Abstract
Prostate cancer is the most commonly diagnosed malignancy among men, but few genetic factors that drive prostate cancer initiation have been identified. The WD repeat domain 77 (Wdr77) protein is essential for cellular proliferation when localizes in the cytoplasm of epithelial cells at the early stage of prostate development. In the adult prostate, it is transported into the nucleus and functions as a co-regulator of the androgen receptor to promote cellular differentiation and prostate function. This developmental process is reversed during prostate tumorigenesis, i.e., Wdr77 is translocated from the nucleus into the cytoplasm to drive proliferation of prostate cancer cells. In this study, we used in vivo genetic studies to further investigate the role of Wdr77 in prostate tumorigenesis. We found that prostate-specific deletion of Wdr77 abolished prostate tumor initiation induced by loss of the tumor suppressor Pten. Mechanistically, Wdr77 ablation inhibited E2F3 activation and enhanced TGFβ signaling, leading to attenuated cellular proliferation induced by loss of Pten. These findings establish a critical role of Wdr77 for prostate tumor initiation.
Similar content being viewed by others
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer-related death among men in the United States [1, 2]. Prostate cancer develops through multiple clinical stages, including prostatic intraepithelial neoplasia (PIN), adenocarcinoma, and metastasis [2]. A few genes that initiate prostate cancer or/and promote prostate cancer progression have been identified, including the tumor suppressor Pten, the gene rearrangement TMPRSS2-ERG, and c-Myc [3, 4]. Some genes (such as Sox9, Hoxb13, and Nkx3.1) and pathways (such as WNT signaling) involved in prostate development are found reactivated or inactivated in cancer initiation and progression [5]. However, there is still a need to determine additional drivers of prostate cancer that could help to understand prostate tumorigenesis and become the targets of new therapies.
The WD repeat domain 77 (Wdr77) protein is composed of 342 amino acid residues and 7 putative WD-40 repeats and was identified as a component (MEP50) of the methylosome complex [6], a subunit (WD45) of the SMN complex [7], and a novel androgen receptor (AR)-interacting protein (p44) [8, 9]. Wdr77 localizes in the cytoplasm of epithelial cells and is required for cell proliferation at the growth stage of prostate development [9,10,11]. In contrast, in the adult prostate, Wdr77 expression is decreased and the Wdr77 protein is translocated into the nucleus to function as an androgen receptor cofactor to establish and maintain luminal epithelia in a growth-arrested fully differentiated state (the G1/G0 cell cycle phase). Thus, Wdr77 provides for the integrated regulation of proliferation and differentiation of prostate epithelial cells through its distinct subcellular localization during prostate development. The increased Wdr77 gene expression and Wdr77 protein translocation from the nucleus to the cytoplasm is associated with age-related prostatic intraepithelial hyperplasia and prostate tumorigenesis [9,10,11]. The cytoplasmic Wdr77 is also required for the proliferation of prostate cancer cells and growth of prostate tumor xenografts [9, 11, 12]. Therefore, the molecular event involving Wdr77 in prostate development is reversed during cancer tumorigenesis. Further studies indicated that Wdr77 is required for cell proliferation via modulating the cell cycle progression [12] and expression of some cell growth regulators [13]. More recently, we found that Wdr77 expression also causes the non-sensitivity of proliferating cells to the TGFβ signaling, thereby contributing to cellular proliferation during tumorigenesis [14].
The tumor suppressor Pten gene encodes a lipid phosphatase that catalyzes the dephosphorylation of phosphatidylinositol-trisphosphate, resulting in the down-regulation of PI3K-AKT signaling pathway [15]. AKT, when activated, modulates a variety of downstream effectors involved in cell proliferation, apoptosis, cell growth, and metabolism [16]. Deletion or mutation of the Pten gene is one of the most frequent genetic alterations in many human cancers, including prostate cancer [17]. Loss of the Pten gene in prostate epithelial cells in the mouse results in the development of prostatic intraepithelial neoplasia (PIN) and prostate cancer with complete penetrance [18,19,20].
To understand whether Wdr77 is essential for prostate tumorigenesis in vivo, we have developed a mouse model in which prostate-specific deletion of both Wdr77 and Pten genes is generated in the epithelium using Cre-loxP site-specific recombination. We report here that deletion of Wdr77 abolished PIN and prostate tumor development initiated by homozygous loss of Pten, demonstrating the essential role of Wdr77 in prostate tumor initiation. We further show that Wdr77 is necessary for maintenance of cellular proliferation induced by loss of Pten. In addition, we demonstrated that Wdr77 is critical for suppressing the TGFβ signaling in vivo, suggesting that Wdr77 plays a role in relieving growth suppression of the TGFβ signaling.
Results
Generation of mice bearing prostate-specific deletion of Pten or Pten and Wdr77 genes
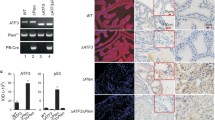
Previous studies have demonstrated that Wdr77 was essential for growth of normal prostate epithelial cells, as well as prostate cancer cells and prostate tumor xenografts [9, 11]. However, it has yet to be shown the role of Wdr77 in prostate tumorigenesis in vivo. To explore this, we used the Pten gene knockout mouse model, which closely mimics human prostate cancer [11, 21]. To generate mice that were homozygous mutants for Pten (Ptenpc−/−) and both Pten and Wdr77 genes (Ptenpc−/−;Wdr77pc−/−) in the prostate, we crossed PtenloxP/loxP and PtenloxP/loxP;Wdr77loxP/loxP mice with PRR2Bi-Cre mice, respectively. The prostate-specific deletion of Pten and Wdr77 genes were confirmed by genomic typing (Supplementary Fig. 1a, lane 6). Consistent with previously published reports [12, 18], Pten protein expression in the cytoplasm of the prostate luminal epithelia with sporadic nuclear staining (Supplementary Fig. 1b, panel A) and Wdr77 protein expression in the nucleus (panel C) were observed. The majority of prostate epithelial cells of Ptenpc−/−;Wdr77pc−/− mice lost both Pten and Wdr77 protein expression (Supplementary Fig. 1b, panels B and D), with a few cells (about 1–3%) still expressing Pten or Wdr77 but at low levels (inserts, indicated by black arrows). As expected, loss of the Pten gene led to up-regulation of the phosphorylated AKT protein in prostate epithelial cells of both prostate epithelial cells of Ptenpc−/− and Ptenpc−/−;Wdr77pc−/− mice relative to the WT mouse (Supplementary Fig. 2a, panels B–E vs. panel A; Supplementary Fig. 2a, lanes 2 and 4 vs. lanes 1 and 3), indicating that Wdr77 gene deletion did not affact AKT activation induced by Pten loss. Loss of the Wdr77 gene did not affect expression of the Pten gene or reverse versa (Supplementary Fig. 2b, lanes 2 and 4 vs. lanes 1 and 3), suggesting no physiological regulation between Pten and Wdr77.
Cytoplasmic Wdr77 is essential for prostate tumor initiation induced by Pten loss
Prostate glands were derived from Ptenpc−/− and Ptenpc−/−;Wdr77pc−/− mice at the ages of 1, 2, and 4 months (Table 1) and submitted to the analyses. Examined following Hematoxylin and Eosin (H&E) staining and consistent with previous observations [18, 22], Pten-null mice exhibited PIN lesions (PIN, circled) and tumors (T, circled) at the age of 2 months (Fig. 1a). The tumor incidence was increased as mice aged (Fig. 2b). Interestingly, examination of Ptenpc−/−;Wdr77pc−/− mouse prostate revealed a few PIN lesions (PIN, circled) and small tumors (T, circled) (Fig. 1b). The incidence of PIN and tumors in Ptenpc−/−;Wdr77pc−/− mice was drastically decreased in mice at the ages of both 2 and 4 months (Fig. 2). Gland sizes are significant increased (Fig. 2c) and epithelial enfoldings are lacking (Fig. 2d) in the DLP prostate of the Ptenpc−/− mouse due to tumor growth. Loss of the Wdr77 gene decreased gland sizes (Fig. 2c), as well as epithelial enfolgings (Fig. 2d), consistent with the critical role of Wdr77 in growth and differentiation of prostate epithelial cells. But Pten gene deletion could not restore grand size and enfolding numbers decreased by Wdr77 deletion (Fig. 2c, d).
Loss of Wdr77 inhibited prostate tumorigenesis induced by Pten gene deletion. The prostate tissue was derived from the Ptenpc−/− (a) or Ptenpc−/−;Wdr77pc−/− (b) mouse at the age of 2 months and stained with H&E. Tumor (T) regions are circled (top panels). The bottom panels show amplification of selected tumor (left) and PIN (right) lesions
The incidence of PIN (a) and prostate tumor (b) in Ptenpc−/− and Ptenpc−/−;Wdr77pc−/− mice. Prostate glands were derived from mice at the ages of 2 (n = 5) and 4 (n = 7) months and PIN and tumor lesions were quantified for each prostate. Tumor or PIN incidence = numbers of glands with tumor or PIN per 100 glands. Data are presented as the means of 5 (at the age of 2 m) or 7 (at the age of 7 m) prostates. Sizes (c) and enfoldings (d) per gland in DLP of WT, Wdr77pc−/−, Ptenpc−/−, and Ptenpc−/−;Wdr77pc−/− mice. Prostates of each genotype were derived from mice (n = 5) at the age of 2 months. Sizes or enfoldings of 10–30 glands in DLP of each mouse were measured and data are presented as mean of data obtained from all mice
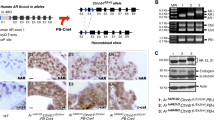
Immunostaining of the prostate derived from Ptenpc−/−;Wdr77pc−/− mouse with anti-Wdr77 antibody revealed that cells in PIN and tumor regions still expressed Wdr77 (Fig. 3a). In contrast, Wdr77 was absent in the benign epithelial cells (Fig. 3a, circled by red lines). These results suggest that PIN and tumors were derived from cells in which Wdr77 was not deleted. Thus, Wdr77 expression is essential for prostate PIN and tumors driven by the Pten gene deletion.
Cytoplasmic Wdr77 is essential for prostate initiation induced by Pten gene deletion. a Immunostaining of Wdr77 (brown) in the prostate tissue derived from the Ptenpc−/−;Wdr77pc−/− mouse at the age of 2 months. Benign regions are circled by red lines (top panel). Red arrows indicate benign epithelial cells expressing Wdr77 in the nucleus. b Wdr77 cytoplasm translocation is associated with prostate tumorigenesis induced by Pten gene deletion. The prostate tissue was derived from the Ptenpc−/− mouse at the age of 2 months and immunostained for Wdr77 (red, left panels). The nucleus was stained with SYTOX green (right panels)
We have previously reported that at the early stage of prostate development, Wdr77 is localized in the cytoplasm to drive cellular proliferation [21]. In contrast, it is transported into the nucleus in adult prostate to drive cellular differentiation. During prostate tumorigenesis, this developmental process is reversed, i.e., Wdr77 is transported from the nucleus into the cytoplasm to initiate cellular proliferation [21]. We investigated whether a similar event happens during prostate tumorigenesis driven by the Pten deletion. Similar to that observed during prostate tumorigenesis [11], Wdr77 is localized in the nucleus of normal prostate epithelial cells (Fig. 3b, top panels) and in contrast, Wdr77 is transported into the cytoplasm in hyperplasia (middle panels) and tumor (bottom panels) lesions. Cells in which Wdr77 was not deleted but localized to the nucleus were also observed in normal luminal epithelia (Fig. 3a, indicated by red arrows). We previously demonstrated that WDR77 in the cytoplasm is essential and sufficient to drive cellular proliferation and, in contrast, when localized in the nucleus, it inhibited cell growth and promoted cellular differention [23]. Thus, WDR77 cytoplasmic translocation may be also required for prostate tumorigenesis driven by Pten gene deletion.
Loss of Wdr77 inhibited cellular proliferation induced by the Pten deletion via E2F transcriptional factor
We previously demonstrated that cytoplasmic Wdr77 is essential for proliferation of prostate epithelial cells, as well as prostate cancer cells [11, 21]. Quantification of proliferation was accomplished by Ki-67 immunostaining (brown, indicated by black arrows) on WT, Wdr77f/f Wdr77pc−/−, Ptenpc−/− and Ptenpc−/−;Wdr77pc−/− prostate sections (Fig. 4a). In WT prostate, about 4% of epithelial cells were Ki-67-positive (Fig. 4b). However, the deletion of the Pten gene significantly enhanced the proliferation rate of epithelial cells in PIN (5.5-fold) and tumor (7.7-fold) regions (Fig. 4b, right panel) but no effect on the proliferation of benign prostate epithelial cells (left panel). Simultaneous deletion of Wdr77 or both Pten and Wdr77 genes resulted in the proliferation rate of benign prostate epithelial cells lower than that observed in the WT mouse (Fig. 4b, left panel). However, the proliferation rate of epithelial cells in PIN and tumor regions of Ptenpc−/−;Wdr77pc−/− prostate is indistinguishable with that observed in the Ptenpc−/− prostate, consistent with the fact that Wdr77 was not deleted in these lesions (Fig. 3a).
Loss of Wdr77 inhibited cellular proliferation induced by Pten gene deletion. a The prostate tissue were derived from the WT, Wdr77f/f, Wdr77pc-/-, Ptenpc−/−, and Ptenpc−/−;Wdr77pc−/− mice at the age of 2 months and stained for Ki-67 (brown). b The percentage of Ki-67 positive cells in WT (n = 2), Wdr77f/f (n = 5), Wdr77pc−/− (n = 5), Ptenpc−/− (n = 5), and Ptenpc−/−;Wdr77pc−/− (n = 7) prostate. c Gene typing of prostate epithelial cells derived from the PtenloxP/loP and PtenloxP/loxP;Wdr77loxP/loxP mice and infected with adenovirus harboring GFP (Ptenf/f, lanes 1, 3, 5) or Cre recombinase (Pten−/−, lanes 2, 4, 6). d The growth curves of prostate epithelial cells derived from the PtenloxP/loxP (left), and PtenloxP/loxP;Wdr77loxP/loxP (right) mice and infected with adenovirus harboring GFP (left: Ptenf/f; Right: Ptenf/f;Wdr77f/f) or Cre recombinase (Left: Pten−/− and Right: Pten−/−;Wdr77-−−). e Percentage of BrdU-positive epithelial cells infected with adenovirus harboring GFP or Cre recombinase. Cell were grown in the presence of BrdU for 2 h and submitted for immunostaining for BrdU. f Western blot of whole cell lystates made from LNCaP, Ptenf/f, and Ptenf/f;Wdr77f/f cells with anti-AR antibody and anti-actin antibodies
Prostate epithelial cells were isolated from PtenloxP/loxP and PtenloxP/loxP;Wdr77loxP/loxP mice and infected with adenovirus harboring the Cre recombinase to delete Pten (Fig. 4c, lane 2) and Pten plus Wdr77 genes (lanes 4 and 6). Of note, the efficiency of Ad-mediated gene deletion is between 95–100% [24]. Pten deletion led to AKT phosphorylation (Supplementary Fig. 3a, lane 2 vs. lane 1) and enhanced growth of prostate epithelial cells (Fig. 4d, left), consistent with previous reports [18, 25]. However, deletion of both Pten and Wdr77 genes also led to AKT phosphorylation (Supplementary Fig. 3a, lane 4 vs. lane 3) but inhibited growth of prostate epithelial cells (Fig. 4d, right). To assess proliferation, we used a BrdU incorporation assay. As reported [25], the percentage of BrdU-positive Pten-null epithelial cells was significantly higher than that of control epithelial cells (Fig. 4e). On the converse, Pten-null and Wdr77-null cells exhibited a significant decrease in cell proliferation relative to that of control cells (Fig. 4e). Thus, Wdr77 mediates cellular proliferation induced by the Pten gene deletion. The isolated epithelial cells lost AR expression (Fig. 4f, lanes 2 and 3), suggesting that the regulation of cell growth by Pten and Wdr77 is AR-independent.
Pten loss promotes phosphorylation of AKT, which in turn phosphorylates multiple targets resulting in an increase in cell proliferation, cell survival, metabolism, and protein synthesis [16]. We next investiageted which signaling(s) activated by Pten loss was affected by Wdr77 gene deletion. As reported [25], Pten loss resulted in phosphorylation of AKT, S6 ribosomal protein (rpS6), and GSK3β (Supplementary Fig. 3a, lane 2) but loss of Wdr77 did not afftect these phosphorylation events (lane 4), indicating that loss of Wdr77 had no affect on Pten-AKT-GSK3β (metabolism) and Pten-AKT-mTOR-S6K (protein synthesis) signalings induced by Pten loss. Loss of Pten gene or Pten and Wdr77 genes did not induce cell apoptosis indicated by DNA fragmentation assay (Supplementary Fig. 3b) and Western blot analysis of PARP protein (Supplementary Fig. 3c). We also observed that loss of Wdr77 gene did not inhibit phosphorylation of the BAD protein induced by Pten deletion (data not shown). So, Wdr77 gene deletion also did not affect cell survival induced by Pten loss. It was reported previously that Pten loss enhanced cellular proliferation by activating the RB-E2F pathway [26, 27]. The pRB-E2F pathway plays a critical role in regulating cellular proliferation because it regulates expression of many genes that are required for cell cycle progression [28]. To determine whether Wdr77 exerts influence on this process, we performed immunostaining of E2F3, a member of the E2F family of transcription factors [29]. As shown in Fig. 5, WT mice presented very low expression of E2F3 (Fig. 5a, panel A; Fig. 5b). However, Pten deletion resulted in strong nuclear expression of E2F3 (Fig. 5a, panel B; Fig. 5b), consistent with previous reports [27]. Interestingly, upon simultaneous deletion of Wdr77 and Pten genes, E2F3 expression in the benign region resembled that of the WT mouse with little to no expression (Fig. 5a, panel C; Fig. 5b). Of note, high levels of E2F3 are observed in the tumor region (Fig. 5a, panel C, circled by red line; Fig. 5b), where Wdr77 is not deleted (Fig. 3a). This finding indicates that Wdr77 is required for expression of E2F3 induced by Pten deletion.
Loss of Wdr77 blocked E2F3 expression induced by Pten gene deletion. a The prostate tissues were derived from the WT, Ptenpc−/−, and Ptenpc−/−;Wdr77pc−/− mice at the age of 2 months and immunostained for E2F3 and phosporylated Rb (pRb). Quantalization of nuclear E2F3 (b) and pRb (c) immunostaining signals using ImageJ32 software (NIH)
Rb interacts with E2F proteins to block its function in the activation of S-phase genes [28]. The phosphorylation of Rb abolishes its interaction with E2F proteins, thereby relieving its inhibitory effect on cell-cycle progression. In WT mice, Rb phosphorylation was minimal (Fig. 5a, panel D; Fig. 5c), as opposed to the strong pRb signal exhibited in the Pten-null mouse prostate (Fig. 5a, panel D; Fig. 5c). This result indicates that Pten loss leads to Rb phosphorylation to relieve its inhibitory effect on E2F transcriptional factors. In the double knockout mutant, pRb presented in epithelial cells of both normal (Wdr77-null) and tumor (circled by red lines, Wdr77-positive) regions (Fig. 5a, panel F; Fig. 5c). Thus, loss of Wdr77 did not affect Rb phosphorylation induced by Pten deletion.
TGFβ signaling is activated in Pten pc−/− ;Wdr77 pc−/− mice
The anti-proliferative effects of TGF-β signaling are lost during tumorigenesis, leading to hyper cellular proliferation [30,31,32]. More recently, we found that Smad2/3 phosphorylation, TGFβ-mediated transcription, and TGFβ2 and TGFβ receptor type II (TβRII) expression were dramatically induced when Wdr77 expression was silenced [14]. We further demonstrated that Wdr77 expression caused the non-sensitivity of proliferating cells to TGFβ signaling, thereby contributing to cellular proliferation during lung tumorigenesis [14]. We analyzed the TCGA prostate cancer data set (https://cancergenome.nih.gov/). Gene set enrichment analysis (GSEA) indicates that genes regulated by TGFβ were over-represented on the gene list, whose expression is altered in prostate cancer (Supplementary Fig. 4a). The gene heatmap reports show the altered expression of TGFβ target genes in majority of prostate cancer patients (Supplementary Fig. 4b). Thus, the TGFβ signaling is lost in prostate cancer. We also analyzed the DNA microarray data set (GSE25140) generated from the mouse with the prostate-specific Pten deletion [33]. GSEA indicates that genes regulated by TGFβ were over-represented on the gene list, whose expression is altered in Pten knockout prostate (Fig. 6a). The gene heatmap reports changes of TGFβ target genes in Pten knockout prostate vs. WT prostate (Fig. 6b). Among these genes, 23 genes (up-regulated by TGFβ in WT prostate) are down regulated and 5 genes (repressed by TGFβ in WT prostate) are up regulated in the Pten-null prostate. This analysis suggests that the TGFβ signaling is down-regulated in the Pten gene knockout prostate. We performed immunostaining of SMAD3 in WT, Ptenpc−/−, and Ptenpc−/−;Wdr77pc−/− prostate sections and detected nuclear SMAD3 staining in normal prostate epithelial cells (Fig. 6c, Some are indicated by black arrows.), indicating that the TGFβ signaling is active. SMAD3 protein was diffusing into the cytoplasm in hyperplasia and tumor cells (Fig. 6c, panels B and C), suggesting loss of the TGFβ signaling. Consistent with this observation, Pten loss led to significant down regulation of TGFβ2, TGFBRII, and TGFBRIII expression in prostate epithelial cells (Supplementary Fig. 5). Loss of Wdr77 reactivated the TGFβ signaling (p-SMAD3 expression) inactivated by Pten loss in vivo (Fig. 6c, panel D, circled) and restored TGFβ2 and TGFBRIII expression in Pten-null epithelial cells (Supplementary Fig. 5). Our findings suggest that Wdr77 may also play an important role to block the anti-proliferative effects of the TGFβ signaling during prostate tumorigenesis.
Loss of Wdr77 prevented inactivation of the TGFβ signaling. a GSEA enrichment plot indicates that genes regulated by TGFβ were over-represented on the gene list, whose expression is regulated by Pten loss. b Genes targeted by TGFβ are visualized by heatmap. The red and blue colors represent higher than average and lower than average expression of particular gene in the Pten-null prostate, respectively. c Immunostaining of phosphorylated SMAD3 (pSMAD3) in prostate tissues derived from WT, Ptenpc−/−, and Ptenpc−/−;Wdr77pc−/− mice at the age of 2 months. d Diagram of the Pten-AKT signaling and its downstream targets
Discussion
Wdr77 regulates proliferation and differentiation of prostate epithelial cells during the development through its subcellular localization. This developmental process is re-activated during prostate tumorigenesis. The data from this study indicate that Wdr77 expression, as well as its cytoplasmic localization is required for prostate tumor initiation induced by Pten loss.
Pten is frequently altered in cancers, suggesting it plays a fundamental role in many malignancies. Pten loss promotes phosphorylation of AKT, which in turn phosphorylates multiple targets resulting in an increase in cell proliferation, cell survival, and protein synthesis (Fig. 6d). An important question is which signaling activated by Pten loss plays an essential role in prostate tumorigenesis. Sox4 expression was up-regulated as a result of activation of Pten-AKT-mTOR signaling induced by Pten loss and loss of the Sox4 gene attenuated invasive phenotype of prostate tumors [34]. β-Catenin, a downstream target of Pten-AKT-GSK3β signaling, is essential for many developmental processes and has been implicated in tumorigenesis in many tissues, including prostate cancer [35, 36]. It has been shown that β-catenin is required for prostate development and its over-expression can promotes invasive prostate cancer in the Pten deletion model [37]. These findings suggest that the activation of Pten-AKT-mTOR or/and Pten-AKT-GSK3B signaling is critical for prostate cancer progression driven by Pten loss. Pten loss resulted in phosphorylation of AKT, S6 ribosomal protein (rpS6), GSK3β, and BAD (data not shown) proteins (Supplementary Fig. 2a, lane 2 vs. lane 1), indicating the activation of Pten-AKT-mTOR, Pten-AKT-BAD, and Pten-AKT-GSK3B signaling pathways. But, deletion of Wdr77 gene did not affect the activation of these pathways (Supplementary Fig. 3a, lane 4 vs. lane 3) and induce cell apoptosis (Supplementary Fig. 3b and 3c), suggesting that Wdr77 did not affect Pten-AKT-mTOR, Pten-AKT-BAD, and Pten-AKT-GSK3B signaling pathways. However, we found that Pten loss induced E2F3 expression and Rb phosphorylation in prostate epithelial cells. As the consequence, cellular proliferation was significantly enhanced. Deletion of Wdr77 abolished prostate tumor initiation induced by Pten loss, correlated with decreased E2F3 expression but not Rb phosphorylation. This finding suggests that Pten-AKT-E2F signaling axis may be required for prostate tumor initiation induced by Pten loss.
We observed that Pten loss significantly enhanced BrdU-positive (proliferative) epithelial cell populations and induced E2F3 expression and Rb inactivation (phosphorylation), consistent with the fact that Pten gene loss induces cellular proliferation [17, 22, 25]. Loss of Wdr77 significantly reduced cellular proliferation induced by Pten loss. This results is consistent with our previous observations that Wdr77 plays an essential role in proliferation of prostate epithelial [23] and cancer cells [21]. Wdr77 deletion reduced E2F3 expression induced by Pten loss, suggesting that Wdr77 is necessary for the maintenance of E2F3 protein levels, which is required for cell cycle progression from G1 to S phase. The mechanism of how Wdr77 regulates E2F3 expression is currently under investigation. Wdr77 also targets several growth factors, as well as growth inhibitors [13]. Whether expression of these factors by Wdr77 contributes to prostate tumorigenesis induced by Pten loss in vivo is also under investigation. It is most likely that Wdr77 is not only required for the tumor onset induced by Pten loss, but also required for tumor initiation by other oncogenic factors because of the critical role of Wdr77 in cellular proliferation. Results supporting this statement include i) a general Wdr77 requirment for cellular proliferation of mutiple tested cancer cell lines (PC3, DU145, LNCaP, A549, PC14, PANC1, ASPC1, mDA MD231, HCT116, and WM2664) independent on the Pten status; ii) an essential role of Wdr77 for growth (proliferation) of mouse tumor xenografts of PC3, LNCaP, A549, and PC13; iii) a requirement of Wdr77 for tumor formation of prostate epithelial cells transformed by large T antigen and Ras (G12V) in the nude mouse.
Most normal adult cells are fully differentiated and generally quiescent. TGFβ acts as a key physiological factor that ensures the maintenance of cell quiescence [38, 39]. Tumorigenesis is involved in the loss of cellular sensitivity to TGFβ signaling [40]. Our analysis of the TCGA prostate cancer dataset across 498 prostate cancer patients and 52 normal prostate tissues supports this conclusion. Down-regulation of the TGFβ signaling was observed in prostate cancer patients when compared to the normal prostate tissues. Similarly, the TGFβ signaling is also down-regulated in the Pten knockout prostate. In this study, we observed inactivation of the TGFβ signaling (loss of p-SMAD3 immunostaining) in prostate epithelial cells following loss of Pten, which was blocked by loss of Wdr77. In the previous study, we described that Wdr77 abrogated TGFβ growth suppression in proliferating cells [14]. These results suggest that Wdr77 cytoplasmic translocation induced by loss of Pten is not only required for cellular proliferation but may also play an important role to block the anti-proliferative effects of the TGFβ signaling, which is essential for prostate tumorigenesis.
It was reported that the loss of the Pten gene resulted in up-regulation of AR expression [41]. We observed the same result in Ptenpc−/− and Ptenpc−/−;Wdr77pc−/− mice (Supplementary Fig. 6, panels B–D vs. panel A). Although AR protein levels were slightly increased, the androgen signaling (reflected by expression of androgen hallmark genes) was down-regulated in the Pten-null mouse prostate (Supplementary Fig. 7). We previously reported that Wdr77 loss selectively affected expression of a set of AR-target genes in the mouse prostate [10] and in prostate cancer cells [9]. We previously observed that Wdr77 regulates cellular proliferation is independent on the AR signaling [11, 12, 42]. We obtained the same result that Wdr77 regulated proliferation of mouse epithelial cells in which AR protein is not expressed. How the AR signaling altered by Pten loss or/and Wdr77 subcellular transportation may contribute to prostate tumorigenesis requires additional studies.
This study suggests that Wdr77 expression and its cytoplasmic translocation are required for cellular proliferation by maintaining E2F3 expression and inactivating the TGFβ signaling, which represents a necessary step in prostate tumor initiation.
Materials and methods
Prostate-specific deletion of Pten and Wdr77 genes in the mouse
PtenloxP/loxP mice (C;129S4-Ptentm1Hwu; Stock Number 006440) were purchased from Jackson Laboratory and Wdr77loxP/loxP mice were generated as previously described by us [10]. The PtenloxP/loxP mouse was crossed with the Wdr77loxP/loxP mouse to generate PtenloxP/loxP;Wdr77loxP/loxP mouse. PtenloxP/loxP or PtenloxP/loxP;Wdr77loxP/loxP mouse was then crossed with PRR2Bi-Cre mouse [43] to generate mice that were prostate-specific deletion of Pten gene (PtenloxP/loxP;Cre or Ptenpc-/-) or both Pten and Wdr77 genes (PtenloxP/loxP;Wdr77loxP/loxP;Cre or Ptenpc-/-;Wdr77pc-/-). For gene typing, the genomic DNA was isolated from tail or prostate gland of mouse at the age of 21 day old and subjected to PCR analysis with primers as described [10, 18]. Prostate glands derived from five to seven aminals per genotype were used for analysis. No randomization and blinding were used. Mice were handled in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Morehouse College School of Medicine’s Institutional Animal Care and Use Committee approved all the experimental procedures used for mice in this study.
Antibodies
Antibodies against Pten (D4.3), Ki-67 (D3B5), Akt (pan C67E7), p-AKT (Ser473), and p-S6 ribosomal protein (Ser235/236) were obtained from Cell Signaling Technology. Antibodies against AR (N-20), E2F3 (N-20), and pSMAD3 (Ser208) were obtained from Santa Cruz Biotechnology. The BrdU antibody was obtained from BD Biosciences. The antigen purified anti-Wdr77 antibody was described previously [8].
Immunohistochemistry
The prostate gland was dissected from WT, Ptenpc−/− or Ptenpc−/−;Wdr77pc−/− mouse at the age of 21, 60, or 120 days and fixed with 10% formalin overnight at 4 oC. The fixed prostate gland was embedded in paraffin and sectioned (5 µm). Prostate sections were subjected to washes in xylene, dehydration in graded ethanol (70–100%), and then washed in phosphate buffered saline (PBS). Following dehydration, the sections were subjected to the citrate antigen retrieval followed by a 12 min treatment with 3% H202 in PBS for endogenous peroxidase blocking. To reduce nonspecific binding of the primary antibody, the sections were blocked with 4% fish gelatin for 30 min. The prostate sections were incubated with the primary antibody overnight at 4 °C and then with a secondary peroxidase-labeled antibody (Biocare Medical) for 30 min, washed with PBS and incubated with 4+ Streptavidin-HRP (Biocare Medical) for 30 min. The sections were then washed with PBS and the signal was visualized by application of 3,3’-Diaminobenzidine (DAB) (Vector Laboratories).
Cell culture and cell growth assay
Mouse prostate epithelial cells were isolated from prostate glands of PtenloxP/loxP and PtenloxP/loxP;Wdr77loxP/loxP mice as described previously by us [23] and cultured in Keratinocyte Serum Free Medium (KSFM) (Gibco) supplemented with 2% Fetal Bovine Serum plus 1% Pen-Strep, epidermal growth factor (0.1 ng/mL), and bovine pituitary extract (23 µg/mL). The Pten gene or both Pten and Wdr77 genes were deleted by infection of epithelial cells with Ad5-CMV-Cre-GFP (15 particles per cell; Vector Development Laboratory, Baylor College of Medicine) as described [23]. The control cells were infected with Ad5-CMV-GFP at the same time with the same viral particles per cell. Cells were harvested at 7 days post adenovirus infection for analysis. For cell growth assay, cells (5 × 105 cells per well) were plated in a 24 well culture plate in triplicate and counted every day for 4 days. For BrdU incorporation assay, cells were plated in a chamber slide (8-well, 1.0 × 104 cells/per well) and grown overnight. BrdU was added to the medium at the final concentration of 10 µM. Cells were incubated for 2 h and immunostained with anti-BrdU antibody (BD Biosciences) according to a previously published protocol [23].
Western blot analysis
Whole cell lysates were obtained using the Passive Lysis Buffer (Promega) supplemented with Protease and Phosphatase inhibitors (Fischer Scientific). Protein concentration was determined using the Bradford Assay (Bio-Rad) with BSA as the standard. Following SDS–PAGE, protein was transferred to a nitrocellulose membrane (NC) at 6 V overnight. NC membrane was blocked for 30 min in 3% milk in TBST (Tris buffered saline with 0.05% Tween-20). Thereafter, the membranes were incubated in primary antibody in 2% BSA–TBST, for 2 h. NC membranes were then washed 3× with TBST in 5 min intervals, followed by incubation in secondary antibody labeled with horseradish peroxidase (HRP) in 2% BSA–TBST, for 1.5 h. NC membranes were then washed 4 × 5 min with TBST. Protein was then detected using an enhanced chemiluminescent substrate (Western Lightning-Plus ECL, Perkin Elmer).
Cell cycle analysis
Cells were trypsinized, washed with PBS and fixed with cold ethanol (70%) for overnight at 4 oC. Cells were pelleted by centrifugation at 2000 r.p.m. for 5 min and washed with PBS. Cells were stained with propidium iodide (PI) and submitted to cell cycle analysis on the Accuri C6 Flow Cytometer (BD Biosciences). Data were analyzed using FloJo software (FloJo).
Real-time PCR
Total RNA was isolated from cells using the TRIzol reagent (Thermofisher). cDNA was generated using the Superscript III First-Strand Synthesis System (Thermofisher). Real-time PCR was performed with Go-Taq qPCR master mix (Promega) and specific primers (Supplementary Table 1) (40 cycles of 15 s at 95 °C and 20 s at 60 °C).
Statistical analysis
Data are presented as the means of three or more independent experiments ± the standard deviation. A 2-tailed unpaired student t-test was used to determine whether differences between control and experiment samples were statistically significant. P values less than 0.05 were considered statistically significant.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000.
Liu W. DNA alterations in the tumor genome and their associations with clinical outcome in prostate cancer. Asian J Androl. 2016;18:533–42.
Koh CM, Bieberich CJ, Dang CV, Nelson WG, Yegnasubramanian S, De Marzo AM. MYC and prostate cancer. Genes Cancer. 2010;1:617–28.
Shtivelman E, Beer TM, Evans CP. Molecular pathways and targets in prostate cancer. Oncotarget. 2014;5:7217–59.
Friesen WJ, Wyce A, Paushkin S, Abel L, Rappsilber J, Mann M, et al. A novel WD repeat protein component of the methylosome binds Sm proteins. J Biol Chem. 2002;277:8243–7.
Meister G, Eggert C, Buhler D, Brahms H, Kambach C, Fischer U. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr Biol. 2001;11:1990–4.
Hosohata K, Li P, Hosohata Y, Qin J, Roeder RG, Wang Z. Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol. 2003;23:7019–29.
Zhou L, Wu H, Lee P, Wang Z. Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells. J Mol Endocrinol. 2006;37:283–300.
Gao S, Wu H, Wang F, Wang Z. Altered differentiation and proliferation of prostate epithelium in mice lacking the androgen receptor cofactor p44/WDR77. Endocrinology. 2010;151:3941–53.
Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, et al. Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci USA. 2008;105:5236–41.
Gao S, Wang Z. Subcellular localization of p44/WDR77 determines proliferation and differentiation of prostate epithelial cells. PLoS ONE. 2012;7:e49173.
Sheng X, Wang Z. Protein arginine methyltransferase 5 regulates multiple signaling pathways to promote lung cancer cell proliferation. BMC Cancer. 2016;16:567.
Yi P, Gao S, Gu Z, Huang T, Wang Z. P44/WDR77 restricts the sensitivity of proliferating cells to TGFbeta signaling. Biochem Biophys Res Commun. 2014;450:409–15.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39.
Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–88.
Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, et al. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24.
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21.
Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55.
Gu Z, Zhou L, Gao S, Wang Z. Nuclear transport signals control cellular localization and function of androgen receptor cofactor p44/WDR77. PLoS ONE. 2011;6:e22395.
Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res. 1998;4:811–5.
Gao S, Wang Z. Subcellular localization of p44/WDR77 determines proliferation and differentiation of prostate epithelial cells. PLoS ONE. 2012;7:e49173.
Prost S, Sheahan S, Rannie D, Harrison DJ. Adenovirus-mediated Cre deletion of floxed sequences in primary mouse cells is an efficient alternative for studies of gene deletion. Nucleic Acids Res. 2001;29:E80.
Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA. 2006;103:1480–5.
Abukhdeir AM, Park BH. P21 andp27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19.
Foster CS, Falconer A, Dodson AR, Norman AR, Dennis N, Fletcher A, et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871–9.
Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–62.
Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–26.
Lebrun JJ. The dual role of TGFbeta in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. 2012;2012:381428.
Massague J. TGFbeta in cancer. Cell. 2008;134:215–30.
Tu WH, Thomas TZ, Masumori N, Bhowmick NA, Gorska AE, Shyr Y, et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–77.
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–73.
Bilir B, Osunkoya AO, Wiles WGt, Sannigrahi S, Lefebvre V, Metzger D, et al. SOX4 is essential for prostate tumorigenesis initiated by PTEN ablation. Cancer Res. 2016;76:1112–21..
Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocr Relat Cancer. 2003;10:537–60.
Yokoyama NN, Shao S, Hoang BH, Mercola D, Zi X. Wnt signaling in castration-resistant prostate cancer: implications for therapy. Am J Clin Exp Urol. 2014;2:27–44.
Francis JC, Thomsen MK, Taketo MM, Swain A. beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9:e1003180.
Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, O’Toole T, et al. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med. 1991;174:925–9.
Sitnicka E, Ruscetti FW, Priestley GV, Wolf NS, Bartelmez SH. Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood. 1996;88:82–8.
Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–23.
Gu Z, Zhang F, Wang ZQ, Ma W, Davis RE, Wang Z. The p44/wdr77-dependent cellular proliferation process during lung development is reactivated in lung cancer. Oncogene. 2012;32:1888–900.
Jin C, McKeehan K, Wang F. Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens. Prostate. 2003;57:160–4.
Acknowledgements
We thank Ms. Shen Gao for animal work and technique support. DOB is supported by NIH NIGMS-RISE R25GM06414 and ZW is supported by W81XWH-12-1-0453 PC111461, NIMHD RCMI 5G12MD007590, and NIMHD 5P20MD002285. The funding agencies had no role in study design, data collection or analysis, decision to publish or manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
O’Bryant, D., Wang, Z. The essential role of WD repeat domain 77 in prostate tumor initiation induced by Pten loss. Oncogene 37, 4151–4163 (2018). https://doi.org/10.1038/s41388-018-0254-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0254-8
- Springer Nature Limited