Abstract
People with depression and other neuropsychiatric disorders can experience motivational dysfunctions such as fatigue and anergia, which involve reduced exertion of effort in goal-directed activity. To model effort-related motivational dysfunction, effort-based choice tasks can be used, in which rats can select between obtaining a preferred reinforcer by high exertion of effort vs. a low effort/less preferred option. Preclinical data indicate that dopamine transport (DAT) inhibitors can reverse pharmacologically-induced low-effort biases and increase selection of high-effort options in effort-based choice tasks. Although classical DAT blockers like cocaine can produce undesirable effects such as liability for misuse and psychotic reactions, not all DAT inhibitors have the same neurochemical profile. The current study characterized the effort-related effects of novel DAT inhibitors that are modafinil analogs and have a range of binding profiles and neurochemical actions (JJC8-088, JJC8-089, RDS3-094, and JJC8-091) by using two different effort-related choice behavior tasks in male Sprague-Dawley rats. JJC8-088, JJC8-089, and RDS3-094 significantly reversed the low-effort bias induced by the VMAT-2 inhibitor tetrabenazine, increasing selection of high-effort fixed ratio 5 lever pressing vs. chow intake. In addition, JJC8-089 reversed the effects of tetrabenazine in female rats. JJC8-088 and JJC8-089 also increased selection of high-effort progressive ratio responding in a choice task. However, JJC8-091 failed to produce these outcomes, potentially due to its unique pharmacological profile (i.e., binding to an occluded conformation of DAT). Assessment of a broad range of DAT inhibitors with different neurochemical characteristics may lead to the identification of compounds that are useful for treating motivational dysfunction in humans.
Similar content being viewed by others
Introduction
Motivational symptoms such as psychomotor slowing, fatigue, anergia, and reduced exertion of effort are debilitating symptoms of major depressive disorder (MDD) and other neuropsychiatric conditions [1,2,3,4,5]. The severity of the motivational symptoms of depression is correlated with problems in daily functioning, social life, and employment [6], making it important to focus on for a more complete remission of symptoms. However, motivational dysfunctions are relatively treatment resistant, and commonly prescribed antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are relatively ineffective at treating motivational symptoms. Moreover, SSRIs have been shown to exacerbate these symptoms [7, 8]. Drugs that stimulate dopamine (DA) transmission such as the DA and norepinephrine transport inhibitor bupropion (Wellbutrin) have been reported to reduce motivational symptoms such as fatigue in patients with MDD [7]. Similarly, the wakefulness agent and DA transport (DAT) inhibitor modafinil was found to reduce depressive symptoms [9] as well as avolition (goal-directed behavior) in schizophrenic patients [10]. Thus, therapeutics that target DAT have been suggested to improve motivational dysfunctions that are observed in multiple psychiatric disorders [1,2,3, 5, 11, 12].
To promote the development of improved therapeutics, preclinical research using paradigms that employ effort-related decision-making tasks can be used as models of effort-related motivational symptoms. These paradigms typically offer a choice between a high-effort instrumental response to obtain a preferred reinforcer versus a low-effort/low-reward alternative [1, 2, 13, 14]. Examples of these animal models of effort-based choice behavior include the fixed ratio (FR) 5/chow feeding choice task [13] and the progressive ratio (PROG)/chow feeding choice task [15,16,17,18]. With the FR5/chow feeding choice task, well-trained rats get most of their food by FR5 lever pressing reinforced by high carbohydrate food pellets, while eating small amounts of the concurrently available but less preferred lab chow. With the PROG/chow choice task, rats prefer to lever press to a certain extent and then reach a break point at which they switch to the concurrently available lab chow when the work requirement is too high [15]. Pharmacological manipulations such as DA D1 or D2 receptor antagonism and neurotoxic or pharmacological depletion of DA can induce a low-effort bias by decreasing the selection of high-effort options in effort-based choice tasks [2, 13, 16, 19,20,21,22,23,24].
Tetrabenazine (TBZ) is a vesicular monoamine transporter type-2 (VMAT-2) inhibitor that increases depressive symptoms including fatigue and apathy in clinical studies [1, 25]. TBZ reversibly depletes neostriatal and accumbens DA in rats [23, 26], and induces low effort bias in effort-related decision-making tasks [16, 23, 27] in male and female rats [24]. Consistent with the clinical literature, SSRIs were found to be ineffective at reversing the effects of TBZ in rats tested on effort-based choice tasks [28, 29]. However, bupropion and DAT inhibitors such as methylphenidate, modafinil, and various modafinil analogs (CE-123, CE-158, MK-26) can reverse the effects of TBZ and enhance the selection of high-effort/high-reward options [1, 3, 23, 28,29,30,31,32,33,34]. Some of these DAT inhibitors also increase the selection of high-effort options in rats tested on the PROG/chow feeding choice task when administered alone [17, 18, 32,33,34]. Overall, these studies illustrate the potential utility of DAT inhibitors as treatments for effort-related motivational dysfunction.
Classical DAT inhibitors like cocaine, as well as substrates that combine DAT inhibition and release stimulation such as amphetamines, have been associated with unfavorable effects such as substance use liability [35, 36] and psychotic symptoms [37, 38], constraining their therapeutic utility. However, not all compounds binding to DAT exhibit the same neurochemical characteristics as cocaine. Benztropine, GBR 12909, and modafinil have been identified as atypical DAT inhibitors that have neurochemical and behavioral characteristics distinct from those of cocaine, and thus could be used as parent compounds for the development of novel drugs [39,40,41,42,43,44]. Atypical DAT inhibitors differ from cocaine in terms of the kinetics of their interaction with the DAT, and their different binding characteristics, resulting in varying behavioral and neurochemical actions [36, 45,46,47,48]. Based on research using mutant mice with alterations of the DAT that influence the conformational state of the protein, as well as computational modeling, Schmitt and Reith [43] concluded that the binding of modafinil gives rise to an inward facing conformation of the DAT, which is different from the open, outward facing conformation that cocaine prefers. Modafinil reversed the low-effort bias induced by TBZ [3], and subsequent research showed that modafinil analogs that are more selective than modafinil for the DAT compared to the norepinephrine or 5-HT transporters also reverse the effects of TBZ and increase selection of high effort PROG lever pressing [30,31,32]. Researchers at the National Institute on Drug Abuse (NIDA) are interested in studying atypical DAT inhibitors, including modafinil analogs, for their potential as treatments for psychostimulant use disorders [36, 40, 49]. For example, JJC8-091 binds to an occluded conformation of the DAT, in contrast to the open conformation that cocaine prefers, and preclinical studies suggest that this compound could be useful for treating stimulant use disorders [36, 40]. Similarly, JJC8-089 was reported to have reduced substance use liability in animal studies when compared to cocaine-like drugs [48]. In contrast, JJC8-088 shows signs of being more cocaine-like in its binding and overall neurochemical and behavioral profile [36]. This spectrum of properties across compounds may offer the potential for finding compounds with an optimal balance between moderate increases in extracellular DA, heightened effort-based motivation, and minimal side effects at therapeutic doses.
Fatigue is the most common psychiatric complaint in general medicine [6], and effort-related motivational symptoms such as fatigue and anergia pose a challenge in achieving more complete remission for MDD and other disorders. Consequently, there is a compelling imperative to use preclinical research to explore novel therapeutic strategies targeting motivational symptoms. The present studies were undertaken to assess the efficacy of novel modafinil analogs that are DAT inhibitors with a broad range of neurochemical and behavioral characteristics, using two tests of effort-based choice behavior, the FR5/chow feeding choice task and the PROG/chow feeding choice task. It was hypothesized that these compounds would show a range of behavioral effects, with some being able to increase selection of high-effort activities due to strong DAergic activity (e.g. JJC8-088), and others being less effective (e.g., JJC8-091).
Materials and methods
Subjects
A total of 87 adult male and 7 adult female Sprague Dawley Rats (Envigo Sprague Dawley, Indianapolis, IN, USA) were pair-housed in a 23 °C colony with a 12-h light/dark cycle. The animals were initially restricted to 85% of their free-feeding weight and then allowed modest growth throughout the experiment. Their weights were recorded and supplemental lab chow was provided as needed, and water was provided ad libitum. All animal procedures were approved by the University of Connecticut Institutional Animal Care and Use Committee (IACUC).
Pharmacological agents and selection of doses
TBZ (Tocris Bioscience; 9,10-dimethoxy-3-(2-methylpropyl)−1,3,4,6,7, 11b hexahydro benzo[a]quinolin-2-one) was dissolved in 20% DMSO, 80% 0.9% saline, and titrated with HCl (pH around 4.5). The DMSO/saline solution was administered as the vehicle control for TBZ. The dose selection for male rats (1.0 mg/kg) was based on previous studies (e.g. [3, 31,32,33]). The 2.0 mg/kg dose used in female rats was based on recent work demonstrating that 1.0 mg/kg was ineffective in females, while 2.0 mg/kg was required for producing both a shift in choice behavior and in increase in nucleus accumbens cFos expression in females [24]. JJC8-088, JJC8-089, JJC8-091, and RDS3-094 were synthesized as previously reported [45, 50] in the Medicinal Chemistry section, NIDA Intramural Research Program, and were dissolved in 10% DMSO, 15% Tween-80, and 75% 0.9% saline. As vehicle for each NIDA compound, a solution consisting of 10% DMSO, 15% Tween-80, and 75% 0.9% saline was used. The doses of all NIDA compounds were based on pilot experiments conducted in our laboratory, previous behavioral studies [48], and information about their relative affinities for DAT [45, 50], with a 30-min lead time for each compound.
Behavioral procedures
FR5/chow feeding choice task
Behavioral sessions (30 min/day, 5 days/week) were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT, USA) in which rats were trained to lever press for reinforcement pellets (Bio-Serv, Flemington, NJ, USA) high-carbohydrate 45 mg pellet. Rats initially had magazine training, which was followed by lever press training with an FR1 reinforcement schedule for one week. Next, rats were trained on FR5 schedule for five weeks followed by the introduction of lab chow to the chamber (Laboratory Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO; about 17–21 g). FR5/chow feeding choice training continued for another five weeks, and drug testing began after this training period was over. At the end of each session, the number of lever presses and the amount of chow consumed (including spillage) were recorded.
PROG/chow feeding choice task
Behavioral sessions (30 min/day, 5 days/week) were conducted in operant chambers (28 × 23 × 23 cm; Med Associates, Fairfax, VT, USA) in which rats were trained to lever press for pellet reinforcement (Bio-Serv, Flemington, NJ, USA) high-carbohydrate 45 mg pellets. Rats initially had magazine training, which was followed by lever press training with an FR1 reinforcement schedule for one week. Next, rats were trained on the PROG schedule [15, 16, 32] in which the ratio started at FR1 and then was increased by 1 additional response every time 15 reinforcements were obtained (FR1 × 15, FR2 × 15, etc.). The response lever was deactivated (i.e., timeout) for the rest of the session whenever 2 minutes elapsed without a completed ratio. After nine weeks of PROG training, lab chow was introduced into the chamber (Laboratory Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO; about 17–21 g). PROG/chow feeding choice training continued for another five weeks and drug testing began after this training period was over. At the end of each session, the number of lever presses and chow intake (including spillage) were recorded.
Experimental design: FR5/chow feeding choice
To assess the effects of the modafinil analogs when co-administered with TBZ on the FR5/chow feeding choice task, 31 trained male rats that were trained on FR5/chow feeding choice procedure were administered either TBZ or vehicle and doses of their respective analog (JJC8-088, JJC8-089, JJC8-091, RDS3-094) or vehicle in separate groups of rats for each compound. All the experiments used a repeated measures design in which each rat received each of the five combined drug treatments in a randomized order once per week. For JJC8-088 (n = 7), rats received: TBZ vehicle + JJC8-088 vehicle, 1.0 mg/kg TBZ + JJC8-088 vehicle, 1.0 mg/kg TBZ + 7.5 mg/kg JJC8-088, 1.0 mg/kg TBZ + 15 mg/kg JJC8-088, 1.0 mg/kg TBZ + 30 mg/kg JJC8-088. For JJC8-089 (n = 8) rats received: TBZ vehicle + JJC8-089 vehicle, 1.0 mg/kg TBZ + JJC8-089 vehicle, 1.0 mg/kg TBZ + 3.75 mg/kg JJC8-089, 1.0 mg/kg TBZ + 7.5 mg/kg JJC8-089, 1.0 mg/kg TBZ + 15.0 mg/kg JJC8-089. For RDS3-094 (n = 8) rats received: TBZ vehicle + RDS3-094 vehicle, 1.0 mg/kg TBZ + RDS3-094 vehicle, 1.0 mg/kg TBZ + 3.75 mg/kg RDS3-094, 1.0 mg/kg TBZ + 7.5 mg/kg RDS3-094, 1.0 mg/kg TBZ + 15.0 mg/kg RDS3-094. For JJC8-091 (n = 8) rats received: TBZ vehicle + JJC8-091 vehicle, 1.0 mg/kg TBZ + JJC8-091 vehicle, 1.0 mg/kg TBZ + 15.0 mg/kg JJC8-091, 1.0 mg/kg TBZ + 30.0 mg/kg JJC8-091, 1.0 mg/kg TBZ + 60.0 mg/kg JJC8-091. In an additional study with female rats (n = 7), animals received: TBZ vehicle + JJC8-089 vehicle, 1.0 mg/kg TBZ + JJC8-089 vehicle, 1.0 mg/kg TBZ + 3.75 mg/kg JJC8-089, 1.0 mg/kg TBZ + 7.5 mg/kg JJC8-089, 1.0 mg/kg TBZ + 15.0 mg/kg JJC8-089. All injections were given intraperitoneally in a total volume of 1.0 ml/kg. TBZ or vehicle were administered 120 min before, and all NIDA compounds were administered 30 min before testing.
Experimental design: PROG/chow feeding choice
For the assessment of each modafinil analog on PROG/chow feeding choice procedure, a total of 56 rats that were trained on PROG/chow feeding choice procedure were administered with doses of their chosen modafinil analog (JJC8-088, JJC8-089, RDS3-094, JJC8-091) or vehicle. All compounds were administered 30 min before testing. In each experiment, a repeated measures design was used in which four drug treatments for each modafinil analog were given in a randomized order, once per week, to separate groups of rats. Drug treatments were as follows: JC8-088 (n = 8), VEH, 7.5, 15.0, and 30.0 mg/kg; JJC8-089 (n = 24), VEH, 3.75, 7.5, and 15.0 mg/kg; RDS3-094 (n = 16), VEH, 3.75, 7.5, and 15.0; JJC8-091 (n = 8) VEH, 15.0, 30.0, and 60.0 mg/kg. All injections were given intraperitoneally in a total volume of 1.0 ml/kg.
Data analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS, IBM, US) software 28. Lever pressing and chow intake were analyzed with repeated measures analysis of variance (ANOVA), and F values, probability, and effect size (ηp2) are reported. To follow up a significant ANOVA, planned comparisons using the overall error term and a restricted number of comparisons (the number of treatments minus one [51]); For the TBZ reversal FR5 experiments, comparisons were made vs. TBZ/VEH and in the PROG experiments, the comparisons were vs. VEH. Power analyses were performed with the G*Power tool and used the effects size values from the ANOVA. This analysis revealed that the sample sizes used in cases where statistical significance was obtained were appropriate. However, in the RDS3-094 PROG experiment, the effect size was so low that a very large sample size (n = 64) would have been required (see below). Graphs were made with GraphPad Prism 8, and include individual rat data (open circles) as well as mean + SEM.
Results
Effects of JJC8-088 on FR5/Chow and PROG/chow feeding choice performance
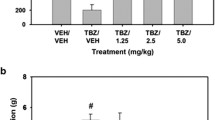
In the JJC8-088 experiment (Fig. 1A, B), there was an overall treatment effect on lever pressing (F(4, 24) = 12.35, p < 0.01, ηp2=0.67). Planned comparisons showed that TBZ/VEH significantly decreased lever pressing compared to VEH/VEH (F (1, 24) = 45.22, p < 0.001), and that the 7.5 and 30.0 mg/kg doses of JJC8-088 plus TBZ significantly increased lever pressing compared to TBZ/VEH (F (1, 24) = 7.82, p < 0.05 and F (1, 24) = 8.39, p < 0.01, respectively). There also was a significant overall treatment effect on chow intake (F (4, 24) = 8.44, p < 0.001, ηp2=0.58). Planned comparisons revealed that TBZ significantly increased chow intake compared to VEH/VEH and increased chow intake (F(1, 24) = 27.68, p < 0.01), and that the 15.0 and 30.0 mg/kg doses of JJC8-088 plus TBZ significantly decreased lever pressing compared to TBZ/VEH (F(1, 24) = 11.74, p < 0.01 and F(1, 24) = 17.31, p < 0.01, respectively).
A Lever presses (mean±SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; *p < 0.05, TBZ/7.5 mg/kg JJC8-088 significantly different from TBZ/VEH; **p < 0.01, TBZ/30.0 mg/kg JJC8-088 significantly different from TBZ/VEH. B Chow intake in grams (mean±SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/15.0 and TBZ/30.0 mg/kg JJC8-088 significantly different from TBZ/VEH. The effects of JJC8-088 on PROG/chow feeding choice procedure during the 30 min session (C, D). C Lever presses (mean±SEM), **p < 0.01, 30.0 mg/kg JJC8-088 significantly different from VEH. D Chow intake in grams (mean ± SEM), **p < 0.01, 15.0 mg/kg, and 30.0 mg/kg JJC8-088 was significantly different from VEH.
The results for the JJC8-088 study on PROG/Chow feeding choice (Fig. 1C, D) showed a statistically significant treatment effect on lever presses (F(3, 21) = 4.34, p = 0.05, ηp2=0.38, Huynh-Fieldt), and planned comparisons showed that there was a significant increase at the 30.0 mg/kg dose (F(1, 21) = 10.74, p < 0.01). There also was a significant overall effect on chow intake (F(3, 21) = 15.95, p < 0.001, ηp2=0.70), with significant decreases seen at the 15.0 and 30.0 mg/kg doses (F(1, 21) = 15.86, p < 0.001 and F(1, 21) = 30.19, p < 0.001, respectively).
Effects of JJC8-089 on FR5/Chow and PROG/Chow feeding choice performance
In the JJC8-089 study (Fig. 2A, B), there was an overall treatment effect on lever pressing (F(4, 28) = 14.22, p < 0.01, ηp2=0.67). Planned comparisons showed that TBZ/VEH significantly decreased lever pressing compared to VEH/VEH (F (1,28) = 43.66, p < 0.01), and that the 7.5 and 15.0 mg/kg doses of JJC8-089 plus TBZ significantly increased lever pressing compared to TBZ/VEH (F(1, 28) = 22.26, p < 0.01 and F (1, 28) = 19.49, p < 0.01, respectively). There was a significant overall treatment effect on chow intake (F(4, 28) = 10.26, p < 0.01, ηp2=0.59). Planned comparisons revealed that TBZ significantly increased chow intake compared to VEH/VEH (F(1, 28) = 17.71, p < 0.001), and that the 7.50 and 15.0 mg/kg doses of JJC8-089 plus TBZ significantly decreased lever pressing compared to TBZ/VEH (F(1, 28) = 11.56, p < 0.01 and F (1,28) = 31.32, p < 0.01, respectively).
A Lever presses (mean±SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/7.5 and TBZ/15.0 mg/kg JJ8-089 significantly different from TBZ/VEH. B Chow intake in grams (mean±SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/7.5 and TBZ/15.0 mg/kg JJ8-089 significantly different from TBZ/VEH. The effects of JJ8-089 on PROG/chow feeding choice procedure during the 30 min session (C, D). C Lever presses (mean ± SEM), *p < 0.05, 15.0 mg/kg JJ8-089 significantly different from VEH. D Chow intake in grams (mean ± SEM), **p <0.01, 7.5, and 15.0 mg/kg JJ8-089 significantly different from VEH.
In the PROG/Chow feeding choice study JJC8-089 (Fig. 2C, D), showed a statistically significant treatment effect on lever presses (F(3, 69) = 3.49, p = 0.04, ηp2=0.24, Huynh-Fieldt), and planned comparisons showed that there was a significant increase at the 15.0 mg/kg dose (F(1, 69) = 7.21, p < 0.05). There also was a significant overall effect on chow intake (F(3, 69) = 19.11, p < 0.001, ηp2=0.62, Huynh-Fieldt), with significant decreases seen at the 7.5 and 15.0 mg/kg doses (F(1, 69) = 17.07, p < 0.001 and F(1, 69) = 49.81, p < 0.001, respectively).
Effects of RDS3-094 on FR5/Chow and PROG/Chow feeding choice performance
For the RDS3-094 experiment (Fig. 3A, B) on reversal of the effects of TBZ on FR5/Chow feeding choice, there was an overall treatment effect on both lever pressing and chow intake F(4, 28) = 9.70, p < 0.01, ηp2=0.58 and F(4, 28) = 9.49, p < 0.01, ηp2=0.58, respectively. TBZ decreased lever pressing and increased chow intake (F(1, 28) = 29.26, p < 0.01 and F(1, 28) = 25.90, p < 0.01, respectively). Coadministration of TBZ and 15.0 mg/kg dose RDS3-094 caused an increase in lever pressing (F(1, 28) = 6.55, p < 0.05) and a decrease in chow consumption (F(1, 28) = 20.95, p < 0.01) compared to TBZ/VEH.
A Lever presses (mean±SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; *p < 0.05, TBZ/15.0 mg/kg RDS3-094 significantly different from TBZ/VEH. B Chow intake in grams (mean ± SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/15.0 mg/kg RDS3-094 significantly different from TBZ/VEH. The effects of RDS3-094 on PROG/chow feeding choice procedure during the 30 min session (C, D). C Lever presses (±SEM), ns. D Chow intake in grams (mean±SEM), *p < 0.05, RDS3-094 is significantly different than VEH; *p < 0.05, 3.75 mg/kg RDS3-094 is significantly different than VEH; **p < 0.01, 7.5 and 15.0 mg/kg RDS3-094 was significantly different from VEH.
Regarding the effects of RDS3-094 (Fig. 3C, D) on PROG/Chow feeding choice performance, no significant effects were observed for RDS3-094 on lever pressing (F(3, 45) = 1.19, p = 0.33, ηp2=0.07). Power analysis revealed that with this very low effect size, an N = 64 would have been required to achieve significance. There was a treatment effect on chow intake (F(3, 45) = 14.47, p < 0.01, ηp2=0.49) in which all the doses (3.75, 7.5. 15.0 mg/kg) were significantly lower than vehicle (F(1, 45) = 5.88, p < 0.05, F(1, 45) = 27.83, p < 0.01 and F (1, 45) = 33.46, p < 0.01, respectively).
Effects of JJC8-091 on FR5/Chow and PROG/Chow feeding choice performance
Analysis of the results from coadministration of TBZ and JJC8-091 on FR5/Chow feeding choice (Fig. 4A, B) showed that there was an overall treatment effect on both lever pressing and chow intake (F(4, 28) = 15.44, p < 0.001, ηp2=0.69 and F(4, 28) = 13.83, p < 0.01, ηp2=0.66, respectively). TBZ was found to decrease lever pressing and increase chow intake (F(1, 28) = 32.67, p < 0.001 and F(1, 28) = 34.43, p < 0.001, respectively). There was no significant increase in lever presses compared to the TBZ/VEH condition, but there was a decrease in chow intake compared to TBZ/VEH at the 60.0 mg/kg dose (F(1, 28) = 15.17, p < 0.01).
A Lever presses (mean ± SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH. B Chow intake in grams (mean ± SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/60.0 mg/kg JJC8-091 significantly different from TBZ/VEH. The effects of JJC8-091 on PROG/chow feeding choice procedure during the 30 min session (C, D). C Lever presses (mean±SEM), *p < 0.05, 60.0 mg/kg JJC8-091 significantly different from VEH. D Chow intake in grams (±SEM), ns.
In the JJC8-091 TBZ reversal study PROG/Chow feeding choice (Fig. 4A, B), there was an overall treatment effect on lever presses (F(3, 21) = 3.08, p = 0.05, ηp2=0.31), which was significant at the 60.0 mg/kg dose, with planned comparisons showing that there was a significant decrease in lever presses (F(1, 21) = 6.36, p < 0.05). There was no significant effect on chow intake (F(3, 21) = 2.58, p = 0.08, ηp2=0.27).
Ability of JJC8-089 to reverse the effects of TBZ on FR5/Chow feeding choice in female rats
In the JJC8-089 female study (Fig. 5), there was an overall treatment effect on lever pressing (F(4, 24) = 3.64, p = 0.019, ηp2=0.38). Planned comparisons revealed that TBZ/VEH significantly decreased lever pressing compared to VEH/VEH (F (1, 24) = 11.03, p < 0.01), and the 7.5 mg/kg dose of JJC8-089 plus TBZ significantly increased lever pressing compared to TBZ/VEH (F(1, 24) = 4.52, p < 0.05). There was a significant overall treatment effect on chow intake (F(4, 24) = 31.46, p < 0.01, ηp2=0.84). Planned comparisons showed that TBZ significantly increased chow intake compared to VEH/VEH (F(1, 24) = 36.21, p < 0.001), and each dose of JJC8-089 plus TBZ significantly decreased chow intake compared to TBZ/VEH (F(1, 24) = 31.73, p < 0.01, F(1, 24) = 77.31, p < 0.01, and F (1,24) = 107.59, p < 0.01, respectively).
A Lever presses (mean ± SEM), #p < 0.01, 2.0 mg/kg TBZ/VEH significantly different from VEH/VEH; *p < 0.05, TBZ/7.5 mg/kg JJ8-089 significantly different from TBZ/VEH. B Chow intake in grams (mean ± SEM), #p < 0.01, TBZ/VEH significantly different from VEH/VEH; **p < 0.01, TBZ/7.5, 3.75 and 15.0 mg/kg JJ8-089 significantly different from TBZ/VEH.
Comparison of effects across compounds
To compare these different compounds in terms of their ability to restore the effects of TBZ on lever pressing, a percent restoration measure was calculated as follows: [Lever pressing numbers under TBZ plus the most effective of the DAT inhibitor/Lever pressing numbers under VEH/VEH * 100] for each compound separately. Table 1 shows that in male rats JJC8-089 had the highest restoration level compared to VEH, followed by JJC8-088 and RDS3-094, and lastly, JJC8-091. In female rats, the 0.75 mg/kg JJC8-089 increased lever pressing by 79.0% compared to TBZ/VEH. Furthermore, to compare these compounds for their capacity to increase the lever pressing in the PROG/Chow feeding choice studies, the percent increase was calculated by dividing [Lever pressing under most effective dose/Lever pressing under VEH*100]. We observed (Table 1) that JJC8-088 had the highest percent increase followed by JJC8-089 and RDS3-094, and with an effect in the opposite direction for JJC8-091.
Discussion
The present study assessed four novel DAT inhibitors using two effort-related choice tasks. In agreement with earlier research [23, 30,31,32,33], administration of 1.0 mg/kg TBZ induced a low-effort bias in effort-based decision-making, significantly reducing FR5 lever pressing while simultaneously increasing intake of the concurrently available chow. Considerable evidence indicates that the effects of TBZ on the FR5/Chow feeding choice task are related to the allocation of time on the lever pressing requirement and not to impairments in primary food motivation or appetite for palatable foods [1, 2, 52]. Previous work has shown that 1.0 mg/kg TBZ did not suppress ‘hedonic eating’ of chocolate in rats [2], and that the low-effort bias induced by TBZ is not due to changes in food intake or preference between the two foods (i.e., high carbohydrate pellets vs. laboratory chow [23]); Two of the DAT inhibitors studied, JJC8-088 and JJC8-089, reversed the effects of TBZ in the FR5/chow feeding choice task, increasing lever pressing and decreasing chow intake in TBZ-treated rats, and also increased the selection of high-effort lever pressing in rats tested on the PROG/chow task. RDS3-094 reversed the TBZ-induced impairment of FR5/Chow feeding choice, but did not enhance high-effort selection in the PROG/chow feeding task. Notably, JJC8-091 did not enhance selection of the high-effort options in any of the tasks, and in fact it reduced lever pressing in the PROG/chow feeding choice study. Overall, JJC8-089 had the greatest percent restoration on the FR5/chow task and JJC8-088 had the highest percent increase in the PROG/chow tasks. RDS3-094 yielded intermediate results, whereas JJC8-091 did not restore or increase lever pressing in any of the tasks. Given the range of behavioral outcomes across compounds, the evaluation of DAT inhibitors with diverse neurochemical properties holds the potential for identifying compounds that are potentially effective in addressing motivational dysfunction in humans.
Previously, the bupropion and DAT inhibitors including modafinil and its analogs (CE-123, CE-158, and MK-26) have been reported to partially reverse the effects of TBZ and increase the selection of high-effort choices [3, 17, 18, 23, 28,29,30,31,32,33,34]. However, the four compounds tested in the present studies showed a wide range of behavioral effects, which is potentially related to their diverse neurochemical characteristics. All four compounds show relatively high selectivity for binding to DAT compared to the norepinephrine and serotonin transporters [36, 45, 48, 50], with DAT binding affinities ranging from 2.6 nM (JJC8-088) to 289 nM (JJC8-091) and JJC8-089 and RDS3-094 being in between (16.7 and 23.1 nM respectively). JJC8-088 appears to share a typical binding profile more akin to cocaine, as characterized by binding more to the outward facing conformation of DAT [36], and administration of this compound leads to a rapid and robust increase in extracellular dopamine levels in the NAc [47]. As expected, it has cocaine-like effects and preclinical signs of substance use liability in rodents, including self-administration and drug-seeking behavior in rats [36, 40]. Furthermore, recent primate studies showed that JJC8-088 suppressed cocaine self-administration, possibly by acting as a substitute [53]. In the present studies, JJC8-088 significantly reversed the effects of TBZ, and produced a very robust increase in PROG lever pressing (Fig. 1, Table 1).
In contrast, JJC8-091 has a different binding profile, with a relatively low DAT affinity and a relative preference for binding to the occluded inward facing conformation of DAT [36]. In microdialysis studies, JJC8-091 produced only a very limited increase in extracellular DA in the NAc compared to JJC8-088 [47]. Newman et al. [36]. reported that JJC8-091 failed to act as a reinforcer, and that IP administration of 30.0 mg/kg JJC8-091 in rats significantly reduced the progressive ratio breakpoint for cocaine reinforcement. Furthermore, JJC8-091 blunted escalation of methamphetamine self-administration and reduced reinstatement of cocaine-seeking behavior in rats [36, 40, 48]. Taken together, these data indicate that JJC8-091 is an atypical DAT inhibitor in both its neurochemical and behavioral characteristics. Consistent with that observation, JJC8-091 differed from the other DAT inhibitors tested in that it failed to increase selection of high-effort activity on either of the behavioral tests used (Fig. 4). It is possible that the relatively low affinity of JJC8-091 for DAT was a factor, and that higher doses should have been used. However, the only significant effect on lever pressing was a decrease in PROG responding at the highest dose, which makes it unlikely that increasing the dose further would have increased lever pressing output. Furthermore, the doses of JJC8-091 used in the present work were based on published reports of behavioral effects of this compound in the 30.0–56.0 mg/kg dose range [36].
JJC8-089 has a robust brain penetration, and was reported to blunt methamphetamine escalation in rats trained with extended access self-administration sessions [48], which is a characteristic also shown by JJC8-091 but not JJC8-088. Moreover, while IP administration of JJC8-088 produced a substantial increase in locomotor activity, 30.0 mg/kg JJC8-089 failed to significantly increase locomotion, which is similar to JJC8-091 (supplementary material shown in ref. [48].). These data suggest that JJC8-089 may be intermediate between JJC8-088 and JJC8-091 in terms of some its neurochemical and behavioral characteristics. Interestingly, this compound also showed positive effects in both effort-based choice tasks, with a robust reversal of the low-effort bias induced by TBZ, and a significant increase in selection of high-effort PROG lever pressing (Fig. 2). Given this overall profile, JJC8-089 appears to be a compound with atypical characteristics (e.g., lack of broad major stimulant effects), which nevertheless does have actions that promote selection of high-effort instrumental activities. Furthermore, RDS3-094 showed a mixed pattern of results, being able to recover effort-related motivation from the challenge provided by TBZ, but not stimulating exertion of effort in the PROG study. This may mean that RDS3-094 would have minimal side effects resulting from DAergic overstimulation, such as mania or compulsive behavior. Additional research with these compounds should focus on their potential as a treatment for effort-related motivational dysfunction.
Given the prevalence of depression in women [54], future animal studies should include both sexes [24, 55,56,57]. Errante et al. [58] reported that haloperidol decreased PROG lever pressing in both male and female rats. Studies with fluoxetine showed that this antidepressant decreased high-effort choices (FR5 lever pressing and wheel running) in both male and female rats, but did not increase intake of food alternatives [59]. Recent research has identified a sex difference in the effects of TBZ, with male rats showing an effect on FR5/chow feeding choice at 1.0 mg/kg, while this dose in females was ineffective [24]. In that study, 2.0 mg/kg TBZ produced a low-effort bias and increased cFos expressing in nucleus accumbens in female rats [24]. Thus, in the present work, the ability of JJC8-089 to reverse the effects of 2.0 mg/kg TBZ in female rats was assessed to explore the feasibility of using a DAT inhibitor to reverse the effects of TBZ in females. As shown in Fig. 5, 7.5 mg/kg JJC8-089 was able to significantly increase lever pressing in TBZ-treated females, a finding that should be explored further in future research.
Taken together, these data support the idea that all drugs that bind to the DAT do not necessarily have the same behavioral characteristics. This likely depends upon a number of neurochemical factors, including the binding kinetics, the conformational changes induced, the resulting impact on extracellular DA, and off target effects. These observations are consistent with what is known about modafinil, which has been shown to bind to DAT though it does not have all the classic characteristics of a major psychomotor stimulant. Observations of the neurochemical and behavioral effects of modafinil, as well as GBR12909 and benztropine, led to the idea that there are typical (i.e., cocaine-like) and atypical DAT inhibitors [39,40,41,42,43]. As more compounds are synthesized and tested, it seems evident that this distinction is not dichotomous, and that there is a continuum of ‘atypicality’ across which many drugs can vary. In the present studies, JJC8-088 and JJC8-089 were active on both tests of effort-based choice, while RDS3-094 was only active on one, and JJC8-091, the most atypical of this series, failed to induce any increase in effort-related responding. The fact that DAT inhibitors vary across a spectrum of neurochemical and behavioral characteristics may allow for exploitation of some compounds for their therapeutic utility. For example, it is possible that one or more DAT inhibitors could be identified that promote effort-related motivational effects, yet have lower substance use liability or fewer side effects than cocaine-like drugs. This balanced profile could yield a drug that would be useful for treating effort-related motivational dysfunctions such as fatigue or anergia in MDD and other disorders.
Data availability
Data files will be provided upon request (john.salamone@uconn.edu).
References
Salamone JD, Correa M. The neurobiology of activational aspects of motivation: exertion of effort, effort-based decision making, and the role of dopamine. Annu Rev Psychol. 2024;75:1–32.
Salamone JD, Ecevitoglu A, Carratala-Ros C, Presby RE, Edelstein GA, Fleeher R, et al. Complexities and paradoxes in understanding the role of dopamine in incentive motivation and instrumental action: exertion of effort vs. anhedonia. Brain Res Bull. 2022;182:57–66.
Salamone JD, Yohn SE, López-Cruz L, San Miguel N, Correa M. Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain. 2016;139:1325–47.
Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6.
Treadway MT, Salamone JD. Vigor, Effort-related aspects of motivation and anhedonia. Curr Top Behav Neurosci. 2022;58:325–54.
Stahl S. The psychopharmacology of energy and fatigue. Clin Neurosci Update. 2002;63:7–8.
Cooper JA, Tucker VL, Papakostas GI. Resolution of sleepiness and fatigue: a comparison of bupropion and selective serotonin reuptake inhibitors in subjects with major depressive disorder achieving remission at doses approved in the European Union. J Psychopharmacol. 2014;28:118–24.
Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31:250–7.
Price CS, Taylor FB. A retrospective chart review of the effects of modafinil on depression as monotherapy and as adjunctive therapy. Depress Anxiety. 2005;21:149–53.
Farrow TFD, Hunter MD, Haque R, Spence SA. Modafinil and unconstrained motor activity in schizophrenia: Double-blind crossover placebo-controlled trial. Br J Psychiatry. 2006;189:461–2.
Stotz G, Woggon B, Angst J. Psychostimulants in the therapy of treatment-resistant depression. Dialog Clin Neurosci. 1999;1:165–74.
Salamone JD, Correa M. Critical review of RDoC approaches to the study of motivation with animal models: effort valuation/willingness to work. Emerg Top Life Sci. 2022;6:515–28.
Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–21.
Salamone JD, Correa M. the mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85.
Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A, et al. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One. 2012;7:e47934.
Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, et al. The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: Reversal with antidepressant drugs. PLoS One. 2014;9:23–6.
Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, et al. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: Implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol. 2015;18:1–11.
Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol. 2014;17:2045–56.
Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–15.
Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharm Biochem Behav. 2001;69:373–82.
Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196:565–74.
Mai B, Sommer S, Hauber W. Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci. 2012;12:74–84.
Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn SE, Baqi Y, et al. Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: Implications for animal models of the motivational symptoms of depression. J Neurosci. 2013;33:19120–30.
Salamone JD, Ecevitoglu A, Beard KR, Srynath S, Edelstein GA, Meka N, et al. Exploring sex differences in the neurochemical and effort-related motivational effects of the VMAT-2 Inhibitor and dopamine depleting agent tetrabenazine. Program No. PSTR100.05. 2023 Neuroscience Meeting Planner. Washington, D.C.: Society for Neuroscience, 2023.
Guay DRP. Tetrabenazine, a monoamine-depleting drug used in the treatment of hyperkinetic movement disorders. Am J Geriatr Pharmacother. 2010;8:331–73.
Pettibone DJ, Totaro JA, Pflueger AB. Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants. Eur J Pharm. 1984;102:425–30.
Yohn SE, Thompson C, Randall PA, Lee CA, Müller CE, Baqi Y, et al. The VMAT-2 inhibitor tetrabenazine alters effort-related decision making as measured by the T-maze barrier choice task: Reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion. Psychopharmacology. 2015;232:1313–23.
Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M, et al. Not all antidepressants are created equal: differential effects of monoamine uptake inhibitors on effort-related choice behavior. Neuropsychopharmacology. 2016;41:686–94.
Yohn SE, Lopez-Cruz L, Hutson PH, Correa M, Salamone JD. Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat. Psychopharmacology. 2016;233:949–60.
Kouhnavardi S, Ecevitoglu A, Dragačević V, Sanna F, Arias-Sandoval E, Kalaba P, et al. A novel and selective dopamine transporter inhibitor, (S)-MK-26, promotes hippocampal synaptic plasticity and restores effort-related motivational dysfunctions. Biomolecules. 2022;12:881.
Rotolo RA, Dragacevic V, Kalaba P, Urban E, Zehl M, Roller A, et al. The novel atypical dopamine uptake inhibitor (S)-CE-123 partially reverses the effort-related effects of the dopamine depleting agent tetrabenazine and increases progressive ratio responding. Front Pharm. 2019;10:1–12.
Rotolo RA, Kalaba P, Dragacevic V, Presby RE, Neri J, Robertson E, et al. Behavioral and dopamine transporter binding properties of the modafinil analog (S, S)-CE-158: reversal of the motivational effects of tetrabenazine and enhancement of progressive ratio responding. Psychopharmacology. 2020;237:3459–70.
Rotolo RA, Presby RE, Tracy O, Asar S, Yang JH, Correa M, et al. The novel atypical dopamine transport inhibitor CT-005404 has pro-motivational effects in neurochemical and inflammatory models of effort-based dysfunctions related to psychopathology. Neuropharmacology. 2021;183:108325.
Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, et al. Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: Implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacology. 2016;109:270–80.
Hersey M, Bartole MK, Jones CS, Newman AH, Tanda G. Are there prevalent sex differences in psychostimulant use disorder? a focus on the potential therapeutic efficacy of atypical dopamine uptake inhibitors. Molecules. 2023;28:5270.
Newman AH, Cao J, Keighron JD, Jordan CJ, Bi GH, Liang Y, et al. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology. 2019;44:1435–44.
Fluyau D, Mitra P, Lorthe K. Antipsychotics for amphetamine psychosis. A systematic review. Front Psychiatry. 2019;10:1–14.
Gallagher KE, Funaro MC, Woods SW. Prescription stimulants and the risk of psychosis: a systematic review of observational studies. J Clin Psychopharmacol. 2022;42:308–14.
Kohut SJ, Hiranita T, Hong S-K, Ebbs AL, Tronci V, Green J, et al. Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biol Psychiatry. 2014;76:802–9.
Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX. New drugs, old targets: tweaking the dopamine system to treat psychostimulant use disorders. Annu Rev Pharm Toxicol. 2021;61:609–28.
Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharm. 2008;75:2–16.
Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, et al. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: Molecular features that differentially determine antagonist-binding properties. J Neurochem. 2008;107:928–40.
Schmitt KC, Reith MEA. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS One. 2011;6:e25790.
Tanda G, Hersey M, Hempel B, Xi Z, Newman AH. Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder. Curr Opin Pharm. 2021;56:13–21.
Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS, et al. Novel and high affinity 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues as atypical dopamine transporter inhibitors. J Med Chem. 2016;59:10676–91.
Hersey M, Chen AY, Bartole MK, Anand J, Newman AH, Tanda G. An fscv study on the effects of targeted typical and atypical dat inhibition on dopamine dynamics in the nucleus accumbens shell of male and female mice. ACS Chem Neurosci. 2023;14:2802–10.
Keighron JD, Quarterman JC, Cao J, Demarco EM, Coggiano MA, Gleaves A, et al. Effects of (R)-modafinil and modafinil analogues on dopamine dynamics assessed by voltammetry and microdialysis in the mouse nucleus accumbens shell. ACS Chem Neurosci. 2019;10:2012–21.
Tunstall BJ, Ho CP, Cao J, Vendruscolo JCM, Schmeichel BE, Slack RD, et al. Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology. 2018;131:96–103.
Mereu M, Hiranita T, Jordan CJ, Chun LE, Lopez JP, Coggiano MA, et al. Modafinil potentiates cocaine self-administration by a dopamine-independent mechanism: possible involvement of gap junctions. Neuropsychopharmacology. 2020;45:1518–26.
Slack RD, Ku TC, Cao J, Giancola JB, Bonifazi A, Loland CJ, et al. Structure-activity relationships for a series of (bis(4-fluorophenyl)methyl)sulfinyl alkyl alicyclic amines at the dopamine transporter: Functionalizing the terminal nitrogen affects affinity, selectivity, and metabolic stability. J Med Chem. 2020;63:2343–57.
Keppel G Design and analysis: A researcher’s handbook, 3rd ed. Englewood Cliffs, NJ, US: Prentice-Hall, Inc; 1991.
Ren N, Carratala-Ros C, Ecevitoglu A, Rotolo RA, Edelstein GA, Presby RE, et al. Effects of the dopamine depleting agent tetrabenazine on detailed temporal parameters of effort-related choice responding. J Exp Anal Behav. 2022;117:331–45.
Rahimi O, Cao J, Lam J, Childers SR, Rais R, Porrino LJ, et al. The effects of the dopamine transporter ligands JJC8-088 and JJC8-091 on cocaine versus food choice in rhesus monkeys. J Pharmacol Exp Ther. 2023;384:372–81.
Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40:219–21.
Maita I, Bazer A, Chae K, Parida A, Mirza M, Sucher J, et al. Chemogenetic activation of corticotropin-releasing factor-expressing neurons in the anterior bed nucleus of the stria terminalis reduces effortful motivation behaviors. Neuropsychopharmacology. 2023; https://doi.org/10.1038/s41386-023-01646-9.
Matas-Navarro P, Carratalá-Ros C, Olivares-García R, Martínez-Verdú A, Salamone JD, Correa M. Sex and age differences in mice models of effort-based decision-making and anergia in depression: the role of dopamine, and cerebral-dopamine-neurotrophic-factor. Psychopharmacology. 2023;240:2285–302.
Carratalá-Ros C, Martínez-Verdú A, Olivares-García R, Salamone JD, Correa M. Effects of the dopamine depleting agent tetrabenazine in tests evaluating different components of depressive-like behavior in mice: sex-dependent response to antidepressant drugs with SERT and DAT blocker profiles. Psychopharmacology. 2023;240:1615–28.
Errante EL, Chakkalamuri M, Akinbo OI, Yohn SE, Salamone JD, Matuszewich L. Sex differences in effort-related decision-making: role of dopamine D2 receptor antagonism. Psychopharmacology. 2021;238:1609–19.
Presby RE, Rotolo RA, Hurley EM, et al. Sex differences in lever pressing and running wheel tasks of effort-based choice behavior in rats: Suppression of high effort activity by the serotonin transport inhibitor fluoxetine. Pharm Biochem Behav. 2021;202:173115.
Acknowledgements
This research was partially supported by grants to JS from NIMH (R01MH121350) and the University of Connecticut Research Foundation.
Funding
Funding from the NIDA-Intramural Research Program was provided to AHN (Z1A DA000389). AO was supported by the NIDA-Intramural Research Program’s Scientific Director’s Fellowship for Diversity in Research
Author information
Authors and Affiliations
Contributions
Alev Ecevitoglu, Renee A. Rotolo, Nicolette Meka: data collection and analysis, manuscript preparation; Gayle Edelstein, Sonya Srinath, Carla Ros, Kathryn Beard, Rose E. Presby: data collection and analysis; Jianjing Cao, Amarachi Okorom: compound synthesis and characterization; Merce Correa: conceptualization, research supervision, manuscript preparation. John D. Salamone, Amy H. Newman: conceptualization, methodology, formal analysis, manuscript preparation; funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ecevitoglu, A., Meka, N., Rotolo, R.A. et al. Potential therapeutics for effort-related motivational dysfunction: assessing novel atypical dopamine transport inhibitors. Neuropsychopharmacol. 49, 1309–1317 (2024). https://doi.org/10.1038/s41386-024-01826-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-024-01826-1
- Springer Nature Switzerland AG