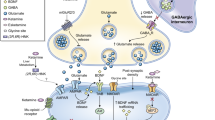
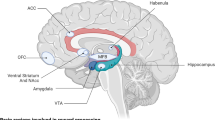
Abstract
The vast majority of treatments for psychiatric and substance use disorders take weeks to work. Notable exceptions to this rule exist, with some treatments such as intravenous ketamine resolving symptoms in minutes to hours. Current research is focused on identifying novel approaches to rapid-acting psychotherapeutics. Promising results from studies of novel classes of drugs and innovative brain stimulation therapies are currently being studied through both clinical and pre-clinical research, as described here. Research focused on understanding neurobiological mechanisms, effective therapeutic context, and implementation approaches are needed to maximize the potential reach of these therapies.
Similar content being viewed by others
The vast majority of drugs for psychiatric and substance use disorders take many days or weeks to work. This simple fact has caused many to assume that rapid-onset therapeutics are impossible; that resolution of complex mood, behavioral and cognitive symptoms unavoidably takes time. Yet this assumption is unmerited, as evidenced by numerous rigorous demonstrations of rapid symptom resolution following a variety of interventions. Depression, for example, can resolve immediately after prolonged sleep deprivation (only to recur equally immediately after a nap) [1]. Neurostimulation can sometimes bring about rapid (within days) or ultra-rapid (minutes to hours) improvement in patients with major depressive disorder or obsessive-compulsive disorder [2,3,4]. And ketamine, esketamine and brexanolone have each demonstrated the potential to resolve depressive symptoms and suicidality in hours [5, 6]. Similarly, evidence that stroke lesions in specific brain regions have been associated with immediate and long-lasting resolution of drug craving is indicative for the possibility of immediate symptom resolution in addiction [7].
Of current psychoactive treatments with the potential for rapid action, there are considerable strengths and opportunities, but also limitations and concerns. Electroconvulsive therapy (ECT) can be tremendously effective, and recent research focused on optimal administration protocols and dosing has reduced side effects considerably [8]. Deep brain stimulation has helped many patients with recalcitrant, treatment-resistant depression, and transcranial magnetic stimulation is generally well tolerated; reports have suggested that both can act rapidly (hours to days) [2, 9] though neither is as clearly effective or rapid as ECT or ketamine [10, 11]. Preliminary evidence also indicates benefits from deep brain stimulation in patients suffering from severe alcohol and opioid use disorders [12] and transcranial magnetic stimulation has been proven effective in the treatment of tobacco cessation [13]. Ongoing research in neurostimulation promises to enhance efficacy and reduce side effects with greater understanding of the crucial circuits to target and improved specificity at targeting these circuits and personalizing their location. Similarly, rapid-acting psychopharmacological agents are generally well-tolerated and can have effects that last weeks after a single administration [14]. Limitations of deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS) include ongoing questions of effectiveness and speed of response. The more established treatments, including ECT, ketamine and brexanolone, while effective and rapid, can be challenging to access for many patients, carry various side effects ranging from memory loss to uncomfortable dissociative experiences, and, for some of the rapid-acting drugs, can be liable to misuse potential [8, 14]. Finally, beyond depression, there remains a paucity of evidence for rapid therapeutic efficacy.
Accordingly, there is a need for additional research to ensure that the fast-acting agents are effective for the indication being used, and to incorporate a wider diversity of patients in clinical trials. Additional treatment avenues are needed to expand options available for rapid-onset relief, including novel classes of pharmacological agents, improved neurostimulation approaches and their combinations. A greater understanding of the neurobiological mechanisms by which existing rapid treatments work will help in the development of novel and effective interventions. Finally, for these new treatments as well as those already available, research that focuses on how to implement them is needed to widen availability, increase equity in access, and ensure their use is effective in real-world settings.
Novel treatment approaches for mental illness
Considerable efforts have been put in to developing novel therapeutic approaches that have the potential to rapidly relieve symptoms and restore function in individuals with mental illnesses. In the psychopharmacology arena, current efforts build upon the success of ketamine and brexanolone. One particularly active area of research is the study of psychedelics as potential therapeutics. Following on promising open-label or small, placebo-controlled trials, larger scale phase 2 and 3 clinical trials of 3,4-Methylenedioxymethamphetamine (MDMA) for post-traumatic stress disorder (PTSD) and psilocybin for major depressive disorder (MDD) have begun to report out efficacy results.
While MDMA appears to be effective for treating PTSD, there is no clear evidence of a rapid-onset effect. In a phase 3 clinical trial of MDMA-assisted psychotherapy for PTSD, Mitchell et al. [15] showed that MDMA plus therapy was more effective than placebo plus therapy. This study utilized an 18 week course that included three 8-hour guided MDMA-assisted therapy sessions, plus a total of 9 weekly 90-min “integration” sessions. The results were promising; nearly 30% of individuals who underwent MDMA-assisted therapy remitted, compared to less than 5% of those who received therapy with placebo. Given the experimental design, however, the rapidity of onset was difficult to assess for the evaluations were made 3–9 weeks after each experimental session. Furthermore, the benefits of treatment (in both placebo and MDMA groups) continued to accrue throughout the 18-week course, suggesting a more gradual onset of improvement.
By contrast, early evidence supports the notion that psilocybin may result in a rapid improvement in MDD symptoms. In a recent phase 2 placebo-controlled clinical trial, a single 25 mg dose of psilocybin resulted in a reduction in depression symptoms compared to a 1 mg dose (placebo) [16]). The protocol included three psychoeducation and trust-building sessions; the 6–8 h drug treatment session, and two integration sessions. Nearly 30% of patients met criteria for remission by the third week, with two thirds of them retained that status at week 12. Notably, the reduction in symptoms was greatest when assessed the day after the drug treatment, suggesting a rapid onset of the antidepressant effect. A larger phase III trial is currently underway.
Neurosteroids are another promising class of drugs with the potential for rapid onset of therapeutic effect. Brexanolone, an intravenous preparation of the neurosteroid hormone allopregnanolone, ameliorates post-partum depression within 3–5 days, and was approved by the Food and Drug Administration in 2019 [6]. Orally bioavailable analogs of brexanolone would help increase access to this novel class of agents. In a phase 2 clinical trial, zuranolone (SAGE-217), one such analog, reduced symptoms in patients with MDD, although the time course was not particularly rapid, with improvement taking place continuously over the course of two weeks [17]; more data is needed to evaluate response onset.
Collectively, these agents suggest the possibility that several different pharmacological approaches may yield novel psychotherapeutics that have the potential to reduce the time it takes for patients to get better. Other novel antidepressant agents with the potential for rapid onset of effect include the combination of dextromethorphan and buproprion, which separated from placebo in the first week after initiation in one study [18].
Another treatment approach with promise is the utilization of various brain stimulation methods to achieve rapid psychotherapeutic effect. It has long been recognized that ECT has antidepressant effects that occur more rapidly than traditional antidepressants, with onset of action in as little as 5 days [1, 3]. A similar time course has been described for deep brain stimulation [2, 4], while the time courses of other, noninvasive brain stimulation modalities are unclear but seemingly not as rapid (see for example, [19]). Current research attempts to build on both the efficacy and speed of brain stimulation therapies by enhancing target specificity and individualized treatment. For example, a recent study using closed-loop stimulation to specifically alter depression-associated neural activity patterns was able to reduce depressive symptoms in a single patient within 1 week [20]. Similarly, a pilot study in 2 patients with binge eating disorders reported that use of a closed-loop system to stimulate the caudate promptly reduced food craving and compulsive eating [21].
Novel treatment approaches for substance use disorders
Multiple targets have been identified for the potential development of medications to treat substance use disorders. Most have targeted the reduction of drug consumption, which is evaluated in clinical trials as the percent of patients that achieve abstinence over a 3 week period or longer. Effects on reducing drug consumption increase over time and are aided by behavioral interventions. Currently, research on validating alternative outcomes (reducing craving, withdrawal, anxiety) is an unmet priority, since these might facilitate studies of treatments that provide immediate relief and facilitate predictions of future drug use. As of now only pilot studies have reported fast relief in some patients with substance use disorders for psylocibin [22], ketamine [23] and ibogaine [24], though the safety of these drugs in these patient populations still needs to be evaluated.
One promising future possibility is the development of immunotherapies. Vaccines or antibodies can sequester drugs in the peripheral circulation, precluding entry into the brain [25]. Such agents might rapidly blunt the effects of drugs. Nonetheless, the relief of cravings and other symptoms would require a longer period of time in order to re-learn the association that taking a drug is no longer rewarding.
Several clinical trials are evaluating the efficacy of invasive and non-invasive neuromodulation to treat various SUDs. Most of the studies that used TMS or transcranial direct current stimulation (TDCS) require repeated treatment to observe a reduction in drug consumption and decreases in craving [26,27,28]. Peripheral nerve stimulation trials have been done predominantly to treat acute withdrawal symptoms and the responses seem to be fast, but its hard to determine their true efficacy since the effects were not compared with placebo particularly since withdrawal symptoms decrease rapidly following drug discontinuation [29]. More recently, pilot studies of deep brain stimulation have reported immediate reduction in craving and anxiety in patients with severe OUD and ongoing trials are evaluating their efficacy in larger patient samples (https://www.clinicaltrials.gov/ct2/show/NCT05303428). Finally, studies are evaluating the efficacy of low energy focused ultrasound (LIFU), which can penetrate into deep brain nuclei non-invasively, to stimulate the nucleus accumbens for the treatment of opioid use disorders (OUDs) (https://www.clinicaltrials.gov/ct2/show/NCT05303428); preliminary non-published findings indicated immediate reduction in craving and anxiety. Trials combining neuromodulation with other substance use disorder treatments, including medication or behavioral interventions, are also ongoing. In general, there is increased recognition that combinations of treatments may accelerate responses and improve outcomes.
Novel treatment approaches for alcohol use disorders
Multiple targets and techniques have been identified for the development of rapid treatments for alcohol use disorder. As of now, only a few psychotherapeutics have shown promise at producing rapid changes in symptoms of substance use disorders (for review see [30]). For instance, in a small trial of 10 subjects with alcohol use disorder (AUD), two supervised sessions of psylocibin treatment resulted in decreases in craving and increases in abstinence that largely persisted over 36 weeks [31]. A meta-analysis of clinical trials using LSD to treat AUD found initial reductions in alcohol consumption that mostly faded over the following year [32]. Research suggests ketamine might have utility for treating AUD [33], though findings are limited [34]. In one study of subjects with AUD, ketamine combined with motivational enhancement therapy (MET) reduced heavy drinking days and increased the likelihood of abstinence compared to subjects receiving a benzodiazepine combined with MET [35]. In a clinical trial involving 96 subjects, three infusions of ketamine resulted in a greater number of days abstinent at 6 month follow-up compared to a placebo [36]. Brain stimulation, either via transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS) or via deep brain stimulation (DBR) with implanted electrodes, has been explored as a means of treating AUD. In a small study, tDCS of the prefrontal cortex decreased the likelihood of relapse to alcohol [37]. Other studies using tDCS have not found positive results for AUD [38]. Stimulating activity in the prefrontal cortex using (TMS) resulted in a reduction in heavy drinking days and an increase in days abstinent for subjects with AUD [39]. A metanalysis of 15 clinical trials concluded that TMS, but not tDCS, of the dorsolateral prefrontal cortex reduced craving in subjects with AUD [40]. In a randomized trial of 12 subjects with treatment resistant AUD, deep brain stimulation of the nucleus accumbens did not result sustained abstinence but did lead to a higher proportion of abstinent days, lower levels of alcohol craving, and improvements in affect across 6 months of treatment [12].
A small number of studies have attempted to reduce craving and subsequent alcohol use by temporarily altering activity in brain neuropeptide systems. Ghrelin is a hormone produced in the gut that stimulates hunger, and has been associated with craving for alcohol in individuals with AUD. In preliminary research, administration of a ghrelin antagonist to heavy drinkers reduced cue-induced craving in a bar lab setting [41]. Oxytocin is a neuropeptide associated with reduced feelings of stress. Findings have been mixed regarding the potential utility of using intranasal oxytocin to reduce craving and use cigarettes, opioids, cocaine and alcohol (e.g., [42,43,44]). Further research is needed to assess the safety and efficacy of antagonists associated with craving as a potential rapid onset treatment for symptoms of AUD.
Finally, in the alcohol field, as with substance use disorders, changes in particular clinical markers, such as reductions in use, decreases in craving, and improvements in affect during withdrawal, can be considered positive outcomes in addition to abstinence as a primary measure of effectiveness in clinical trials. Acceptance of more “harm reduction” endpoints could influence the likelihood of recovery in the long-term and may reveal a larger toolbox of rapid treatments.
Strengthening and modernizing research approaches
A strengthened and modernized research agenda is needed to capitalize on these promising new treatment modalities and maximize the potential benefit of rapid acting treatments for mental illnesses and substance and alcohol use disorders. Going forward, research must focus on understanding how to apply these treatments with equity in real world settings, on developing biomarkers and other tools to identify who will benefit from which treatments and on how best to personalize interventions, and clarifying the mechanisms by which the treatments work to enable further improvements in reach and efficacy.
The most immediate need is for research that focuses on how these rapid acting treatments can be used in the real world. Treatments that work in hours or days instead of weeks have the potential to significantly reduce morbidity and mortality and to improve care and reduce the need for intensive interventions such as inpatient hospitalization. But this potential can only be realized if research answers key questions about how to use them effectively. For example, intravenous ketamine can reduce depressive symptoms including suicidal thoughts within a few hours of administration [45]. If ketamine or other rapidly acting agents could be administered in the emergency room, would they enable patients at high risk to be discharged to an outpatient setting, reducing the need for inpatient admissions? What safeguards would be needed to enable safe referrals in this situation? How long does the reduction in suicidal ideation last? Answering these questions could radically alter care for the depressed patient at risk of suicide, but only if it is safe to do so.
A second issue of near-term importance is understanding how long the therapeutic effects of these treatments last. The best studied of these treatments — ketamine and ECT — typically last a few days to a few weeks, requiring chronic intermittent treatment or additional agents to sustain a response. Early data on the psychedelics suggest that responses might last weeks or even months. Clarifying how long these effects last and what is needed to sustain them, as well as the safety of chronic intermittent dosing if it proves necessary, is needed in order for patients and care providers to make appropriate clinical decisions regarding how best to use these approaches.
Finally, cost-effectiveness and equity considerations must be thoroughly studied. For some of these treatments, the amount of time and/or financial resources required look to be considerable. Cost-effectiveness studies similar to that conducted for the phase 3 MDMA trial [46] would assist in understanding the true costs of these new approaches, including potential benefits in terms of increased productivity and reduced health care costs. The costs of implementing these novel treatments, inclusive of clinical training of personnel involved in treatment delivery, equipment and space, and administrative and other costs need also to be considered to determine the true cost-effectiveness balance. Demonstrating cost-effectiveness would enable public mental health systems including those that fund treatment for substance use disorders and as well as private funders to justify adopting these novel approaches.
Extending beyond generic cost-effectiveness, it is already clear that any given rapid-acting treatment approach, like all currently available psychotherapeutics, will not work for everyone. Understanding who will most likely benefit from each treatment will require innovation and experimentation. There is some progress on this front, with electroencephalography [47] and functional magnetic resonance imaging [48] measures demonstrating promise as potential biomarkers for treatment responses to antidepressants. Prospective studies are needed to determine whether these or other approaches could, on an individual patient level, predict response to different treatment approaches with sufficient specificity to be helpful in clinical decision-making.
Mechanistically there is also much work to be done. There is already some intriguing convergence between studies of ketamine and psychedelics, which both appear to have effects at the level of neural circuit plasticity [49]. More basic work remains, including which receptors and second messenger systems are crucial for the psychotherapeutic effect of the psychedelics, or what neural pathways are necessary to engage with brain stimulation paradigms. At a clinical mechanistic level, it will also be important to clarify the role of context in treatment, particularly regarding the various “set and setting” parameters and psychotherapy elements included, but not controlled for, in current clinical trials of MDMA and psilocybin.
Finally, it is clear that the dependent variable used to characterize the success of a rapid treatment should be considered. For example, suicidal ideation may remit earlier than other symptoms associated with depression and a decrease in heavy drinking may follow a more rapid time course in alcohol use disorder than abstinence or remission of all the major symptoms of a moderate to severe alcohol use disorder. Advances in understanding the nosology of mental illness and alcohol and drug disorders will not only advance individualized approaches to treatment but also facilitate the discovery of more rapid treatments.
Conclusions
The pathway forward to ensuring the reach and effectiveness of rapid-acting psychotherapeutic approaches seems clear. Promising areas have been identified and strong research programs established, particularly in depression, PTSD, and substance use disorders. Further research is needed in implementation science, biomarkers discovery, and therapeutic mechanism to help ensure these early efforts are sustained and transformed into improved mental health and substance use disorder care and strengthened public health. Yet gap areas remain, particularly with regards to comorbidities and polysubstance use, objective metrics for measurement of functional recovery, biomarkers to aid treatment decisions, and approaches to sustained rapid effects to accelerate recovery. Additional attention to these areas would strengthen the overall approach, unlocking the previously unthinkable potential for rapidly effective and sustainable treatments for individuals bearing the burden of mental illness and substance and alcohol use disorders.
References
Post RM, Uhde TW, Rubinow DR, Huggins T. Differential time course of antidepressant effects after sleep deprivation, ECT, and carbamazepine: clinical and theoretical implications. Psychiatry Res. 1987;22:11–9.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Rodger CR, Scott AI, Whalley LJ. Is there a delay in the onset of the antidepressant effect of electroconvulsive therapy? Br J Psychiatry. 1994;164:106–9.
Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–12.
Yavi M, Lee H, Henter ID, Park LT, Zarate CA Jr. Ketamine treatment for depression: a review. Discov Ment Health. 2022;2:9.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480–89.
Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4.
Hermida AP, Glass OM, Shafi H, McDonald WM. Electroconvulsive therapy in depression: current practice and future direction. Psychiatr Clin North Am. 2018;41:341–53.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26.
Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2016;16:1–66.
Figee M, Riva-Posse P, Choi KS, Bederson L, Mayberg HS, Kopell BH. Deep brain stimulation for depression. Neurotherapeutics. 2022;19:1229–45.
Bach P, Luderer M, Müller UJ, Jakobs M, Baldermann JC, Voges J, et al. Deep brain stimulation of the nucleus accumbens in treatment-resistant alcohol use disorder: a double-blind randomized controlled multi-center trial. Transl Psychiatry. 2023;13:49.
Zangen A, Moshe H, Martinez D, Barnea-Ygael N, Vapnik T, Bystritsky A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. 2021;20:397–404.
Iqbal SZ, Mathew SJ. Ketamine for depression clinical issues. Adv Pharm. 2020;89:131–62.
Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27:1025–33.
Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387:1637–48.
Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381:903–11.
Iosifescu DV, Jones A, O’Gorman C, Streicher C, Feliz S, Fava M, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83:21m14345.
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16.
Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27:1696–700.
Shivacharan RS, Rolle CE, Barbosa DAN, Cunningham TN, Feng A, Johnson ND, et al. Pilot study of responsive nucleus accumbens deep brain stimulation for loss-of-control eating. Nat Med. 2022;28:1791–96.
Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28:983–92.
Jones JL, Mateus CF, Malcolm RJ, Brady KT, Back SE. Efficacy of ketamine in the treatment of substance use disorders: a systematic review. Front Psychiatry. 2018;9:277.
Mash DC, Duque L, Page B, Allen-Ferdinand K. Ibogaine detoxification transitions opioid and cocaine abusers between dependence and abstinence: clinical observations and treatment outcomes. Front Pharm. 2018;9:529.
Luba R, Martinez S, Jones J, Pravetoni M, Comer SD. Immunotherapeutic strategies for treating opioid use disorder and overdose. Expert Opin Investig Drugs. 2023;32:77–87.
Belgers M, Van Eijndhoven P, Markus W, Schene AH, Schellekens A. rTMS reduces craving and alcohol use in patients with alcohol use disorder: results of a randomized, sham-controlled clinical trial. J Clin Med. 2022;11:951.
Gay A, Cabe J, De Chazeron I, Lambert C, Defour M, Bhoowabul V, et al. Repetitive transcranial magnetic stimulation (rTMS) as a promising treatment for craving in stimulant drugs and behavioral addiction: a meta-analysis. J Clin Med. 2022;11:624.
Qin J, Chen J, Wang Y, Zou Z. Effects of psychoeducation combined with transcranial direct current stimulation on reducing cigarette craving and consumption in male smokers. Addict Behav. 2023;141:107643.
Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abus. 2018;44:56–63.
da Costa SC, Oesterle T, Rummans TA, Richelson E, Gold M. Psychedelic drugs for psychiatric disorders. J Neurol Sci. 2022;440:120332.
Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–99.
Krebs TS, Johansen P-Ø. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. 2012;26:994–1002.
Worrell SD, Gould TJ. Therapeutic potential of ketamine for alcohol use disorder. Neurosci Biobehav Rev. 2021;126:573–89.
Garel N, McAnulty C, Greenway KT, Lesperance P, Miron J-P, Rej S, et al. Efficacy of ketamine intervention to decrease alcohol use, cravings, and withdrawal symptoms in adults with problematic alcohol use or alcohol use disorder: a systematic review and comprehensive analysis of mechanism of actions. Drug Alcohol Depend. 2022;239:109606.
Dakwar E, Levin F, Hart CL, Basaraba C, Choi J, Pavlicova M, et al. A Single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: a randomized midazolam-controlled pilot trial. Am J Psychiatry. 2020;177:125–33.
Grabski M, McAndrew A, Lawn W, Marsh B, Raymen L, Stevens T, et al. Adjunctive ketamine with relapse prevention-based psychological therapy in the treatment of alcohol use disorder. Am J Psychiatry. 2022;179:152–62.
Holla B, Biswal J, Ramesh V, Shivakumar V, Bharath RD, Benegal V, et al. Effect of prefrontal tDCS on resting brain fMRI graph measures in alcohol use disorders: a randomized, double-blind, sham-controlled study. Prog Neuro Psychopharmacol Biol Psychiatry. 2020;102:109950.
den Uyl TE, Gladwin TE, Lindenmeyer J, Wiers RW. A clinical trial with combined transcranial direct current stimulation and attentional bias modification in alcohol-dependent patients. Alcohol Clin Exp Res 2018;42:1961–69.
Mahoney JJ 3rd, Hanlon CA, Marshalek PJ, Rezai AR, Krinke L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: Review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149.
Sorkhou M, Stogios N, Sayrafizadeh N, Hahn MK, Agarwal SM, George TP. Non-invasive neuromodulation of dorsolateral prefrontal cortex to reduce craving in alcohol use disorder: a meta-analysis. Drug Alcohol Depend Rep. 2022;4:100076.
Kharbanda KK, Farokhnia M, Deschaine SL, Bhargava R, Rodriguez-Flores M, Casey CA, et al. Role of the ghrelin system in alcohol use disorder and alcohol-associated liver disease: a narrative review. Alcohol Clin Exp Res. 2022;46:2149–59.
Melby K, Gråwe RW, Aamo TO, Skovlund E, Spigset O. Efficacy of self-administered intranasal oxytocin on alcohol use and craving after detoxification in patients with alcohol dependence. a double-blind placebo-controlled trial. Alcohol Alcohol. 2020;56:565–72.
Noël Raby W, Heller M, Milliaressis D, Jean Choi C, Basaraba C, Pavlicova M, et al. Intranasal oxytocin may improve odds of abstinence in cocaine-dependent patients: results from a preliminary study. Drug Alcohol Depend Rep. 2022;2:100016.
Van Hedger K, Bershad AK, Lee R, de Wit H. Effects of intranasal oxytocin on stress-induced cigarette craving in daily smokers. Nicotine Tob Res. 2018;22:89–95.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–58.
Marseille E, Mitchell JM, Kahn JG. Updated cost-effectiveness of MDMA-assisted therapy for the treatment of posttraumatic stress disorder in the United States: Findings from a phase 3 trial. PLoS One. 2022;17:e0263252.
Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, et al. An electroencephalographic signature predicts antidepressant response in major depression. Nat Biotechnol. 2020;38:439–47.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharm Sci. 2021;42:929–42.
Author information
Authors and Affiliations
Contributions
JAG, NDV, and GFK contributed equally to the manuscript. All authors conceived the content, wrote equal parts of the first draft, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gordon, J.A., Volkow, N.D. & Koob, G.F. No time to lose: the current state of research in rapid-acting psychotherapeutics. Neuropsychopharmacol. 49, 10–14 (2024). https://doi.org/10.1038/s41386-023-01627-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01627-y
- Springer Nature Switzerland AG