Abstract
High-intensity sweet-liking has been linked to alcohol use disorder (AUD) risk. However, the neural underpinning of this association is poorly understood. To find a biomarker predictive of AUD, 140 participants (social and heavy drinkers, ages 21–26) underwent functional magnetic resonance imaging (fMRI) during a monetary incentive delay (MID) task and stimulation with high (SucroseHigh)- and low-concentration sucrose, as well as viscosity-matched water. On another day after imaging, and just before free-access intravenous alcohol self-administration, participants experienced a 30 mg% alcohol prime (10 min ascent) using the Computerized Alcohol Infusion System. Principal component analysis (PCA) of subjective responses (SR) to the prime’s ascending limb generated enjoyable (SRenjoy) and sedative (SRsed) intoxication components. Another PCA created one component reflective of self-administered alcohol exposure (AE) over 90 min. Component loadings were entered as regressors in a voxel-wise general linear fMRI model, with reward type as a fixed factor. By design, peak prime breath alcohol concentration was similar across participants (29 ± 3.4 mg%). SRenjoy on the prime’s ascending limb correlated positively with [SucroseHigh > Water] in the supplementary motor area and right dorsal anterior insula, implicating the salience network. Neither SR component correlated with the brain’s response to MID. AE was unrelated to brain reward activation. While these findings do not support a relationship between alcohol self-administration and (1) subjective liking of or (2) regional brain response to an intensely sweet taste, they show that alcohol’s enjoyable intoxicating effects on the rising limb correspond with anterior insular and supplementary motor area responses to high-concentration sucrose taste. No such associations were observed with MID despite robust activation in those regions. Insula and supplementary motor area responses to intense sensations relate to a known risk factor for AUD in a way that is not apparent with a secondary (monetary) reward.
Similar content being viewed by others
Introduction
Sweet-liking has been linked to alcohol consumption and AUD risk in both humans and animals [1, 2], but with less attention to related brain mechanisms. We initially reported that daily drinking intensity was associated with greater orbitofrontal activation [3] from high-concentration sucrose—a finding that did not survive in a larger sample [4]. Rudenga and Small [5] also showed no association between the brain response to sweet taste and self-reported alcohol use. However, self-reported drinking can vary across participants [6] and its temporal resolution for subjective response (SR) to alcohol exposure is poor. We therefore employed laboratory-based alcohol self-administration to examine the relationship between the brain’s response to sweet taste and SR to alcohol.
Both consummatory (e.g., sweet taste, alcohol) and abstract rewards (such as money) engage common brain systems [7, 8], but with key differences [9]. Thus, while reward mechanisms in AUD have been studied using mostly monetary reward tasks, a salient consummatory reward could offer greater external validity for investigating brain mechanisms underlying alcohol use. In fact, some argue that a similar brain response across consummatory rewards reflects a mechanism for transference of reward drive between sweet rewards and alcohol [2, 10]. Thus, we posit that the neural processing of sweet rewards may better parallel those of alcohol reward and therefore prove a useful surrogate.
While the relationship between sweet and alcohol reward is poorly understood, that between SR to alcohol exposure and AUD risk is better studied. Schuckit, et al. [11] first reported that the sons of fathers with AUD had greater tolerance to alcohol’s adverse effects and were more likely to develop future alcohol problems. Recently, King et al. [12] showed that greater sensitivity to alcohol’s pleasant effects was related to future AUD. Both findings relate to Newlin and Thomson’s “differentiator” model [13], which hypothesizes that a predisposition to AUD includes sensitivity to alcohol’s pleasant effects during the rising limb (ascent) in breath alcohol (BrAC) and resistance to the aversive effects on the descending limb.
To clarify brain mechanisms that mediate alcohol reward-sensitivity and sweet-liking, we tested for associations between SR to alcohol and brain responses to sweet taste (primary reward), as well as both secondary reward anticipation and receipt using the monetary incentive delay (MID) task. We employed the laboratory-based computerized alcohol infusion system (CAIS; [14]), which permits ad libitum intravenous alcohol self-administration using individual physiologically-based pharmacokinetic modeling to standardize each BrAC increment and avoid the inter-individual BrAC variability inherent to oral consumption [15, 16]. Intravenous administration also separates intoxicating effects from confounding rewarding sensations, such as flavor [17].
We tested four hypotheses: (1) Participants who like intensely sweet tastes self-administer a greater alcohol-exposure and (2) responses to an intensely sweet taste in reward-related brain areas positively predict self-administration. (3) Reward region responses to an intensely sweet taste correlate with SR change on alcohol’s ascending limb. (4) The association between reward-related brain response and SR is specific to primary (consummatory) rewards (sweet taste) and absent from monetary reward.
Methods
Procedures
Interview, MRI, and alcohol self-administration occurred on separate days (Figs. S1–S3). Assessments included the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; [18]), a 35-day version of the Timeline Followback interview [19], the revised NEO-Personality Inventory (NEO; [20]), the Center for Epidemiological Studies-Depression Scale (CES-D; [21]), the Short Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation seeking Personality scale (SUPPS-P; [22]), and the Alcohol Use Disorders Identification Test (AUDIT; [23]). Procedures occurred only following participants’ written consent approved by the Indiana University IRB.
Participants
One hundred seventy-two healthy participants (a subsample of whom were reported previously [4]) were balanced by family history of AUD, recent drinking, and sex (supplement for inclusion/exclusion criteria). Eight were excluded for incomplete imaging, excessive motion, or technical problems prior to data quality review, described below. A further 21 were excluded for incomplete alcohol self-administration data from not returning for the session (n = 3), insufficient alcohol supply (n = 4), nausea/investigator concerns about safety (n = 8), intravenous insertion site problems (n = 2), or technical problems (n = 4). Three had data quality problems involving both self-administration and imaging.
The family history assessment module [24] of the SSAGA [18] interview determined family history status, with family history negative (FHN) defined as no first- or second-degree relatives with AUD. Family history positive (FHP) status required at least one first-degree relative with AUD. Those with a maternal history of AUD (n = 11) reported that their mothers abstained during pregnancy (n = 7) or that alcohol problems were post-natal (n = 5; i.e., one reported both). The final sample (Table 1) included 140 participants; 71 (50.7%) were female, and 66 (47.1%) family history positive. Family history groups did not differ in average drinks per week, average drinks per drinking day, or sex [χ2 = 0.27] (ps > 0.46).
Imaging day
Taste test
Before imaging, participants rated perceived intensity and pleasantness of five concentrations of sucrose-sweetened water. Intensity ratings used a labeled magnitude scale [25] and pleasantness used a visual analog scale. Solutions were presented in three blocks of five volumes, with order in each block pseudorandomized and participants blind to order and concentration (range 0.05 M to 0.83 M). Participants were given 15 mL of each solution in a cup and instructed to “swish” for 5 s and spit without swallowing. Following ratings, participants cleansed their palates using ~10 mL of deionized water before the next cup.
Sucrose stimulation task (fMRI)
A mouthpiece delivered tastant sprays from a computer-controlled five-channel gustometer [3, 26]. In six functional scans, three solutions were delivered: a 0.1 M (low) sucrose solution (SucroseLow), a 0.83 M (high) sucrose solution (SucroseHigh), and a water control. SucroseLow and water were viscosity-matched to SucroseHigh using a tasteless thickening agent (ThickenUp Clear®, Nestlē Health Sciences, Vevey, Switzerland). SucroseHigh and SucroseLow were delivered in separate, alternating scans (3 each), with water as an activated baseline control (Fig. S4 and [3, 4] for detail). Participants were randomized to scan order, balancing across family history and drinking groups.
Monetary incentive delay task (fMRI)
Participants performed the monetary incentive delay (MID; Fig. S5) task [27]. A cue signaled the ability to win (Win) or avoid losing one or five (5) dollars (Win5; Fig. S5) by responding during a reaction time target’s display. A neutral cue signaled a control trial without monetary gain/loss. The task adjusted reaction time to approximate 66% accuracy for each trial type. After each trial, participants received performance feedback (trial outcome plus cumulative winnings). Participants practiced a shortened task outside the scanner before imaging. Winnings were paid at the end of the study day.
Image acquisition and analysis
Imaging used a Siemens 3T Magnetom Prisma (Erlangen, Germany) scanner and a 64-channel head coil array. Blood oxygenation level dependent (BOLD) contrast-sensitive images were acquired using a product echo planar imaging (EPI) sequence (gradient echo, repetition/echo time (TR/TE) 2110/29 ms, flip angle 78°, field-of-view 220 × 220 mm2, matrix 80 × 80, 39 interleaved 3 mm thick slices, 2.75 × 2.75 × 3.0 mm3 voxels, GRAPPA acceleration factor 2, 164 measurements). A high-resolution anatomical image (1.05 × 1.05 × 1.2 mm3 voxels, T1-weighted 3D magnetization prepared rapid gradient echo) was acquired for co-registration with the BOLD images. Functional imaging included one MID task and six sucrose stimulation BOLD fMRI scans, brief instructions, intra-MRI assessment questions, and time for post-scan water rinse delivery and post-scan subjective ratings (~65 min). Prior to the MID and sucrose scans, a gradient echo field mapping scan (TR = 355 ms, TE1/TE2 = 3.86/6.32 ms, advanced B0 shim mode adjustment, same imaging volume and voxel size as BOLD EPI) optimized field homogeneity and facilitated BOLD EPI volume distortion evaluation/correction. Foam pads and real-time prospective acquisition motion correction reduced head movement [28].
Image preprocessing used the FMRIB Software Library (FSL version 6.0; [29], including BOLD volume geometric distortion correction with fugue that utilized distortion field estimates from a field mapping scan, slice time acquisition correction with slicetimer, motion correction with mcflirt [30], brain extraction with bet [31], registration to each participant’s T1-weighted image and MNI152 standard space with flirt and fnirt, and 6 mm FWHM Gaussian filter spatial smoothing. FSL’s MELODIC version 3.15 automatically estimated and retained an optimal number of independent components for each BOLD scan, which was subsequently denoised using an unsupervised ICA-AROMA [32] classifier. Denoised data were projected into standard MNI space and interpolated to 2 mm isotropic voxels for statistical analyses.
Given potential effects from swallowing during sucrose fMRI, quality control (QC) screening (blind to participant characteristics) assessed both [Sucrose>Implicit Baseline] activation in primary somatosensory cortex from the intraoral stimulation (Figs. S9–S11) and excessive activation within the cerebrospinal fluid and/or white matter. To maximize sample size and include the same number of sucrose scans for each participant, we included only two QC-verified sucrose scans at each concentration for each participant. Considering potential habituation in those with three QC-verified scans at each concentration, we counter-balanced scan-pair orders (i.e., first/second, first/third, second/third) across family history, sex, and drinking groups. Five participants failed QC criteria for SucroseHigh scans and two participants for SucroseLow scans resulting in a total of seven participants excluded from analyses of both concentrations.
Intravenous alcohol self-administration paradigm
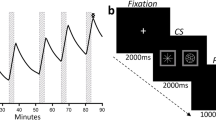
Alcohol sessions began with a 30-min “prime” immediately followed by 90 min ad libitum alcohol self-administration. CAIS [15] allowed participants to choose an intravenous “drink” (described as “bits” to avoid implying a drinking glass volume) by depressing a button, with each press raising BrAC by a targeted 7.5 mg% over 2.5 min [14]. During this 2.5 min, the button was inactive, and the screen displayed a message that the bar was closed.
The prime began with participants being asked to depress the button four consecutive times to achieve a targeted peak BrAC of ~30 mg% in 10 min. Following this ascent, alcohol became unavailable for 20 min and CAIS maintained a controlled linear descent of ~1 mg/dl/min to reach 10 mg% BrAC. Ad libitum self-administration immediately followed. Breath readings occurred near the peak of every self-administration for the first six requests and then every other request for feedback. This generated a latent BrAC curve throughout the session, up to a 150 mg% maximum safety limit when the “bar-closed” message appeared until BrAC declined.
Participants rated on a visual analog scale subjective enjoyment, sedation, anxiousness, stimulation, intoxication, and fatigue (each defined using synonyms; Supplementary Materials and Methods). Participants also rated perceived number of drinks (to the nearest ½ drink) from 0 to 10 + . Ratings occurred at baseline just prior to the prime, at the estimated prime peak, prime end, and at 20-minute intervals during self-administration near a local peak when applicable. Question order was randomized at each acquisition.
Statistical analysis
Principal component analysis
To reduce dimensionality we performed principal component analyses (PCA) with Varimax rotation [33] (SPSS 28, IBM 2021) on variables characterizing the self-administration profile and, separately, SR to the fixed 30 mg% alcohol prime exposure, retaining components with eigenvalues > 1.0 (Table S9).
Inputs for alcohol self-administration included three variables— area under the curve during self-administration, peak BrAC, and BrAC slope during the first 75 min of self-administration (time for the fastest participant to reach the 150 mg% limit).
SR variables for the prime’s ascending limb to 30 mg% comprised perceived intoxication, number of drinks, enjoyment, stimulation, sedation, and tiredness (all calculated as peak—baseline). Anxiousness was excluded for zero inflation (>70% at all timepoints). Although data were acquired during self-administration, we limited SR analyses to the prime given its standard BrAC exposure across participants. PCA prerequisites were satisfied (linearity; Kaiser-Meyer-Olkin measure = 0.73 for SR and 0.59 for self-administration; Bartlett’s test of sphericity, ps < 0.001). Higher scores reflect greater SR and self-administration. Pearson coefficients evaluated correlations between principal components.
Non-normally distributed variables
Square root transforms of drinks/drinking day and drinks/week were used in linear models.
BOLD fMRI models
Within-subject fixed effects of the BOLD response to trials were estimated in SPM12 [34] using the canonical hemodynamic response function (HRF) with time and dispersion derivatives. Sucrose and water trial onsets (duration = 3 s) coincided with pump activation. MID trial conditions modeled cue onsets, and in a separate model, feedback (wins and neutral outcomes), using only canonical HRF. Swallowing (sucrose) and button presses (MID) were conditions of no interest, using visual cue onsets. Six head motion parameters from realignment served as multiple regressors. An autoregressive AR(1) model accounted for serial correlations, while a high-pass filter (1/128 Hz) removed low-frequency noise. Contrasts of interest were SucroseHigh relative to water, as well as Win5 relative to neutral (i.e., [SucroseHigh > Water], [Win5 > Neutral]). Water immediately following sucrose was excluded to maximize taste contrast [4].
Contrasts were compared in group random effects one-way analysis of variance (ANOVA) models: ([SucroseHigh > Water] vs [SucroseLow > Water]), ([SucroseHigh > Water] vs [Win5 > Neutral]cue), and ([SucroseHigh > Water] vs [Win5 > Neutral]feedback). PCA-derived component weights for each participant were covariates. While the self-administration component was tested as a covariate in the same model as the SR measures, separate models compared SucroseHigh to (1) SucroseLow (2) Win5 anticipation and (3) Win5 feedback.
Per a priori hypotheses, we created an explicit mask of the frontal lobe, insula, and striatum (Harvard-Oxford parcellation, ≥99% probability; Fig. S6) as well as the pallida and amygdalae (Melbourne Subcortex Atlas Scale I [35]). The significance criterion was family-wise error-corrected (FWE) voxel-level, pFWE < 0.05 [36], correcting for multiple comparisons within the mask volume, and minimum cluster size k ≥ 5. Effects of interest were tested further in other statistical models by extracting mean activation from 5 mm radius spheres (Figs. S7, S8).
Results
Alcohol self-administration
Ad libitum alcohol self-administration varied widely (e.g., peak BrAC Range = 14.8–154.2 mg%, average = 89.3 mg%, n = 140). PCA (n = 140) on the three self-administration variables yielded one principal component reflecting alcohol exposure (AE; Table S9), while PCA on SR revealed two principal components, interpretable as enjoyable intoxication (SRenjoy) and sedative intoxication (SRsed; Table S9).
SRenjoy did not correlate with AE, AUDIT, drinks/week, or drinks/drinking day (ps > 0.10). SRsed negatively correlated with AE (r = −0.17, p = 0.047), AUDIT (r = −0.29, p < 0.001), drinks/week (r = −0.33, p < 0.001), and drinks/drinking day (r = −0.29, p < 0.001). AE correlated with drinks/drinking day (r = 0.39, p < 0.001), drinks/week (r = 0.41, p < 0.001), and AUDIT (r = 0.34, p < 0.001).
AE was examined in a stepwise FHA(2) × Sex(2) × Sweet-liking(2) factorial model using as predictors SRenjoy, SRsed, depression, AUDIT problem subscale, SUPPS-P average urgency, anxiety, drinks/week, and drinks/drinking day. Only SRenjoy, drinks/week, and drinks/drinking day explained significant variance in self-administration. Linear regression model with these three variables and 1000 bootstrap repetitions showed significant associations between all three variables and AE (SRenjoy β = −0.19, p = 0.014, drinks/week β = 0.28, p = 0.014, and drinks/drinking day β = 0.21, p = 0.048), collectively explaining 22.4% of variance in AE.
Effect of FHA, Sweet-liking, and sex on AE, SRenjoy, and SRsed
One participant did not complete the taste test (n = 139). Forty-three percent rated the 0.83 M solution highest, qualifying as sweet-liking. Nicotine use within the last 6 weeks was unassociated with sweet-liking (χ2 = 0.115, p = 0.74). FHA(2) × Sex(2) × Sweet-liking(2) factorial models with SRenjoy, SRsed, and AE as dependent variables showed a significant main effect of Sex on SRenjoy (p = 0.05; men higher), without other main effects. However, a significant interaction between sweet-liking and FHA (p = 0.03) reflected FHP sweet-likers administering less alcohol (estimated marginal mean (EMM) = − 0.18) than FHP sweet dislikers (EMM = 0.16) while FHN sweet-likers administered more (EMM = 0.17) than FHN sweet dislikers (EMM = − 0.23). There were no other significant interactions (ps > 0.08; Figs. S12, S13).
BOLD activation to sucrose stimulation
High-concentration sweet-taste ([SucroseHigh > Water]) elicited robust activation in taste- and reward-related regions (Fig. 1, Table 2). The effects of [SucroseHigh > Water] and [SucroseLow > Water] were similar, apart from nonsignificant amygdala activation in [SucroseLow > Water] (Table 2).
Blue = SucroseHigh greater than water activation ([SucroseHigh > Water]). Green = [SucroseHigh > Water] activation that predicted the enjoyable intoxicating effects of intravenous ethanol (peak-level significance pFWE < 0.05, family-wise error corrected for the frontal/insular/striatal/amygdala/pallidal brain mask, k = 108,962 = 2472.07 cm3; Fig. S13). Pink = the same as Green, but at p < 0.001 uncorrected, for context.
BOLD activation from either concentration versus water did not correlate with AE or SRsed. However, [SucroseHigh > Water] positively correlated with SRenjoy in both the inferior supplementary motor area (SMA; pFWE = 0.006; k = 9) and dorsal anterior insula (daINS; pFWE = 0.014; k = 7). [SucroseLow > Water] did not show a similar correlation. A direct comparison within 5 mm radius spheres defined by the peaks of correlation between [SucroseHigh > Water] and SRenjoy showed that SRenjoy was significantly more associated with [SucroseHigh > Water] than [SucroseLow > Water] in both the right daINS (pFWE < 0.001; k = 57) and the SMA (pFWE = 0.008; k = 15). The reverse contrast (greater correlation between SRenjoy and [SucroseLow > Water] compared to that between SRenjoy and [SucroseHigh > Water]) showed no significant foci.
BOLD activation: monetary reward compared to sucrose stimulation
Despite substantial spatial overlap between sweet ([SucroseHigh > Water]) and monetary reward ([Win5 > Neutral]) responses (Fig. 2), MID task responses were unassociated with AE, SRenjoy, and SRsed.
On the other hand, feedback of monetary reward receipt (successful trials) differs substantially from sucrose taste in these areas. In both cases sucrose dominates the middle insulae where the primary taste area is thought to be located (top left, bottom left). Further, although both the Win5 cue and SucroseHigh activate the ventral striatum (VST), SucroseHigh activation is more circumscribed, while the Win5 cue activates the striatum broadly (top right). By the time of Win5 successful feedback, the striatum is less active than during sucrose administration. Threshold for all contrasts shown is pFWE < 0.05.
We next tested if the correlation between the [SucroseHigh > Water] contrast and SRenjoy was significantly different than correlations between [Win5 > Neutral] and SRenjoy by applying a small volume correction within two 5 mm radius spherical ROIs defined by the peaks of correlation between [SucroseHigh > Water] and SRenjoy. Both the [SucroseHigh > Water] and [Win5 > Neutral]cue contrasts produced significant activation in the daINS (psFWE < 0.001, ks ≥ 40) and SMA (psFWE < 0.001, ks ≥ 24), while [Win5 > Neutral]feedback was significant only in the daINS (pFWE < 0.001, k = 36). The sucrose response was significantly greater than that from monetary reward in the daINS when the MID was modeled at both the cue ([[SucroseHigh > Water] > [Win5 > Neutral]cue]; pFWE < 0.001, k = 50) and feedback ([[SucroseHigh > Water] > [Win5 > Neutral]feedback]; pFWE < 0.001, k = 47). The sucrose response was also greater than that from monetary feedback in the SMA ([SucroseHigh > Water] > [Win5 > Neutral]feedback, pFWE < 0.001, k = 60).
Correlations between [SucroseHigh > Water] and SRenjoy were greater than correlations between [Win5 > Neutral] for cue or feedback in both regions (psFWE < 0.007, ks ≥ 23; Fig. 3).
Recent drinking
Given the findings, we tested if extracted activation from the insula and SMA (at the peak of the association with SRenjoy) correlated with drinking behavior and related problems. Extracted [SucroseHigh > Water] activity was non-significantly correlated with AUDIT (r = 0.14, p = 0.099) and at trend level with drinks/week (r = 0.16, p = 0.052) in the SMA sphere. AUDIT correlated non-significantly with [SucroseHigh > Water] in the daINS (r = 0.15, p = 0.083). [Win5 > Neutral]cue correlated non-significantly with drinks/week (r = 0.15, p = 0.083) in the SMA. There were no correlations with [Win5 > Neutral]feedback.
Family history of AUD, Sweet-liking, and sex
Extracted spheres of mean BOLD responses in insula and SMA were analyzed in FHA(2) × Sex (2) × Sweet-liking (2) factorial models. There were no significant effects (ps > 0.093).
Recent nicotine use
In those without recent nicotine use (n = 102; Table S10), correlations between SRenjoy and [SucroseHigh > Water] remained significant (ps < 0.003). There were no additional significant associations (ps > 0.072).
Discussion
High-concentration sucrose, but not low-concentration sucrose or monetary reward, elicited right daINS and SMA responses that predicted alcohol’s pleasant intoxicating effects. Those with greater activation to intensely sweet taste reported greater enjoyable intoxicating effects to a fixed intravenous alcohol exposure, a risk for AUD [12].
We found no association between alcohol’s sedative effects and the brain response to sucrose. This is perhaps unsurprising as sucrose is an intense primary reward that would involve salience, stimulation, and reward [37], rather than sedation. Enjoyable intoxication was, however, related to SucroseHigh-induced daINS and SMA activation. The right daINS correlation with a principal component including perceived stimulation is consistent with this area’s role in sympathetic autonomic sensation [38,39,40,41]. The ventral SMA neighbors the dorsal anterior cingulate considered responsible for reward-based decision-making and limbic motor responses [41, 42]. Both of these midcingulo-insular salience network [43] areas are implicated in addiction maintenance [44].
Moreover, the association between daINS and SMA activation and enjoyment was unique to SucroseHigh, even though SucroseLow and MID reward cues evoked robust BOLD daINS activation. This 1) suggests a stimulus intensity threshold below which these regions’ responses do not correlate with subjective response to alcohol, even when activation is prominent and 2) illustrates the potential importance of reward type in understanding brain markers of AUD risk. As noted by Sescousse and colleagues [9], regional responses to food and money differ [9] despite overlap [7, 45,46,47].
As above, both the daINS and ventral SMA are nodes of the salience network [48] that is implicated in AUD risk [44, 47, 49,50,51], and where lesions can result in addiction remission [44, 51]. This network is thought to orient individuals to external stimuli [52,53,54], with the daINS serving as a hub between multiple functional networks [38, 55,56,57]. It is also thought to integrate internal states with external stimuli to evaluate their relevance [38, 40], thus maintaining allostasis by comparing predicted and actual states [58], and directing organisms toward or away from stimuli to match a predicted outcome.
In particular, Feldman-Barrett and Simmons [59] proposed a predictive interoceptive model in which the anterior insula and cingulate’s agranular/dysgranular cortices hold predictions that are compared to sensory information in granular cortices. When afferent information differs from the prediction, mechanisms 1) move or change body states to align the afferent information to the prediction, 2) reinterpret the sensory input, or 3) alter the prediction in a Bayesian manner. These authors suggest that the relative lack of granular cells in these areas increases the inertia of the predictions so that signals from the afferent granular regions are altered more often while the prediction is unchanged. Our results could then reflect a process in which intense sensory stimuli induce a body state that deviates from the allostatic prediction. This discrepancy could then increase salience region activity as they move to align sensation and prediction.
Our findings add to mixed results for sweet-liking, familial AUD, and drinking [2, 60,61,62,63,64,65,66,67,68,69]. We found no main effects of family history or sweet-liking on self-administration, but family history interacted with sweet-liking: FHP sweet-likers administered less alcohol than FHP sweet-dislikers (and vice-versa for FHN). Sweet-liking was also unassociated with recent drinking or family history. This is inconsistent with findings where FHP sweet-likers drink more than FHP sweet-dislikers [70], and where sweet-liking is a heritable trait linked with AUD [2, 61, 64, 69, 70]. Our recruitment strategy of balancing family history by recent drinking may explain the discrepancy.
Alcohol self-administration’s external validity [71,72,73,74,75] was evident in its association with recent drinking, while the prime’s sedative effects inversely correlated with subsequent self-administration, recent self-reported drinking, and problematic drinking. Enjoyable intoxication was, however, negatively related to self-administration only when covarying for recent drinking, when the association between sedation and self-administration was no longer statistically significant. This implies that those less sensitive to the intoxicating effects of alcohol’s rising limb, whether from acquired or innate tolerance, subsequently administer more. While Newlin & Thomson’s differentiator model [13] would seemingly suggest the opposite, their model did not propose controlling for recent drinking history, nor did it rely on data from the same principal component used here. Similarly, Schuckit’s [11] Low Level of Response Model appears a better fit for these data, but Schuckit again asked different questions (body sway, high, etc..), and used an alcohol challenge method that would cause wide variation in brain alcohol exposure level and slope at measured time points [17].
In distinction to our result, meta-analyses show stimulation is positively related to recent drinking [76], with longitudinal studies [12, 77, 78] showing that stimulation from an 80 mg% BrAC oral challenge predicts binge drinking and AUD progression. Here again, SRenjoy was more complex than stimulation alone, and its relationship to intravenous self-administration during this one session may not reflect broader drinking patterns as they evolve over time. In that regard, not only is route of administration different (including effects of flavor, gastric sensation, cephalic phase of ingestion, first-pass metabolism, etc.), but level of exposure (80 mg% target vs. 30 mg%) and environment (living room-like vs. hospital room) are differences that could contribute to the discrepant results.
Study limitations
We used only sweet solutions and cannot confirm if our findings are sweet-specific or if other intense tastes (e.g., bitter, umami) would show similar associations. This relationship may also not be specific to gustation. Our prime was modest in magnitude, complicating comparisons to higher oral alcohol challenges (e.g., 80 mg%). Interesting trends reflected correlations between SucroseHigh-induced right daINS and SMA responses and problematic drinking. However, the cross-sectional design cannot determine if this is a cause or consequence of alcohol consumption, or if it predicts future AUD. Due to time constraints, our fMRI paradigm included only two concentrations of oral sucrose administration, making it impossible to assess effects from intermediate concentrations.
Conclusion
daINS and SMA responses to a highly intense sweet taste are associated with self-reported enjoyable alcohol intoxication, a known risk for AUD. This association was not evident with a mildly sweet taste or monetary reward. Our data do not, however, support hypotheses about relationships between alcohol self-administration and (1) liking of and (2) regional brain responses to an intensely sweet taste. Future research into brain processing of other primary rewards could be useful, with potential as a biomarker that scales with enjoyable intoxication, and which can be used in children.
Change history
28 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41386-023-01713-1
References
Kampov-Polevoy AB, Overstreet DH, Rezvani AH, Janowsky DS. Saccharin-induced increase in daily fluid intake as a predictor of voluntary alcohol intake in alcohol-preferring rats. Physiol Behav. 1995;57:791–5.
Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–95.
Kareken DA, Dzemidzic M, Oberlin BG, Eiler WJ 2nd. A preliminary study of the human brain response to oral sucrose and its association with recent drinking. Alcohol Clin Exp Res. 2013;37:2058–65.
Eiler WJA 2nd, Dzemidzic M, Soeurt CM, Carron CR, Oberlin BG, Considine RV, et al. Family history of alcoholism and the human brain response to oral sucrose. Neuroimage Clin. 2018;17:1036–46.
Rudenga KJ, Small DM. Ventromedial prefrontal cortex response to concentrated sucrose reflects liking rather than sweet quality coding. Chem Senses. 2013;38:585–94.
Carney MA, Tennen H, Affleck G, Del Boca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time "electronic interviews". J Stud Alcohol. 1998;59:447–54.
Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60.
Sescousse G, Caldu X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–96.
Abrantes AM, Kunicki Z, Braun T, Miranda R Jr., Blevins CE, Brick L, et al. Daily associations between alcohol and sweets craving and consumption in early AUD recovery: results from an ecological momentary assessment study. J Subst Abus Treat. 2022;132:108614.
Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–9.
King A, Vena A, Hasin DS, deWit H, O’Connor SJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder. Am J Psychiatry. 2021;178:560–71.
Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402.
Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–8.
Plawecki MH, Decarlo R, Ramchandani VA, O’Connor S. Improved transformation of morphometric measurements for a priori parameter estimation in a physiologically-based pharmacokinetic model of ethanol. Biomed Signal Process Control. 2007;2:97–110.
Ramchandani VA, Plawecki M, Li TK, O’Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009;33:938–44.
Cyders MA, Plawecki MH, Corbin W, King A, McCarthy DM, Ramchandani VA, et al. To infuse or ingest in human laboratory alcohol research. Alcohol Clin Exp Res. 2020;44:764–76.
Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr., et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58.
Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–61.
Costa PT, McCrae RR. Normal personality assessment in clinical practice: the NEO Personality Inventory. Psychol Assess. 1992;4:5–13.
Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401.
Cyders MA, Littlefield AK, Coffey S, Karyadi KA. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addict Behav. 2014;39:1372–6.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection Of Persons With Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804.
Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–23.
Green B, Shaffer G, Gilmore M. A semantically-labeled magnitude scale of oral sensation with apparent ratio properties. Chem Senses. 1993;18:683–702.
Marciani L, Pfeiffer JC, Hort J, Head K, Bush D, Taylor AJ, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. J Neurosci Methods. 2006;158:186–94.
Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7.
Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–65.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–90.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77.
Liu RX, Kuang J, Gong Q, Hou XL. Principal component regression analysis with SPSS. Comput Methods Prog Biomed. 2003;71:141–7.
Ashburner J, Barnes G, Chen C-C, Daunizeau J, Flandin G, Friston K, et al. SPM12 manual. London, UK: Wellcome Trust Centre for Neuroimaging; 2014. p. 4.
Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci. 2020;23:1421–32.
Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113:7900–5.
Roberts CA, Giesbrecht T, Fallon N, Thomas A, Mela DJ, Kirkham TC. A systematic review and activation likelihood estimation meta-analysis of fMRI studies on sweet taste in humans. J Nutr. 2020;150:1619–30.
Centanni SW, Janes AC, Haggerty DL, Atwood B, Hopf FW. Better living through understanding the insula: why subregions can make all the difference. Neuropharmacology. 2021;198:108765.
Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71.
Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci. 2011;1225:72–82.
Vogt BA. Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28–46.
Uddin LQ, Yeo BTT, Spreng RN. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32:926–42.
Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69.
Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. 2019;99:2115–40.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Grodin EN, Cortes CR, Spagnolo PA, Momenan R. Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend. 2017;179:100–8.
Halcomb ME, Chumin EJ, Goni J, Dzemidzic M, Yoder KK. Aberrations of anterior insular cortex functional connectivity in nontreatment-seeking alcoholics. Psychiatry Res Neuroimaging. 2019;284:21–28.
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–55.
Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum Brain Mapp. 2014;35:2265–84.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15.
Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–9.
Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–89.
Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Wang Y, Zhu L, Zou Q, Cui Q, Liao W, Duan X, et al. Frequency dependent hub role of the dorsal and ventral right anterior insula. Neuroimage. 2018;165:112–7.
Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–29.
Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16:419–29.
Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–9.
Lange LA, Kampov-Polevoy AB, Garbutt JC. Sweet liking and high novelty seeking: independent phenotypes associated with alcohol-related problems. Alcohol Alcohol. 2010;45:431–6.
Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 1997;154:269–70.
Kampov-Polevoy AB, Garbutt JC, Davis CE, Janowsky DS. Preference for higher sugar concentrations and Tridimensional Personality Questionnaire scores in alcoholic and nonalcoholic men. Alcohol Clin Exp Res. 1998;22:610–4.
Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27:1743–9.
Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, et al. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol. 2007;42:75–9.
Krahn D, Grossman J, Henk H, Mussey M, Crosby R, Gosnell B. Sweet intake, sweet-liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav. 2006;31:622–31.
Kranzler HR, Sandstrom KA, Van Kirk J. Sweet taste preference as a risk factor for alcohol dependence. Am J Psychiatry. 2001;158:813–5.
Tremblay KA, Bona JM, Kranzler HR. Effects of a diagnosis or family history of alcoholism on the taste intensity and hedonic value of sucrose. Am J Addict. 2009;18:494–9.
Kampov-Polevoy A, Lange L, Bobashev G, Eggleston B, Root T, Garbutt JC. Sweet-liking is associated with transformation of heavy drinking into alcohol-related problems in young adults with high novelty seeking. Alcohol Clin Exp Res. 2014;38:2119–26.
Kampov-Polevoy A, Tzoi M, Zvartau E, Crews F, Neznanov N. Sweet liking, family history of alcoholism, blood glucose level, and alcohol craving in hospitalized alcoholics. Alcohol: Clin Exp Res. 1998;22:197А–97А.
Bujarski S, Jentsch JD, Roche DJO, Ramchandani VA, Miotto K, Ray LA. Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology. 2018;43:1891–9.
Sloan ME, Gowin JL, Janakiraman R, Ester CD, Stoddard J, Stangl B, et al. High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addict Biol. 2020;25:e12734.
Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094–101.
Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, et al. Exposure-response relationships during free-access intravenous alcohol self-administration in nondependent drinkers: influence of alcohol expectancies and impulsivity. Int J Neuropsychopharmacol. 2017;20:31–39.
Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, et al. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE). Psychopharmacology. 2009;202:689–97.
Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1759–70.
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99.
King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806.
Acknowledgements
The authors gratefully acknowledge Azziza Ahdoot, Rachel Baum, Paige Erb, Tetlu Myint, Caron Peper, Kathryn Snyder, Christina Souert, Shreya Patel. Dr. Claire Carron, and Cari Tsinovoi for subject recruiting and management, Drs. Sarine Janetsian-Fritz and William J.A. Eiler for intellectual contributions to previous iterations of this project, Dr. Yu-Chien Wu, Dr. Sourajit Mustafi, Michele Dragoo, Traci Day, and Robert Bryant Jr. for technical assistance with MRI, Keegan Sawin and Hannah Marotta for assistance with data management and study day expertise, and Dwight Hector, Ph.D. for design refinements and construction of the gustometer. We further thank Dr. Brian Knutson for permitting us the use of his monetary incentive delay task as well as Dr. James Bjork for early discussions on its use.
Funding
This work was supported by an NIAAA fellowship T32 AA07462 to JPA, and by R01 AA022476, P60 AA007611 to DAK.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: DAK, MD, JH, SJO’C, AEKK; data collection: GC, KB; analysis and interpretation of results: JPA, DAK, MD, GC, KB. Draft manuscript preparation: JPA, MD, DAK. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the publication date for reference 21 was corrected from 2016 to 1977. The correct reference should read “Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401”.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alessi, J., Dzemidzic, M., Benson, K. et al. High-intensity sweet taste as a predictor of subjective alcohol responses to the ascending limb of an intravenous alcohol prime: an fMRI study. Neuropsychopharmacol. 49, 396–404 (2024). https://doi.org/10.1038/s41386-023-01684-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01684-3
- Springer Nature Switzerland AG