previous data showing increased protein levels of MKRN1 in cervical cancers [45]. Based on these observations, the effects of MKRN1 on cervical cancer cell lines, including HeLa and ME-180, were investigated. Under MKRN1 ablation, APC levels were significantly increased with a concomitant decrease in β-catenin. In accordance with these findings, cellular proliferation was hindered, which was reversed by the simultaneous knockdown of APC (Supplementary Figure S11). These findings demonstrate that physiologically, MKRN1 might have oncogenic effects in cervical cancer by modulating the activities of β-catenin.
Discussion
Canonical Wnt/APC/β-catenin signaling plays an important role in regulating cellular and systemic homeostasis [19, 23, 51]. It is well known that dysregulation of this pathway causes tumors and developmental defects. For these reasons, investigating molecules that regulate Wnt/APC/β-catenin signaling has been a major interest in the biological science field [25, 49, 53, 54]. In particular, APC is a potent tumor suppressor that negatively regulates Wnt/β-catenin by destabilizing β-catenin via a destruction complex containing CK1, Axin, and GSK3β. The role of APC as a tumor suppressor is well established based on the observation that germline and somatic APC mutations result in neoplasia. For example, familial adenomatous polyposis is derived from an autosomal dominant inherited disease, which is triggerd by germline mutations in the APC gene. Loss of the WT APC allele via somatic mutations is also considered a major factor in most sporadic colorectal adenomas and carcinomas [55,56,57]. Epigenetically, methylation of the APC promoter silences APC expression, which activates Wnt signaling [58]. While the critical role of APC as a master tumor suppressor in the Wnt signaling pathway is well established, regulation of APC by post-translational modifications require further investigation. Specifically, while APC ubiquitylation and subsequent degradation is continually observed, the specific factors involved in this mechanism remain to be elucidated [21, 24].
In the present study, we identified MKRN1 as a new regulatory component of the Wnt signaling pathway. Our results show that MKRN1 plays a crucial role in regulating cellular proliferation, migration, and invasion in association with Wnt stimulation. MKRN1 depletion leads to a downregulation of Wnt-dependent biological processes because APC protein is polyubiquitylated by MKRN1 and subsequently degraded by the proteasome, leading to the stabilization and activation of β-catenin. Our observation that the MKRN1 H307E, the E3 ligase activity-defective mutant, was not able to induce APC ubiquitylation and degradation suggests that MKRN1 is the bona fide E3 ligase of APC.
APC consists of multifunctional domains, including an oligomerization domain and an Arm domain in the N terminus. Domain mapping studies indicated that MKRN1 directly interacts with amino acids 1–733 of APC, which includes the Arm domain. Furthermore, the Arm domain appears to be the minimal region required for the interaction with MKRN1 (Supplementary Figures S3a-c). The Arm domain is the most conserved region among vertebrate and invertebrate APC homologs, and it mediates the association with several binding partners, including WTX, Asef, Sam68, striatin, and IQGAP1 [59,60,61,62,63]. For example, WTX enhances APC/Axin-dependent β-catenin ubiquitylation and degradation through the interaction with the Arm domain of APC, thereby negatively regulating Wnt signal transduction [64]. Asef, a Rac-specific guanine nucleotide exchange factor, associates with the Arm domain of mutant APC and promotes cancer migration by regulating the actin cytoskeleton [65]. Thus, the interaction of APC with MKRN1 and its subsequent degradation would not exclude other factors from binding to the Arm domain, thereby altering cellular responses to a number of stimuli, including Wnt.
An interesting observation is that MKRN1 was able to induce the ubiquitylation and degradation of an APC deletion mutant containing only amino acids 1–733. The Arm repeat domain alone (amino acids 407–775) was not degraded by MKRN1, while the deletion mutant 1–733 was degraded, which could indicate that MKRN1 might be able to target the region between amino acids 1 and 406 for ubiquitylation while it binds to the Arm domain. Further analyses of the ubiquitylation sites are required. Notably, cancers such as colorectal cancer can occur in response to the expression of truncated N-terminal forms of APC. Because MKRN1 induced the degradation of both WT and truncated N-terminal forms of APC, it might display oncogenic and/or tumor-suppressive properties in tissue- and cancer-specific ways. Furthermore, in vivo studies are required to test this hypothesis. Using various Arm domain-binding proteins, as mentioned above, studies on possible exclusive or cooperative interactions of these molecules with MKRN1 toward the regulation of APC activity would elucidate additional Wnt signaling pathway complexity. Finally, the evaluation of MKRN1’s ability to regulate other proteins harboring the Arm domain would aid in discovering roles of MKRN1 in other cellular processes.
The high frequency at which the Wnt pathway is activated in diverse human carcinomas positions this signaling pathway as an essential target for therapies to decrease cancer morbidity and mortality. Pharmaceutically, several drugs targeting Wnt signaling components, including GSK, Axin, and receptors, have been developed as cancer therapeutic drugs, a few of which are being evaluated in clinical trials [24, 25, 54, 66]. Identifying a novel E3 ligase that destabilizes APC could also represent an important therapeutic target for repressing cancers that exhibit high β-catenin activity, even in the absence of mutations in APC or β-catenin.
Materials and Methods
Cell culture and transfection
The cell lines L929, RKO, HeLa, HEK293T, HEK293FT, and HEK293-TOP-FLASH [HEK293 cells with an integrated Super8XTopFlash reporter (STF293)] were cultured in Dulbecco’s modified Eagle’s medium (Corning Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum (Corning Cellgro) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37 °C. NCI-H1299 (human lung carcinoma) and ME-180 (human cervical cancer) cells were grown in RPMI 1640 with the same supplements listed above. NCI-H1299, HeLa, and HEK293T cells were purchased from the American Type Culture Collection (Manassas, VA, USA). We also purchased 293FT cells from Invitrogen. ME-180 cells (human cervical cancer cell lines) were obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). L929, L929-hWnt3a, and STF293 cells were kindly provided by Dr. Kang-Yell Choi (Yonsei University, Korea). Dr. Jong-In Yook (Yonsei University College of Dentistry) kindly offered a panel of colorectal cancer cell lines (RKO, DLD1, SW480, and SW620). All cell lines in this study were negative for mycoplasma when confirmed by an e-Myco™ plus Mycoplasma PCR Detection Kit (Intron), and cell lines were maintained with plasmocin (Invivogen). Wnt3a-CM was generated from L929-Wnt3a cells as previously described [67]. PEI (Sigma-Aldrich, St. Louis, MO, USA) was utilized to transfect HEK293FT cells with plasmids. Cells were transfected with siRNAs using Lipofectamine RNAiMax (Invitrogen) via the reverse-transfection method according to the manufacturer’s protocols.
Antibodies and chemicals
The following antibodies were used: APC (Santa Cruz, sc-7930 or Abcam, ab58, Cambridge, UK) for IP; APC (1:500, Abcam, ab58) for WB; MKRN1 (1:1000, Bethyl Laboratories, A300-990A); β-catenin (1:4000, Cell Signaling, C19220); Axin1 (1:1000, Cell Signaling, C76H11); MYC (1:1000, Santa Cruz, sc-40 or 1:1000, Roche, 9E10, 11667149001); HA (1:1000, Santa Cruz, sc-805 or Roche, 12013819001, Mannheim, Germany); FLAG (1:1000, Sigma-Aldrich, F3165 or F7425); horseradish peroxidase-conjugated-α-Ub FK2 (1:3000, Biomol-Enzo, PW0150, Farmingdale, NY, USA); GST (1:3000, Santa Cruz, sc-138); GFP (1:5000, Santa Cruz, sc-8334); β-actin (1:10 000, Sigma-Aldrich, A5316); normal mouse IgG (Santa Cruz, sc-2025); normal rabbit IgG (Santa Cruz, sc-2027); PARP (1:2000, Cell Signaling, 9532); GAPDH (1:5000, Santa Cruz, sc-25778); GSK3β (1:1000, Cell Signaling, 27C10, 9315); phospho-LRP6 (1:1000, Cell Signaling, Ser1490, #2568); Dvl3 (1:1000, Cell Signaling, 3218); LEF (1:1000, Cell Signaling, C12A5, Rabbit mAb, 2230); TCF1/TCF7 (1:1000, Cell Signaling, C63D9 Rabbit mAb, 2203); and non-p-β-catenin (1:1000, Cell Signaling, non-phospho (Active) β-catenin (Ser33/37/Thr41) (D13A1) Rabbit mAb #8814).
Recombinant human Wnt3a was acquired from R&D Systems (5036-WN-010/CF, Minneapolis, MN, USA). MG132 was purchased from A.G. Scientific (San Diego, CA, USA). CHX (C4859); N-ethylmaleimide (NEM, E3876), trypan blue (T8154), and dimethylsulfoxide (D8418) were purchased from Sigma-Aldrich.
Plasmids
The MKRN1 constructs (pcDNA3.1-MKRN1 WT, pcDNA3-HA-MKRN1 WT, and H307E, and deletion mutants pcDNA3-FLAG-MKRN1 WT and H307E, pcDNA-FLAG-siR-MKRN1, and pGEX-4T-1-GST-MKRN1 WT and H307E) [44,45,46], pcDNA3-His-Ub [44], and pCS2-MT-MYC-APC WT and its mutants [52] have been previously described. The APC 1–733 fragment was generated from pCS2 + MT-APC by PCR and subcloned into pCS3-MT-BX and pGEX-4T-1 vectors (Invitrogen). The pET28a-APC-Arm repeat domain (407–751) construct, which was subcloned into the pCS3-MT-BX-APC-Arm repeat domain (407–751), was kindly provided by Prof. Geng Wu (Shanghai Jiao Tong University, China) [68]. His-T4L-MKRN1 was generated by PCR and subcloned into a bacterial expression vector, His-PH-4TL-THR, which was kindly provided by Prof. HS. Cho (Yonsei University) [69]. pEGFP-C2 (Clontech, San Diego, CA, USA) was used as a transfection control. All constructs were sequenced to verify their identity (Genotech, Korea). Various mutants were produced by PCR and subcloned into mammalian expression vectors.
qRT-PCR and siRNA
For qRT-PCR analysis, total RNA was prepared employing TRIzol reagent (Invitrogen) following the manufacturer’s protocols. cDNA was generated from 1 μg of total RNA using PrimeScript Reverse Transcriptase (2680A, Takara Bio, Shiga, Japan). The amplified cDNA was analyzed using a QuantiTect SYBR Green PCR Kit and for qRT-PCR (Qiagen) analysis employing the following primers: c-Jun (5′-GTCCACGGCCAACATGCTCA-3′ and 5′-TGTTTGCAACTGCTGCGTTAG-3′); LEF1 (5′-CATCCTCCAGCTCCTGATATC-3′ and 5′-CTGACCTTGCCAGCCAAGAG-3′); and Nkd1 (5′-GGTCGAGGCACTCGGGAACTCGT-3′ and 5′-GTCCACTCTTGCCGGCTGTCCTC-3′). The primers for Axin2, CyclinD1, c-MYC, APC, and MKRN1 were obtained from Integrated DNA Technologies. Relative mRNA expression was calculated using the ΔΔCt method. Control siRNA (siCtrl, 1027281), siMKRN1#5 (5′-ACCGATTCTATTGGCCTAGTA-3′), and siMKRN1#7 (5′-CTGCGTCGGGTACGTGATGAA-3′) were aquired from Qiagen-Xeragon. ON-TARGET plus SMARTpool for human APC siRNA (L-003869-00-0005), human AXIN1 siRNA (L-009625-00-0005), and non-targeting pool (siNON, D-001810-10) were purchased from Dharmacon/Thermo Scientific (Waltham, MA, USA).
Wnt luciferase reporter assays
For cell-based luciferase assays, cells were plated and transfected with siRNA or DNA as described above. L-CM or Wnt3a-CM was added to HEK293 STF cells for 24 h after transfection, and luciferase activity was assayed using a dual luciferase assay kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. Luciferase activities were normalized to the viable cells using a CellTiter-Glo Assay (Promega).
Cell lysis and western blotting
For WB of APC protein, cells were lysed using lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% NP-40, 0.5% Na-deoxycholate, 0.5% Triton X-100, 1 mM EDTA, and a protease inhibitor cocktail [2 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin A, and 100 mg/mL phenylmethane sulfonyl fluoride, Sigma-Aldrich]). Cell lysates were subjected to WB with a monoclonal APC antibody (Ab58, Abcam) or a polyclonal APC antibody (H290, Santa Cruz) for human APC overnight at 4 °C. For APC detection, lysates were separated by 5% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes in transfer buffer containing 20 mM CAPS buffer (C2632, Sigma), 10% methanol, and 0.01% SDS (pH 11) at 100 mA for 1000 min at 4 °C. To detect the ubiquitylation of WT APC, a 3–8% Criterion XT Tris-acetate gradient gel (18-well, #345-0130, Bio-Rad) was employed for protein separation.
Immunoprecipitation
HEK293FT cells were collected in phosphate-buffered saline (PBS) and lysed using the above-described lysis buffer. Cell lysates were incubated with antibodies for 2 h, and incubated with protein G Sepharose beads (GE Healthcare, Buckinghamshire, UK) for 2 h at 4 °C. Immunoprecipitates were washed and then boiled in 2× sample buffer for 5 min and analyzed by WB.
Ubiquitylation analyses
The in vivo ubiquitylation assay was conducted as described previously [44, 46]. Briefly, cells were prepared in 6 M guanidinium-HCl buffer (pH 8) with 5 mM NEM (Sigma-Aldrich) to prevent deubiquitylation. His-ubiquitin-conjugated proteins were pulled down by Ni2+-NTA agarose beads (Qiagen), followed by washing and then elution in sample buffer with 10 mM β-mercaptoethanol and 200 mM imidazole. Proteins were then analyzed by WB. To evaluate endogenous APC ubiquitylation, cells were collected in PBS with 10 nM NEM and lysed in 1% SDS followed by 10 min boiling. Cell lysates were dissolved in 0.1% SDS followed by addition of lysis buffer by adding containing NEM and protease inhibitors. The lysates were immunoprecipitated with α-APC antibodies and analysed by WB. The ubiquitylation analyses were done as described before [44].
For the in vitro ubiquitylation assay, GST, GST-MKRN1 WT, GST-MKRN1 H307E, and GST-APC 1–733 or purified APC from HEK293FT cells were used. APC 1–733 and purified APC were incubated in 5 µg of ubiquitin (Sigma), 2 mM ATP (Fermentas), 100 ng of an E1 enzyme (UBE1; E-305; Boston Biochem), and 250 ng of an E2 enzyme (UbcH5c; E2-627; Boston Biochem) together with GST, GST-MKRN1 or GST-H307E in 40 mM Tris-HCl (pH 7.6), 50 mM NaCl, and 1 mM dithiothreitol for 3 h. The mixture was boiled in sample buffer and analyzed by WB.
Protein purification and in vitro binding assays
Recombinant human GST-MKRN1, GST-H307E, and GST-APC 1–733 proteins were purified as previously reported [46]. Recombinant His-T4L-MKRN1 was purified using Ni-NTA affinity chromatography as described before [69]. For in vitro binding assays, recombinant His-T4L-MKRN1 was incubated with GST or GST-APC for 2 h, and the proteins were precipitated by glutathione sepharose beads. Proteins were analyzed by WB.
Subcellular fractionation assays
Cytoplasmic and nucleic fractionations of HEK293FT was done using NE-PER Nuclear and Cytoplasmic Extraction Reagents following the manufacturer’s protocol (Thermo Scientific, 78835).
Immunocytochemistry
H1299 cells were transfected with siCtrl, siMKRN1#5, or siMKRN1#7. L-CM and Wnt3a-CM were added 48 h after transfection, and cells were then fixed with 4% paraformaldehyde followed by staining with α-β-catenin antibodies and 4′,6-diamidino-2-phenylindole (DAPI). For immunofluorescence staining, the cells were incubated with an Alexa Fluor 488-conjugated secondary antibody (1:500) at room temperature for 1 h, counterstained with DAPI and mounted. Fluorescence was visualized by immunofluorescence microscopy (Carl Zeiss Vision, San Diego, CA, USA) at excitation wavelengths of 488 nm (Alexa Fluor 488) and 405 nm (DAPI).
Colony-formation assays
HEK293FT cells were transfected with siCtrl, siMKRN1#5, or siMKRN1#7. After 24 h, the cells were detached with trypsin/EDTA, seeded (103 cells/35-mm plate) with L-CM (as a control) or Wnt3a-CM and incubated for 1 week. After incubation, the cells were fixed in 10% paraformaldehyde for 10 min and washed three times with PBS before crystal violet staining.
Scratch wound-healing, cell migration, and invasion assays
For the scratch wound-healing assays, HEK293FT cells were transfected with siCtrl or siMKRN1#7 together with siAPC using the above-described methods. After 24 h, a wound was introduced into a confluent monolayer of cells by scraping a pipette tip across the cell layer, and the cells were then rinsed with fresh medium removing cell debris. The wound-healing process was evaluated by imaging at the indicated times followed by microscopic analysis. The wound widths were measured and determined as a percentage of wound confluence. Wnt3a-induced cell migration and invasion were evaluated by modifying previously described methods [42]. Briefly, migration or invasion assays were performed using transwell chambers (for 24-well plates, #3422, Corning Costar, Cambridge, MA, USA). Both sides of the filter membrane were coated with 10 µg/mL collagen type 1 for migration assays or Matrigel (BD) for invasion assays. HEK293FT cells transfected with siRNA (2 × 104 cells/well) were plated onto each upper chamber in serum-free medium, and L-CM or Wnt3a-CM was added to the lower chamber. These cells were grown on the transwell upper chamber for 24 h. Transwell membranes were fixed in 10% paraformaldehyde for 10 min, washed three times with PBS, stained with crystal violet solution, and subjected to histologic analysis. The stained cells from different area were imaged under light microscopy and quantified by measuring the staining intensity using ImageJ (NIH). All experiments were independently performed three times.
Generation of MKRN1 knockout cell lines using CRISPR/Cas9
For vector construction, the pLentiCRISPR v2 vector containing Cas9 sequences (GeCKO, Plasmid #52961) that was used to knock out human MKRN1 via the CRISPR/Cas9 system was cloned and purchased from Genscript (USA). Correct insertion of the sgRNA sequences was validated by Genscript (USA).
For the second-generation lentiviral vectors, the pLentiCRISPR v2 containing individual sgRNAs, pMD2-VSVG and psPAX2, HEK293T cells were transfected with a mixture of DNAs using Lipo3000 (Invitrogen) according to the manufacturer’s protocol. At 48 h after transfection, the produced viruses were used to infect target cells. At 48 h after infection, the cells were cloned by serial dilution. Three different clones for MKRN1 were used for functional experiments.
In silico analysis
To examine the differential expression of MKRN1 and APC in cancer, data from the Gene Expression Omnibus were analyzed. GDS3233 includes 33 primary cervical cancers and 24 normal cervical epithelial samples.
Statistical analysis and reproducibility
The values are presented as the mean ± SD. Significance was determined with an unpaired two-tailed t-test using Prism (version 5.0; GraphPad). P values < 0.05 were considered significant.
References
Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723.
Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109.
Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–87.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98.
Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–15.
Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–8.
Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harbor Perspect in Biology. 2012;4:a008086
Berndt JD, Moon RT. Making a point with Wnt signals. Science. 2013;339:1388–9.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26.
Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–56.
Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33.
van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60.
Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84.
Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7.
Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–20.
Nusse R. Cell signalling: disarming Wnt. Nature. 2015;519:163–4.
Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Saito-Diaz K, Chen TW, Wang XX, Thorne CA, Wallace HA, Page-McCaw A, et al. The way Wnt works: components and mechanism. Growth Factors. 2013;31:1–31.
Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–84.
Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28.
Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31:2737–46.
Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51.
Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–33.
Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson’s ‘two-hit’ hypothesis. Nat Med. 1999;5:1071–5.
Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–45.
Rubinfeld B, Tice DA, Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1 epsilon. J Biol Chem. 2001;276:39037–45.
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3 beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–6.
Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–6.
Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol Cell. 2004;15:511–21.
Tran H, Polakis P. Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the beta-catenin destruction complex. J Biol Chem. 2012;287:28552–63.
Tran H, Bustos D, Yeh R, Rubinfeld B, Lam C, Shriver S, et al. HectD1 E3 ligase modifies adenomatous polyposis coli (APC) with polyubiquitin to promote the APC-axin interaction. J Biol Chem. 2013;288:3753–67.
Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898.
Tauriello DVF, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9:3700–9.
Tran H, Hamada F, Schwarz-Romond T, Bienz M. Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 2008;22:528–42.
Choi J, Park SY, Costantini F, Jho EH, Joo CK. Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. J Biol Chem. 2004;279:49188–98.
Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10.
Gao C, Xiao G, Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4:13.
Lee MS, Jeong MH, Lee HW, Han HJ, Ko A, Hewitt SM, et al. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015;6:7769.
Kim JH, Park KW, Lee EW, Jang WS, Seo J, Shin S, et al. Suppression of PPARgamma through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 2014;21:594–603.
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, et al. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun. 2012;3:978.
Ko A, Shin JY, Seo J, Lee KD, Lee EW, Lee MS, et al. Acceleration of gastric tumorigenesis through MKRN1-mediated posttranslational regulation of p14ARF. J Natl Cancer Inst. 2012;104:1660–72.
Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28:2100–13.
Kim JH, Park SM, Kang MR, Oh SY, Lee TH, Muller MT, et al. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev. 2005;19:776–81.
Cassar PA, Carpenedo RL, Samavarchi-Tehrani P, Olsen JB, Park CJ, Chang WY, et al. Integrative genomics positions MKRN1 as a novel ribonucleoprotein within the embryonic stem cell gene regulatory network. EMBO Rep. 2015;16:1334–57.
Bastakoty D, Young PP. Wnt/beta-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016;30:3271–84.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Choi J, Park SY, Costantini F, Jho EH, Joo CK. Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by axin. J Biol Chem. 2004;279:49188–98.
Valkenburg KC, Steensma MR, Williams BO, Zhong Z. Skeletal metastasis: treatments, mouse models, and the Wnt signaling. Chin J Cancer. 2013;32:380–96.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32.
Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5.
Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–33.
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600.
Bol GM, Suijkerbuijk KP, Bart J, Vooijs M, van der Wall E, van Diest PJ. Methylation profiles of hereditary and sporadic ovarian cancer. Histopathology. 2010;57:363–70.
Grohmann A, Tanneberger K, Alzner A, Schneikert J, Behrens J. AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J Cell Sci. 2007;120:3738–47.
Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642–5.
Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, et al. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–7.
Morishita EC, Murayama K, Kato-Murayama M, Ishizuka-Katsura Y, Tomabechi Y, Hayashi T, et al. Crystal structures of the armadillo repeat domain of adenomatous polyposis coli and its complex with the tyrosine-rich domain of Sam68. Structure. 2011;19:1496–508.
Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–83.
Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–6.
Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol. 2003;5:211–5.
Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, et al. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103.
Kim W, Kim SY, Kim T, Kim M, Bae DJ, Choi HI, et al. ADP-ribosylation factors 1 and 6 regulate Wnt/beta-catenin signaling via control of LRP6 phosphorylation. Oncogene. 2013;32:3390–6.
Zhang Z, Chen L, Gao L, Lin K, Zhu L, Lu Y, et al. Structural basis for the recognition of Asef by adenomatous polyposis coli. Cell Res. 2012;22:372–86.
Tong J, Yang H, Yang HY, Eom SH, Im YJ. Structure of Osh3 reveals a conserved mode of phosphoinositide binding in oxysterol-binding proteins. Structure. 2013;21:1203–13.
Acknowledgements
We thank Prof. KY Choi (Yonsei University) for providing L929, L929-hWnt3a, and HEK293-TOP-FLASH stable cell lines. This research was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A3A2066581) to J Song.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lee, HK., Lee, EW., Seo, J. et al. Ubiquitylation and degradation of adenomatous polyposis coli by MKRN1 enhances Wnt/β-catenin signaling. Oncogene 37, 4273–4286 (2018). https://doi.org/10.1038/s41388-018-0267-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0267-3
- Springer Nature Limited
This article is cited by
-
New insights in ubiquitin-dependent Wnt receptor regulation in tumorigenesis
In Vitro Cellular & Developmental Biology - Animal (2024)
-
MKRN1 promotes colorectal cancer metastasis by activating the TGF-β signalling pathway through SNIP1 protein degradation
Journal of Experimental & Clinical Cancer Research (2023)
-
Regulating tumor suppressor genes: post-translational modifications
Signal Transduction and Targeted Therapy (2020)