Abstract
Sleep problems are a prominent feature of mental health conditions including post-traumatic stress disorder (PTSD). Despite its potential importance, the role of sleep in the development of and/or recovery from trauma-related illnesses is not understood. Interestingly, there are reports that sleep disruption immediately after a traumatic experience can reduce fear memories, an effect that could be utilized therapeutically in humans. While the mechanisms of this effect are not completely understood, one possible explanation for these findings is that immediate sleep disruption interferes with consolidation of fear memories, rendering them weaker and more sensitive to intervention. Here, we allowed fear-conditioned mice to sleep immediately after fear conditioning during a time frame (18 h) that includes and extends beyond periods typically associated with memory consolidation before subjecting them to 6-h of sleep disruption. Mice exposed to this delayed regimen showed dramatic reductions in fear during tests conducted immediately after sleep disruption, as well as 24 h later. This sleep disruption regimen also increased levels of mRNA encoding brain-derived neurotrophic factor (BDNF), a molecule implicated in neuroplasticity, in the basolateral amygdala (BLA), a brain area implicated in fear and its extinction. These findings raise the possibility that the effects of our delayed sleep disruption regimen are not due to disruption of memory consolidation, but instead are caused by BDNF-mediated neuroadaptations within the BLA that actively suppress expression of fear. Treatments that safely reduce expression of fear memories would have considerable therapeutic potential in the treatment of conditions triggered by trauma.
Similar content being viewed by others
Introduction
Fear- and trauma-related learning and memory alter—and are altered by—sleep patterns. Abnormal sleep is a characteristic feature of many fear- and trauma-related disorders. Diagnostic criteria for Generalized Anxiety Disorder (GAD) and Post-Traumatic Stress Disorder (PTSD) include nightmares, difficulty falling or staying asleep, and restless unsatisfying sleep [1]. These disorders appear to have a reciprocal relationship with sleep, whereby sleep problems worsen symptom severity; indeed, the consequences of insufficient sleep on mood and cognitive function are well documented [2,3,4,5]. The ability to sleep after experiencing a traumatic event is often compromised due to various situational parameters, such as the need for medical or police intervention, the need to remain on duty, or subsequent trauma-related insomnia. Interestingly, there are reports that sleep disruption immediately after a traumatic experience can reduce fear memories, which could be utilized therapeutically in humans [6, 7]. Despite its potential importance, however, the role of sleep in the development of and/or recovery from trauma-related illnesses is not well understood.
In research settings, Pavlovian fear conditioning is commonly used as a model to study threat- and trauma-related experiences [8, 9]. Studies of sleep disruption on fear memory frequently report memory deficits, but typically focus on the time immediately after trauma, which prevents sleep-based interventions from benefiting clinical populations in most instances [7, 10,11,12]. When sleep is restricted immediately preceding or following fear conditioning, impairments in contextual memory are observed [7, 10]. Studies in mice show reductions in fear expression if sleep disruption occurs during the 0–5 h period after Fear Conditioning, but no effect if delayed until the 5–10 h period [7]. Depriving rodents specifically of rapid eye movement (REM) sleep produces similar results, including impairments in cued fear memories and induction of long-term potentiation (LTP) [11, 12]. The mechanism by which sleep disruption immediately following fear conditioning impairs fear memories is thought to involve interference with memory consolidation. Seminal findings show that blocking consolidation through protein synthesis or transcription inhibition in immediate hours after learning or LTP induction prevents memory formation and LTP persistence [13,14,15,16]. There is also evidence that some additional consolidation occurs over a longer time frame (~12 h) [17, 18]. Sleep disruption itself can serve as a type of stress [19, 20], and stress is known to alter memory function [21,22,23], as well as induce neuroplasticity in brain cells and circuits that regulate the development and expression of fear-related behaviors, including the amygdala [9, 24]. Regardless of mechanism, the possibility that early (immediate) sleep disruption could be used therapeutically to reduce the formation and expression of traumatic memories has important—and potentially exciting—implications for mental health. However, this approach would require rapidly organized interventions beginning immediately following trauma exposure, since the presumed mechanism of action would be preventing the initial formation of fear memories rather than reducing or eliminating already-stabilized fear memories. Approaches that enable intervention at more distal time points would have a transformational impact on the treatment of conditions such as PTSD.
Here, we examined in mice the effects of a delayed, next-day regimen of sleep disruption on the expression of fear. We allowed fear-conditioned mice to sleep immediately after fear conditioning during a time frame (18 h) that extends beyond periods typically associated with memory consolidation before subjecting them to 6-h of sleep disruption the next day. We used a method of sleep disruption (gentle stimulation) that does not produce stress responses, to avoid adding a secondary stressor that could complicate interpretation of our findings. In parallel, we examined the effects of our sleep disruption regimen on expression of mRNA encoding brain-derived neurotropic factor (BDNF), a molecule implicated in neuroplasticity, in brain areas involved in the development, expression, and extinction of fear-related behaviors [17, 25, 26]. Considering sex differences in baseline sleep parameters [27,28,29,30,31], interactions between sleep and gonadal hormones [28, 30, 32,33,34,35,36], and the prevalence of trauma-related illnesses such as PTSD [37,38,39,40,41,42,43,44], we designed the studies to enable qualitative and quantitative comparisons between males and females. Our findings show that delayed sleep disruption can reduce expression of conditioned fear in both sexes, and raise the possibility that the effects of this regimen are not due to disruption of memory consolidation but instead caused by active processes related to BDNF-mediated neuroadaptations.
Materials and methods
Subjects
Adult (6–8 weeks) male and female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were maintained on a 12-h light/dark cycle (07:00 On-19:00 Off) in a temperature-controlled vivarium. Mice were singly-housed in standard Plexiglas home cages with ad libitum food and water and weekly cage changes (including new bedding and nesting materials), and provided one week acclimation to the vivarium prior to experiments. Procedures were approved by McLean Hospital Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health’s (NIH) Guide for the Care and Use of Animals.
Sleep disruption: gentle stimulation and sweeper bar
Initial studies compared two methods of sleep disruption: gentle stimulation and sweeper bar. Both procedures started at lights-on (07:00) and lasted 6-h, with one mouse per holding cage. For both methods, the goal was to prevent mice from sleeping for this duration of time. For the gentle stimulation method, the procedure began by moving the mouse to a new cage with new nesting materials. Upon completion of the nest—in general, ~2 h into the procedure—mice were then kept awake by placing objects within the cage to encourage voluntary activity, as well as gently tapping the cage or softly touching the hindquarters. Objects were selected to be familiar to the mice (e.g., a rubber stopper from a drinking bottle, a loose metal rod similar to those comprising the cage top). The minimum amount of stimulation needed to ensure wakefulness was used; all mice received the objects and similar amounts of tactile stimulation. In contrast, the sweeper bar method was automated and involved a sleep fragmentation apparatus (Lafayette Instrument; Lafayette, IN, USA). The apparatus closely resembled the home cage but was outfitted with a sweeper bar that alternated across the bottom of the cage at the minimum frequency (7.5 s/cycle) necessary to ensure continuous movement. Mice actively avoid contact with the moving bar, which prevents sleep onset. Considering differences in stress responses caused by these methods (see below), the gentle stimulation method was selected for use in fear conditioning studies.
Corticosterone ELISA
To determine if the methods of sleep disruption produce stress-like responses, we examined their effects on circulating plasma corticosterone (CORT). Immediately following sleep disruption, mice were sacrificed by rapid decapitation, and trunk blood was collected and centrifuged to obtain plasma. CORT levels were quantified using enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Science, Ann Arbor, MI, USA) according to manufacturer instructions.
Fear conditioning and extinction paradigms
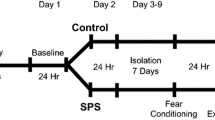
Behavioral testing was performed 6 h after lights on so that sleep disruption (on Day 4) would not shift the time of testing. As described previously [8, 45], mice were habituated to a conditioning chamber (Context A) for 15 min on two consecutive days (Days 1–2). Context A consisted of a free-standing chamber (no external sound-attenuating chamber) with a shocker grid floor, house lights on, and lightly scented with quatricide (used as a cleaner). For Fear Conditioning (Day 3), mice were placed in Context A and received 5 conditioned stimulus (CS)-unconditioned stimulus (US) pairings: the CS was a 30-s, 6000-Hz, 75-dB tone, and the US was a 1-s, 0.7-mA footshock. Tone-shock trials were presented on a variable ITI, ranging from 1 to 3 min, and began after a 3-min context exposure. Mice were then returned to their home cages and left undisturbed. The next morning (Day 4), at the start of lights-on—18 h after Fear Conditioning—half of the mice underwent gentle stimulation sleep disruption for 6-h. Control mice were left undisturbed. Immediately after this regimen, mice were tested for Fear Recall in Context B, which was a novel chamber inside an external sound-attenuating box, with smooth black plastic flooring, house lights off and lightly scented with 70% ethanol (used as a cleaner). Fear Recall tests, which also served as a mild extinction protocol, consisted of 2-min acclimation, followed by 15 CS presentations with a 90-s ITI. This shortened protocol was used because it is sufficient to cause some level of extinction while avoiding the tendency for sleep-deprived mice to fall asleep during longer test sessions. For Extinction Recall (Day 5), the Fear Recall procedure was repeated.
To explore if our sleep disruption regimen interferes with initial consolidation of fear memories, a separate cohort of mice was used to test for fear reinstatement after extinction. These mice did not receive Extinction Recall on Day 5; instead, they were placed in Context C, with black and white striped walls, pine scented bedding under the shock grid floor and lightly scented with Clidox (used as a cleaner). In Context C, mice received two un-signaled (reminder) 1-s, 0.7 mA footshocks 1-min apart after a 3-min baseline period. On Day 6, recall was tested in Context C with 2-min baseline, followed by 5 CS presentations with a 90-s ITI. The ability of the reminder footshocks to reinstate fear behavior in response to the CS is considered evidence of initial consolidation.
The primary endpoint for all fear conditioning studies was freezing behavior, which was quantified using FreezeFrame software (Coulbourn) with thresholds set by a trained observer unaware of treatment conditions.
Sleep transmitter surgery
To examine fear conditioning effects on sleep, mice were implanted with wireless transmitters (PhysioTelTM HD-X02, Harvard Bioscience, Inc. dba Data Sciences International [DSI], St. Paul, MN) to enable continuous collection of EEG (electroencephalography) and EMG (electromyography) data, as described [31, 46,47,48]. Mice were anesthetized via intraperitoneal (IP) injections of 100 mg/kg ketamine/10 mg/kg xylazine mixed in saline. EEG leads were attached to the skull with screws over the frontal lobe (+1 mm anterior/posterior, +1 mm medial/lateral) and the contralateral parietal lobe (−3 mm anterior/posterior, -3mm medial/lateral) and secured using dental cement. EMG wires were threaded through the trapezius muscle, the remaining incision was sutured closed and antibiotic ointment was applied. Antibiotic (sulfamethoxazole and trimethoprim) was provided in water and ketofen was given subcutaneously as an analgesic (5.0 mg/kg). After surgery, mice recovered for 2 weeks prior to baseline sleep recordings.
Physiological recordings
Mice implanted with sleep transmitters were housed in standard cages placed on top of receiver platforms (RPC-1; DSI) for wireless data collection, as previously described [31, 46,47,48]. Collection of EEG and EMG data occurred continuously except when the mice were in the fear conditioning apparatus, enabling analyses after habituation and for 24 h after Fear Conditioning. Data from habituation Day 2 were used as the baseline for comparison of vigilance states before and after fear conditioning. Vigilance states were quantified using software Neuroscore (DSI) to visualize EEG and EMG signals, allowing a trained scorer unaware of treatment conditions to manually assign vigilance states in 10-s epochs.
Quantitative polymerase chain reaction (qPCR) to quantify mRNA encoding BDNF
Considering previous work implicating BDNF in the development, expression, and extinction of conditioned fear [25, 26, 49, 50], we examined the effects of our 6-h gentle stimulation-induced sleep disruption regimen on BDNF mRNA in brain areas previously implicated in fear memory processing, including basolateral amygdala (BLA), medial prefrontal cortex (PFC), bed nucleus of the stria terminalis (BNST), and dorsal hippocampus (HIP) [9, 51]. These studies quantified gene expression immediately following sleep disruption (without confounding effects of fear conditioning). Mice were sacrificed by rapid decapitation immediately after sleep disruption or uninterrupted sleep. Brains were flash frozen using 2-methylbutane and stored at −80°. Brains were sectioned on a cryostat to enable precision dissections with 0.8 mm tissue punches. RNA was extracted with RNeasy Micro Kit (QIAGEN, Germantown, MD, USA) according to manufacturer instructions, cDNA was synthesized with iScript cDNA Synthesis Kit (BIO-RAD, Hercules, CA, USA) and qPCR was performed with iQ SYBR green supermix (BIO-RAD) on MyiQ Single Color Real-Time PCR Detection System (BIO-RAD) with coordinating software. Primers were acquired through Integrated DNA Technologies, Inc. (IDT; Coralville, IA, USA) (Table 1). BDNF expression was quantified using the Pfaffl method relative to housekeeping gene 7SK [52]. Values were normalized to the control group for analyses, which were performed on males and females combined (normalized to combined controls) and as males and females separately (each sex normalized to their same-sex controls).
Statistical analyses
Analyses were performed using GraphPad Prism 9 with significance set to P < 0.05. Outliers were determined using ROUT outlier detection test (Q = 1). Outlier exclusions are noted in Results. Behavioral data were measured as percent freezing for the duration of the CS presentation or baseline. A 2-way ANOVA (Sleep Condition x Trial/Trial Block) with repeated measures was used to determine main effects and Bonferroni’s multiple comparisons were used to compare between trials/trial blocks or sleep conditions. Two-tailed unpaired t-tests assessed CORT and BDNF levels. Two-tailed, paired t-tests analyzed changes in vigilance states after Fear Conditioning relative to baseline.
Results
Sleep disruption by gentle stimulation and sweeper bar methods differentially affect CORT
To determine if our sleep disruption methods cause stress responses in mice, we measured plasma CORT in mice sacrificed immediately after each 6-h regimen. For the gentle stimulation method, when sexes were combined, there were no overall differences in CORT levels between mice that had received sleep disruption (N = 16; 8 males, 8 females) or undisturbed controls (N = 18; 8 males, 10 females) (t(32) = 1.39, not significant [ns]) (Fig. 1A). Likewise, there were no differences between conditions when males (t(14) = 1.87, ns) and females (t(16) = 0.36, ns) were analyzed separately. Low variability among mice in the sleep disruption condition suggests that minor individual differences in the amount of stimulation received did not produce substantive physiological differences by reflected as by CORT levels. These data indicate that neither male nor female mice perceived the gentle stimulation method of sleep disruption as stressful. In contrast, for the sweeper bar method, when sexes were combined, CORT levels were significantly elevated in mice that received sleep disruption (N = 18; 9 males, 9 females) compared to controls (N = 17; 9 males, 8 females) (t(33) = 3.99, P = 0.0003) (Fig. 1B). This was also seen when males (t(16) = 3.14, P = 0.0064) and females (t(15) = 2.4, P = 0.03) were analyzed separately. These data indicate that both male and female mice perceived the sweeper bar method of sleep disruption as stressful. Considering these data, we selected the gentle stimulation method of sleep disruption for further studies, with the justification that a stress-free approach would provide a clearer signal and enable more straightforward data interpretation.
A For the gentle stimulation method, CORT levels in sleep-disrupted ice did not differ from those in control (undisturbed) mice when data from the sexes were combined (left) or analyzed separately (right). B For the sweeper bar method, CORT levels were significantly higher in sleep-disrupted mice compared to controls when data from the sexes were combined (left) or analyzed separately (right). N’s = 8–10/sex/group, *P < 0.05, **P < 0.01, ns not significant, t-tests.
Sleep disruption prior to fear recall tests reduces expression of conditioned fear
Confirming previous work [8, 45], our Fear Conditioning regimen (Fig. 2A) produced progressive increases in freezing behavior, our operational measure of fear. During Fear Conditioning (Day 3)—but prior to sleep disruption—mice did not differ by treatment assignment (N = 14/condition; 7 male, 7 female), as would be expected before our experimental intervention (Fig. 2B). A 2-way ANOVA revealed a main effect of Trials (F(5,130) = 34.69, P < 0.0001), but no main effect of Sleep Condition (F(1,26) = 0.16, ns) nor Trial x Sleep Condition interaction (F(5,130) = 0.21, ns). Bonferroni comparisons revealed significant increases in freezing during trials 3–5 compared to baseline (BL) (P’s < 0.0001) and trials 1–2 (P’s < 0.001). Additionally, freezing during trial 5 was significantly higher than trial 3 (P = 0.03). A similar pattern was seen when sexes were analyzed separately: there was a significant main effect of Trials in males (F(5,60) = 16.14, P < 0.0001) (Fig. 2C) and females (F(5,60) = 16.89, P < 0.0001) (Fig. 2D), and no main effect of Sleep Condition or interaction. In both males and females, post hoc tests revealed significant increases in freezing during trials 3–5 when compared to BL (P’s < 0.001) and trial 1 (P’s < 0.01). In males, freezing was also increased during trials 3-5 compared to trial 2 (P’s < 0.01), whereas in females, freezing during trials 4–5 was increased compared to trial 2 (P’s < 0.01). These data indicate that our Fear Conditioning regimen produces comparable fear acquisition in male and female mice.
A Schematic of the experimental (“A-B-B”) design. B Fear conditioning develops with repeated pairing of the CS (tone) and UCS (footshock), reflected by progressive increases in freezing, in groups where sexes are combined or when data from (C) males or (D) females are analyzed separately. E In Fear Recall tests, fear responses diminish progressively over trials, but sleep-disrupted mice showed reduced responses immediately, even without extinction trials. This pattern was seen when sexes are combined or data from (F) males or (G) females are analyzed separately. H In Extinction Recall tests, sleep-disrupted mice continued to show reduced responses, even without further extinction trials, helping to rule out non-specific effects of sleep disruption in the Fear Recall tests on the previous day. This pattern was seen when sexes are combined or (I) males or (J) females are analyzed separately. Combined N’s = 14/condition, individual N’s = 7/sex, ++P < 0.01, main effect of trials; *P < 0.05, **P < 0.01 between-group differences.
Immediately after 6-h sleep disruption (Day 4), mice underwent Fear Recall testing—which also serves as Extinction training—with 15 presentations of the CS without the US (footshock) in Context B. For clarity, data were consolidated into 3-blocks of 5 CS presentations (Fig. 2E). A 2-way ANOVA of data with sexes combined revealed main effects of Trial Block (F(2, 52) = 20.45, P < 0.0001) and Sleep Condition (F(1, 26) = 19.46, P = 0.0002), but no Trial Block x Sleep Condition interaction (F(2, 52) = 0.68, ns). Bonferroni post hoc comparisons revealed significant reductions in freezing during trial blocks 2–3 compared to trial block 1 (P’s < 0.001), indicating that fear decreased across the test session. Subsequent tests revealed less freezing in sleep-disrupted mice compared to controls on trial blocks 1 (P < 0.0001), 2 and 3 (P’s = 0.0018). A similar pattern was seen when the sexes were analyzed separately. In males, there were main effects of Trial Block (F(2, 24) = 8.84, P = 0.0013) and Sleep Condition (F(1, 12) = 12.52, P = 0.004), with reduced freezing during trial blocks 2 (P = 0.02) and 3 (P = 0.0014) compared to trial block 1, and lower levels of freezing in sleep-disrupted mice compared to controls during all trial blocks (P’s < 0.05) (Fig. 2F). In females, there were also main effects of Trial Block (F(2, 24) = 11.8, P = 0.0003) and Sleep Condition (F(1, 12) = 7.39, P = 0.019) (Fig. 2G). Like males, females displayed significantly reduced freezing in trial blocks 2 (P = 0.036) and 3 (P = 0.0002) compared to trial block 1, and lower levels of freezing in sleep-disrupted mice compared to controls on trial block 2 (P = 0.044). Importantly, no main effect of sleep condition was seen during inter-trial intervals (F(1, 26) = 0.88, ns), suggesting that sleep disruption did not produce non-specific effects on locomotor activity that would be incompatible with freezing behavior (not shown). These data indicate that sleep disruption produces immediate reductions in freezing, even prior to extinction training, with minimal qualitative differences between sexes.
On the following day (Day 5), mice were returned to Context B for Extinction Recall, which was performed and analyzed exactly as Fear Recall/Extinction. Consistent with the Fear Recall, a 2-way ANOVA found main effects of Trial Block (F(2, 52) = 42.32, P < 0.0001) and Sleep Condition (F(1, 26) = 17.94, P = 0.0003), but no Trial Block x Sleep Condition interaction (F(2, 52) = 2.99, ns) (Fig. 2H). Again, post hoc tests of Trial Blocks revealed significant reductions in freezing during blocks 2–3 compared to trial block 1 (P’s < 0.0001), indicating further extinction of fear. Bonferroni’s multiple comparisons revealed significantly reduced freezing in sleep-disrupted mice compared to controls on all trial blocks: 1 (P < 0.0001), 2 (P = 0.003), and 3 (P = 0.03). A similar pattern was seen when sexes were analyzed separately. In males, there were main effects of Trial Block (F(2, 24) = 18.41, P < 0.0001) and Sleep Condition (F(1, 12) = 10.83, P = 0.0064), with reduced freezing during trial blocks 2–3 compared to 1 (P’s < 0.0001), and lower levels of freezing in sleep-disrupted mice compared to controls during Trial Block 1 (P = 0.0014) and 2 (P = 0.039) (Fig. 2I). In females, there were again main effects of Trial Block (F(2, 24) = 26.29, P < 0.0001) and Sleep Condition (F(1, 12) = 6.43, P = 0.026). Females also displayed significantly reduced freezing in trial blocks during trial blocks 2–3 (P’s < 0.001) compared to trial block 1 and on trial block 3 compared to trial block 2 (P = 0.029), as well as lower levels of freezing in sleep-disrupted mice compared to controls on trial block 1 (P = 0.025). These data indicate that the effects of sleep disruption on expression of conditioned fear are sustained, while also mitigating concerns that reductions in fear expression during Fear Recall the previous day reflect a transient artifact of acute sleep disruption.
Sleep disruption does not affect reinstatement of fear or sleep patterns
To examine if our sleep disruption regimen might be interfering with a late-phase memory consolidation, we tested for fear reinstatement after sleep disruption-enhanced Fear Recall (Fig. 3A,B) [49, 53]. Mice (n = 14/condition, 8 males, 6 females) received Fear Conditioning (Day 3), sleep disruption and Fear Recall/Extinction (Day 4) as previously, which replicated the finding of reduced fear expression. A 2-way ANOVA of Fear Conditioning Trials x Sleep Condition prior to sleep disruption found a main effect of Trials, (F(5, 130) = 89.73, P < 0.0001), but no main effect of Sleep Condition (F(1, 26) = 0.04, ns) nor Trial x Sleep Condition interaction (F(5, 130) = 0.17, ns). Post hoc comparisons found significantly increased freezing on trials 3-5 compared to BL, and trials 1 and 2 (P’s < 0.0001), and on trials 4 and 5 compared to trial 3 (P’s < 0.001). Immediately after sleep disruption, mice underwent Fear Recall testing in Context B. A 2-way ANOVA again showed a significant main effect of Sleep Condition (F(1, 26) = 5.24, P = 0.030), and a main effect of Trial Block (F(2, 52) = 26.04, P < 0.0001), indicating extinction of fear across the session. There was no significant Sleep Condition x Trial Block interaction (F(2, 52) = 1.06, ns). Post-hoc tests found that controls significantly differed from sleep-disrupted mice on trial block 1 (P = 0.03), and that freezing significantly reduced on trial blocks 2 (P = 0.031) and 3 (P < 0.0001) compared to trial block 1, and on trial block 3 compared to trial block 2 (P = 0.0001). On Day 5, mice were placed in Context C and received 2 un-signaled (reminder) footshocks. On Day 6, mice were returned to Context C and presented 5 CS presentations, equivalent to trial block 1 on Day 4. As evidence of reinstatement, a 2-way ANOVA revealed a main effect of Trial (F(1, 26) = 47.44, P < 0.0001), with significantly increased freezing to the CS presentations (averaged) compared to BL. There was no main effect of Sleep Condition (F(1, 26) = 3.78, ns) or Trial x Sleep Condition interaction (F(1, 26) = 0.023, ns), indicating that sleep-disrupted mice retained the same initial fear response to the CS as controls and suggesting intact consolidation of the fear memories produced by the original fear conditioning session.
A Behavioral timeline for fear reinstatement experiment. B Freezing response during fear conditioning (FC) at baseline (BL) and over 5 CS-US pairings, extinction (Ext) in trial blocks of 5 CS presentations and during a reinstatement test at BL and during 5 CS presentations (averaged). +P < 0.05, ++P < 0.01 indicates main effect of Trials/Trial Block; compared to BL (FC and Test) or Trial Block 1 (Ext). *P < 0.05 indicates main effect of Sleep Condition. n = 14/condition, 8 males, 6 females. C Representative EEG and EMG traces for wake, SWS and REM vigilance states. D There are no significant changes in sleep architecture during the 18 h after fear conditioning (N = 29, 15 males, 14 females).
Considering the effect of sleep disruption on the expression of conditioned fear, we assessed vigilance states to confirm that sleep patterns are not fundamentally different during the 18 h after Fear Conditioning (N = 29, 15 males, 14 females). Vigilance state durations during the 18 h after Fear Conditioning were compared to the same 18 h time period following context habituation the previous day (Day 2) (Fig. 3C). Within-subject analyses revealed no changes in duration of wakefulness (Wake; t(28) = 1.54, ns), slow wave sleep (SWS; t(28) = 1.85, ns), or rapid eye movement sleep (REM; t(28) = 0.97, ns). Results were similar when males and females were analyzed separately (not shown). These findings indicate that mice sleep normally—without evidence of insomnia—in the hours following Fear Conditioning, when sleep can enhance memory consoliation [43, 54].
Sleep disruption produces changes in BDNF expression
We next explored whether our sleep disruption regimen might be triggering neuroplasticity within fear-related circuits that leads to suppression of fear responses. Since work from our group and others have implicated BDNF in regulation of fear expression [25, 49], we examined the effects of 6-h gentle stimulation sleep disruption on BDNF mRNA in brain areas implicated in fear and trauma memory processing, including the BLA, PFC, HIP, and BNST (see Supplemental Fig. 1 for depiction of the tissue-punch dissections). These studies examined gene expression immediately following sleep disruption, without the confounding effects of fear conditioning, in males (N = 8/condition) and females (N = 8/condition). For BLA, one male (control) and one female (sleep-disrupted) were identified as statistical outliers and excluded from analyses. Between-group analysis combining sexes revealed significant increases in BDNF mRNA (t(28) = 3.23, P = 0.0032) after sleep disruption (Fig. 4A). Similar results were seen when sexes were analyzed individually: BDNF mRNA levels were higher following sleep disruption in males (t(13) = 2.36, P = 0.035) and females (t(13) = 2.19, P = 0.047). For PFC, one male (control) was excluded as a statistical outlier. Analysis combining sexes revealed significant increases in BDNF mRNA after sleep disruption (t(29) = 2.20, P = 0.036) (Fig. 4B), however, analysis of the sexes individually revealed a sex-dependent effect. Sleep disruption did not alter BDNF mRNA levels in males (t(13) = 0.55, ns), whereas it elevated levels in females (t(14) = 2.38, P = 0.032). For BNST, combining sexes revealed no effects of sleep disruption (t(30) = 1.63, ns) (Fig. 4C). However, analysis of the sexes individually revealed a sex-dependent effect opposite to that seen in the PFC: sleep disruption elevated BDNF mRNA levels in males (t(14) = 3.03, P = 0.0089), but not in females (t(14) = 0.05, ns). For HIP, one male control was identified as a statistical outlier and excluded. Analyses found no effect of sleep disruption on BDNF mRNA, regardless of whether sexes were combined (t(29) = 0.15, ns) (Fig. 4D), or separated: males (t(13) = 0.49, ns), females (t(14) = 0.31, ns). Similar non-significant results were found in ventral HIP (Supplemental Fig. 2). To address the possibility that our gentle stimulation regimen includes elements that represent environmental enrichment, we conducted parallel studies in males during the dark phase and found no effects on BDNF expression in BLA or plasma CORT levels (Supplemental Fig. 3). These data demonstrate that our sleep disruption regimen can trigger increases in BDNF gene expression in brain areas implicated in fear expression, but of the areas examined in this report, only the changes in the BLA were consistent across sexes.
A In the BLA, sleep disruption produced significant increases in BDNF gene expression when sexes are combined (left) or analyzed separately (right). B There were also significant increases in the PFC when sexes were combined, but this effect was carried by females, with no effects in males. C In the BNST, increases were seen in males only, with no effects in females alone or when the sexes were combined. D There were no effects in the HIP. Combined N’s = 16, individual N’s = 8/sex, ns not significant, *P < 0.05, **P < 0.01, t-tests.
Discussion
We report that a delayed, non-stressful form of sleep disruption can reduce the expression of conditioned fear in mice. A novel and distinguishing feature of these studies is that the sleep disruption was performed on the day after fear conditioning, following an 18-h period when the mice were left undisturbed and able to sleep normally. While there have been other reports that sleep disruption can disrupt fear behaviors, those have generally demonstrated that it is necessary to perform the sleep disruption immediately, beginning within minutes after fear conditioning [6, 7]. The effects were similar in males and females, suggesting that they involve parallel mechanisms across sexes. Importantly, the effects were seen in recall tests conducted immediately following sleep disruption as well as the day after, ruling out the possibility that is due to a transient, non-specific artifact of the sleep disruption regimen itself. In addition, we demonstrate that mice do not perceive the sleep disruption regimen as stressful, which rejects the possibility that the effects are due to stress interactions. This approach has translational relevance and benefits: the ability to reduce fear at time points that do not require intervention immediately after trauma exposure would have considerable therapeutic potential, and if it someday becomes possible to utilize sleep disruption in traumatized humans for treating conditions like PTSD, procedures that minimize stress would be preferable.
Previous reports describing the effects of sleep disruption on expression of conditioned fear generally attribute reductions in fear behavior to disruption in memory consolidation. Synaptic consolidation—which is required for a lasting memory—is thought to begin immediately following learning and complete within a few hours [13]. Studies in mice enable exquisite resolution of this effect, showing reductions the expression of fear memories if sleep disruption occurs during the 0–5 h period after Fear Conditioning, but no effect if delayed until the 5–10 h period [7]. Several observations suggest that the effect of our sleep disruption effect is not due to disruption of consolidation. Our sleep disruption regimen began the next day, 18 h after Fear Conditioning. During the 18-h period, the mice are left undisturbed and allowed to sleep, which is known to enhance memory consolidation [7, 43, 54]. Using mice implanted with wireless telemetry devices that enable continuous monitoring of sleep architecture, we show that sleep after Fear Conditioning is essentially normal; if anything, there are nominal (though not statistically significant) increases in REM sleep, which in humans has been associated with enhanced consolidation of emotional memories [55]. Our work also shows comparable reinstatement of fear responses in sleep-disrupted and control mice, providing evidence that fear to the CS was learned and consolidated prior to sleep disruption [53]. In addition, we did not observe differences in freezing behavior during inter-trial intervals in Fear Recall, ruling out the possibility that sleep disruption produces non-specific locomotor-activating effects.
There are potential explanations for these findings that do not involve disruption of fear memory consolidation. It has been reported that administration of neurotrophic factors can reduce the expression of fear, effectively serving as a “substitute” for extinction training. In a seminal study in rats, it was shown that infusion of BDNF directly into the PFC on the day after Fear Conditioning reduces expression of fear, even without extinction training [49]. Interestingly—and similar to our current findings—complete reductions in fear were seen immediately (in the first trials), as opposed to enhancement of a more prototypical extinction response in which fear responses decay with subsequent trials. While the neural mechanisms of this effect are not completely understood, there is evidence that PFC projections to BLA are involved in fear inhibition and extinction [9]. Specifically, projections from the PFC provide strong and preferential input to BLA “Extinction/Fear-off” cells [9, 56, 57]. If the BLA is downstream of other brain areas implicated in fear extinction, effects occurring in this region would prevail over those in upstream regions. This formulation may provide insight into why sleep disruption-induced increases in BDNF mRNA within the BLA align so closely with reductions in fear expression across sexes. While effects on BDNF were also seen in other brain areas (PFC, BNST), only the effects in the BLA were consistent in males and females, matching the consistency across sexes in the behavioral studies. Our finding of increased BDNF gene expression in BLA aligns with previous data supporting critical roles for BDNF and its receptor (TrkB) within this region in fear behavior and extinction [9, 25, 26, 50, 58]. An intriguing possibility is that BDNF systems are involved in active processes occurring within the BLA itself that enable rapid transitions between defensive and exploratory behavior. By extension, transitions between high and low fear are triggered by switches in the balance of activity in two distinct populations of BLA neurons, conceptualized as “Fear” and “Extinction” cells [9, 56, 57]. Together, our behavioral and molecular findings provide the basis for mechanistic studies that explore the possibility that our sleep disruption regimen recruits BDNF-dependent processes in the BLA that actively suppress fear memories, thereby substituting for extinction training.
It is possible that our method of placing objects in the cage during the gentle stimulation procedure could represent environmental enrichment, adding complexity to interpretation of our findings. While published work indicates that neuroplasticity is seen following long-term environmental enrichment [59], it is important to emphasize that the putative enrichment portion of our procedure (placing objects into the cage) is less than 4 h. In our vivarium, mice receive cage changes and new nesting materials on a regular weekly schedule, so the initial ~2 h element of the procedure is unlikely to represent environmental enrichment. In addition, environmental enrichment is known to prominently affect the hippocampus [60]. However, we report that the hippocampus is the only brain region studied where gentle stimulation fails to produce any effect on BDNF. We also show that performing the putative enrichment element during the dark phase—when it does not promote sleep disruption—has no effect on BDNF in the BLA or plasma CORT levels. These findings provide strong evidence that stress-free sleep disruption differs from enrichment and represents the element of our gentle stimulation procedure that drives the behavioral and molecular effects.
In summary, we have discovered a sleep disruption regimen in mice that can be used the day after Fear Conditioning to reduce expression of fear memories. These findings provide the basis for future work designed to understand the basic molecular, cell-type specific, and circuit mechanisms underlying threat regulation in mammalian amygdala. Furthermore, this work may also provide a basis for new (non-pharmaceutical) therapeutic approaches that could be rapidly translated to the clinic and transformational with respect to the prognosis for people with conditions such as PTSD, even as neural mechanisms are dissected and thoroughly characterized.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington D.C.: American Psychiatric Association; 2013.
Watling J, Pawlik B, Scott K, Booth S, Short MA. Sleep loss and affective functioning: more than just mood. Behav Sleep Med. 2017;15:394–409.
Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117.
Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;433:104–15.
Raven F, Van der Zee EA, Meerlo P, Havekes R. The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Med Rev. 2018;39:3–11.
Cohen H, Ephraim-Oluwanuga OT, Akintunde OT, Gureje O, Matar MA, Todder D, et al. The potential beneficial effect of sleep deprivation following traumatic events to preventing PTSD: Review of current insight regarding sleep, memory, and trauma resonating with ancient rituals-Àìsùn Oku (African) and Tsuya (Japanese). Neuropsychopharmacol Rep. 2023;43:2–11.
Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76.
Maddox SA, Hartmann J, Ross RA, Ressler KJ. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron. 2019;102:60–74.
Ressler KJ, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol. 2022;18:273–88.
Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus‐mediated contextual learning but not amygdala‐mediated cued learning in rats. Eur J of Neuroscience. 2004;19:3121–4.
Ravassard P, Hamieh AM, Joseph MA, Fraize N, Libourel P-A, Lebarillier L, et al. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex. 2016;26:1488–1500.
Hunter AS. REM deprivation but not sleep fragmentation produces a sex-specific impairment in extinction. Physiol Behav. 2018;196:84–94.
Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86.
Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–6.
Frey U, Morris RG. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37:545–52.
Davis HP, Rosenzweig MR. Recovery as a function of the degree of amnesia due to protein synthesis inhibition. Pharmacol Biochem Behav. 1978;8:701–10.
Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–77.
Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM. A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J Neurosci. 2014;34:12547–59.
McEwen BS, Karatsoreos IN. Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep Med Clin. 2015;10:1–10.
Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210.
Anisman H, Zacharko RM. Behavioral and neurochemical consequences associated with stressors. Ann N Y Acad Sci. 1986;467:205–25.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Schwabe L, Hermans EJ, Joëls M, Roozendaal B. Mechanisms of memory under stress. Neuron. 2022;110:1450–67.
Wu M, Zhang X, Feng S, Freda SN, Kumari P, Dumrongprechachan V, et al. Dopamine pathways mediating affective state transitions after sleep loss. Neuron. 2024;112:141–154.e8.
Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–92.
Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–2.
Ehlen JC, Hesse S, Pinckney L, Paul KN. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One. 2013;8:e62205.
Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–85.
Kostin A, Alam MA, Siegel JM, McGinty D, Alam MN. Sex- and age-dependent differences in sleep-wake characteristics of Fisher-344 Rats. Neuroscience. 2020;427:29–42.
Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150110.
Foilb AR, Taylor-Yeremeeva EM, Fritsch EL, Ravichandran C, Lezak KR, Missig G, et al. Differential effects of the stress peptides PACAP and CRF on sleep architecture in mice. NPP—Digit Psychiatry Neurosci, 2024;2;3.
Koehl M, Battle SE, Turek FW. Sleep in female mice: a strain comparison across the estrous cycle. Sleep. 2003;26:267–72.
Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O’Hara BF, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci USA. 2006;103:7118–23.
Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–23.
Lord C, Sekerovic Z, Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol. 2014;62:302–10.
Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 1980;185:385–98.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60.
Shansky RM. Sex differences in PTSD resilience and susceptibility: challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65.
Cover KK, Maeng LY, Lebrón-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry. 2014;4:e422.
Straus LD, Norman SB, Risbrough VB, Acheson DT, Drummond SPA. REM sleep and safety signal learning in posttraumatic stress disorder: a preliminary study in military veterans. Neurobiol Stress. 2018;9:22–28.
Kobayashi I, Mellman TA. Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behav Sleep Med. 2012;10:180–90.
Kobayashi I, Cowdin N, Mellman TA. One’s sex, sleep, and posttraumatic stress disorder. Biol Sex Differ. 2012;3:29.
Pace-Schott EF, Germain A, Milad MR. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015;141:835–57.
Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24:1–15.
McCullough KM, Chatzinakos C, Hartmann J, Missig G, Neve RL, Fenster RJ, et al. Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning. Nat Commun. 2020;11:5180.
Missig G, Mokler EL, Robbins JO, Alexander AJ, McDougle CJ, Carlezon WA. Perinatal immune activation produces persistent sleep alterations and epileptiform activity in male mice. Neuropsychopharmacology. 2018;43:482–91.
McCullough KM, Missig G, Robble MA, Foilb AR, Wells AM, Hartmann J, et al. Nucleus accumbens medium spiny neuron subtypes differentially regulate stress-associated alterations in sleep architecture. Biol Psychiatry. 2021;89:1138–49.
Wells AM, Ridener E, Bourbonais CA, Kim W, Pantazopoulos H, Carroll FI, et al. Effects of chronic social defeat stress on sleep and circadian rhythms are mitigated by kappa-opioid receptor antagonism. J Neurosci. 2017;37:7656–68.
Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–90.
Heldt SA, Zimmermann K, Parker K, Gaval M, Weinshenker D, Ressler KJ. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34:2444–50.
Ressler RL, Maren S. Synaptic encoding of fear memories in the amygdala. Curr Opin Neurobiol. 2019;54:54–59.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
Goode TD, Maren S. Animal models of fear relapse. ILAR J. 2014;55:246–58.
Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9.
Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66.
Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–6.
Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33:10396–404.
Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–806.
Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52.
Zhang T-Y, Keown CL, Wen X, Li J, Vousden DA, Anacker C, et al. Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nat Commun. 2018;9:298.
Funding
P50MH115874 (to WAC/KJR), R01MH063266 (to WAC), and a Rappaport Mental Health Research Scholar Award (to ARF).
Author information
Authors and Affiliations
Contributions
ARF, KJR, and WAC designed the studies; ARF, EMTY, and BDS conducted the experiments; ARF and WAC performed the statistical analyses; ARF and WAC wrote early drafts of the manuscript; and all authors provided editing and approval of the final version.
Corresponding author
Ethics declarations
Competing interests
WAC and KJR are members of the NPP editorial board. WAC is a consultant for Psy Therapeutics and has sponsored research agreements with Cerevel Therapeutics and Delix Therapeutics. KJR has performed scientific consultation for Bioxcel, Bionomics, Acer, Takeda, and Jazz Pharma; serves on Scientific Advisory Boards for Sage and the Brain Research Foundation, and he has received sponsored research support from Takeda, Brainsway, and Alto Neuroscience. ARF, EMTY, and BDS report no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foilb, A.R., Taylor-Yeremeeva, E.M., Schmidt, B.D. et al. Acute sleep disruption reduces fear memories in male and female mice. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01978-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01978-0
- Springer Nature Switzerland AG