Abstract
The serotonin deficit hypothesis explanation for major depressive disorder (MDD) has persisted among clinicians and the general public alike despite insufficient supporting evidence. To combat rising mental health crises and eroding public trust in science and medicine, researchers and clinicians must be able to communicate to patients and the public an updated framework of MDD: one that is (1) accessible to a general audience, (2) accurately integrates current evidence about the efficacy of conventional serotonergic antidepressants with broader and deeper understandings of pathophysiology and treatment, and (3) capable of accommodating new evidence. In this article, we summarize a framework for the pathophysiology and treatment of MDD that is informed by clinical and preclinical research in psychiatry and neuroscience. First, we discuss how MDD can be understood as inflexibility in cognitive and emotional brain circuits that involves a persistent negativity bias. Second, we discuss how effective treatments for MDD enhance mechanisms of neuroplasticity—including via serotonergic interventions—to restore synaptic, network, and behavioral function in ways that facilitate adaptive cognitive and emotional processing. These treatments include typical monoaminergic antidepressants, novel antidepressants like ketamine and psychedelics, and psychotherapy and neuromodulation techniques. At the end of the article, we discuss this framework from the perspective of effective science communication and provide useful language and metaphors for researchers, clinicians, and other professionals discussing MDD with a general or patient audience.
Similar content being viewed by others
Introduction: the need to communicate an accessible and accurate framework of depression
Major depressive disorder (MDD) is one of the most common psychiatric conditions, yet it is highly heterogenous. What researchers and clinicians consider to be MDD is the presentation of a set of symptoms, defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), that must include persistent depressed mood and/or loss of interest or pleasure (anhedonia), as well as a certain number of symptoms such as excessive guilt, hopelessness and pathologically altered sleep, appetite, motivation, and cognition. However, patients who meet the criteria for MDD can have drastically different presentations and combinations of symptoms. For instance, one patient may experience too little sleep, while another experiences too much, though both can share a diagnosis of MDD.
Given the heterogeneous, multifactorial nature of MDD, a cohesive yet understandable conceptualization of the condition has been historically elusive. Before clinical depression was even known as MDD, it was hypothesized in the 1960s to arise from a deficit in monoamines, including norepinephrine, dopamine, and serotonin [1, 2]. Over the next several decades, evidence demonstrated that drugs that decrease serotonin produce depression symptoms while drugs that increase serotonin alleviate them, and the serotonin deficit hypothesis became colloquially known as the “chemical imbalance” hypothesis (reviewed in France et al. [3]). Typical antidepressants—such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors (MAOIs)—were said to treat depression by restoring normal levels of serotonin and other monoamines. However, the serotonin deficit hypothesis has been considered incorrect for at least the last 20 years due to insufficient evidence [4, 5]. Despite this, the serotonin deficit hypothesis has persisted in the public psyche, bolstered by pharmaceutical company advertisements for conventional antidepressants [6, 7]. Therefore, when a recent meta-analysis denounced the serotonin deficit model of MDD for lack of evidence [8], the media responded as if a major theory had just now been debunked [9,10,11,12,13,14], contributing to eroding public trust in science and medicine. Furthermore, the authors of the meta-analysis concluded that the lack of evidence for the serotonin deficit hypothesis suggested that typical serotonergic antidepressants must be ineffective and should not be prescribed [8], a dangerous message amid rising mental health crises around the world. Although the serotonin deficit model has been oversimplified and over-sold, serotonin is still critical for conventional treatments for MDD, as many others point out in responses to the meta-analysis [15,16,17]. Classic antidepressants that enhance serotonin transmission out-perform placebos in clinical studies and are effective in up to two-thirds of patients suffering from MDD [18].
The challenge with putting forth an updated model of MDD is that it must be simple enough to understand but complex enough to be accurate, as well as adaptable enough to incorporate future research discoveries. In this article, we describe a framework for understanding and communicating MDD pathophysiology and treatment based on current preclinical and clinical research findings. First, we summarize the multiple contributing biopsychosocial factors that contribute to MDD symptomology. Although MDD is highly heterogenous, a common feature is dysfunction in cognitive and emotional brain circuits—including the prefrontal cortex, hippocampus, and amygdala—that leave someone “stuck” in a state of maladaptive information processing that involves a persistent negativity bias [19]. Second, we give examples of how current therapeutic approaches, both pharmacological and non-pharmacological, have both acute and sustained effects that restore healthy cognitive and emotional processing—a process broadly defined as “neuroplasticity.” Neuroplasticity represents a category of mechanisms that alter synaptic strength and functional connectivity. The foundations for this neuroplasticity framework of MDD have been comprehensively reviewed in mechanistic detail by others [20,21,22,23]; in this article, our purpose is to summarize the key concepts with the intent to offer a high-level synthesis of MDD pathophysiology and treatment in the context of neuroplasticity. Finally, we discuss this neuroplasticity framework from the perspective of science communication, offering ways for researchers and clinicians to talk about MDD with the general public that are simple yet accurate.
A note on nomenclature
Throughout this article, the focus will be primarily on MDD as a DSM-defined diagnosis, as opposed to depression used in the general sense, which typically describes low mood states in the absence of a known diagnosis. When reviewing human studies, we will specify whether the findings pertain to MDD or to depressive symptoms. When discussing rodent studies, we will refer to depression-like effects or behaviors.
Pathophysiology of MDD understood as inflexible brain states
Biopsychosocial contributors to MDD
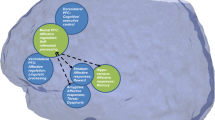
As with many disorders, genetic predispositions, environmental factors, and developmental windows all contribute to and interact with one another in the pathophysiology and treatment of MDD [24]. Genome-wide association studies (GWAS) have identified numerous genes associated with overall risk for MDD [25]; however, the contribution of each gene is small and genetic variations interact with environmental exposures [26]. Chronic stress exposure and traumatic experiences, particularly during childhood, are among the strongest environmental risk factors [27, 28]. Increased neuroinflammation, due to chronic stress or disease states, also contributes to the pathophysiology of MDD [29]. These myriad contributing factors converge in ways that are not yet fully understood to cause the symptoms of MDD. Although heterogeneous and distinct for each patient, these symptoms share features of dysfunction in brain circuits that process emotional and cognitive information and regulate survival functions like energy and motivation (Fig. 1). These dysfunctional circuits leave individuals “stuck” in a state characterized by negativity bias, depressed mood, anhedonia, and dysfunction in several other behavioral domains, such as motivation, appetite, and sleep [20, 24]. In contrast, non-depressed individuals retain the potential to shift fluidly between different environmental contexts and respond appropriately and adaptively to the negative and positive features of their internal and external environments.
MDD is a complex, multifactorial, biopsychosocial disorder with no single cause or homogenous presentation of symptoms. Factors such as stress, trauma, genetics, and more can contribute to MDD pathophysiology, which involves symptoms such as anhedonia, low affect, negativity bias, and cognitive and emotional inflexibility. Though the contributing factors and presentation of symptoms vary from patient to patient, MDD can be understood as inflexibility in circuits that process cognitive and emotional information and regulate motivation and arousal. MDD major depressive disorder.
Considerations regarding MDD heterogeneity and other depressive disorders
Despite some shared biopsychosocial risk factors and symptoms, MDD is a highly heterogeneous condition. For instance, childhood trauma is not a universal experience for adults with MDD. Furthermore, MDD can present with insomnia or hypersomnia as well as increased or decreased appetite. MDD is heterogeneous enough that it may be better characterized as multiple distinct categories, as considered in an editorial by Shorter [30]. For instance, a meta-analysis showed that the prevalence of suicidal ideation varies among different types of depression, and treating all instances of MDD identically with SSRIs may be problematic [30, 31]. Future understanding of the risk factors and mechanisms underlying heterogeneity in MDD, perhaps facilitated by advancements in precision psychiatry, will further refine the neuroplasticity framework of MDD pathophysiology and treatment.
Additionally, the biopsychosocial factors contributing to MDD are also involved in other depressive disorders that have similar symptoms, such as premenstrual dysphoric disorder (PMDD) and postpartum depression (PPD). The neuroplasticity framework that we discuss in this article is also relevant to the pathophysiology and treatment of PMDD and PPD, with a heightened emphasis on the role of hormones, such as estrogen, progesterone, and neuroactive steroids, that play an important role in neuroplasticity in cognitive and emotional circuits [32]. Although unique considerations of PMDD and PPD pathophysiology and treatment is beyond the scope of the current article, we refer interested readers to comprehensive reviews on these topics [33,34,35].
Behavioral level: negativity bias and impaired emotional and cognitive flexibility
A unifying feature of MDD, and depressive symptoms more generally, is a negativity bias in processing emotionally salient information, supporting the notion of being “stuck” in a negative brain state [19]. People with MDD are more likely to interpret neutral or ambiguous facial expressions as displaying a negative emotion, such as sadness [36]. They also pay more attention to negative information and remember it better. Additionally, people with MDD do not respond as positively to rewards, have decreased motivation to pursue them, and increased sensitivity to punishment [37, 38]. When negativity bias is heightened and reward sensitivity is dampened, people not only view the world and themselves through a negative lens but also struggle to benefit from positive experiences in their environment.
The negativity bias in MDD is a cognitive problem as well as an emotional one. Cognitive deficits are a feature of MDD, including executive dysfunction and attention and memory impairments, as previously reviewed [20, 39]. In particular, being stuck in a rut of negative information processing can be understood as an impairment in cognitive and emotional flexibility. Cognitive flexibility, a domain of executive functioning dependent on the prefrontal cortex (PFC; Fig. 2), is defined as the ability to adjust attention, goals, and actions according to a changing environment, either consciously or unconsciously [40]. Participants with MDD are slower to adapt to changing rules in cognitive flexibility tasks [41]. Similarly, emotional flexibility allows someone to switch between processing emotional and non-emotional information [42], as well as to experience and shift between a range of responses, rather than being stuck with a narrow or rigid repertoire of emotions [43]. People with a diagnosis of MDD or depression symptoms show impairments in both types of emotional flexibility [42, 43]. Collectively, these findings result in a tendency to perseverate on negative information and an impaired ability to respond to positive input in people with MDD.
A The prefrontal cortex (PFC; yellow) is a part of the neocortex involved in executive functioning, including cognitive and emotional flexibility. The hippocampus (blue) is important for learning and memory. The nucleus accumbens (NAc) is a region at the forefront of the caudate nucleus (orange) and is important for responding to rewards and generating motivation to pursue rewards. The amygdala (light green) is important for processing and regulating emotional information, both positive and negative. Each of these brain regions are connected with one another by cortico-mesolimbic pathways, which are impaired in MDD. In this image, the neocortex, including the PFC, has been removed from the right hemisphere of the brain for better visibility of the NAc, hippocampus, and amygdala. B MDD and its specific symptoms are associated with changes in volume, activity, and/or connectivity in brain regions and networks. The PFC and hippocampus show decreased volume. The PFC, hippocampus, NAc, and amygdala all primarily show decreased activity, though rumination is associated with increased PFC activity and negativity bias is associated with increased amygdala activity. Connectivity is decreased between the PFC, hippocampus, NAc, and amygdala. MDD major depressive disorder. A created by Clint Carlson, MS; B created with BioRender.com.
Network level: altered activity and connectivity in emotional and cognitive brain networks
The behavioral symptoms of MDD, including the negativity bias, come from changes in the volume, activity, and connectivity of brain regions and networks involved in emotional salience, reward processing, motivation, and executive functioning. The neurocircuitry of MDD is highly complex and involves many brain regions and networks, but key brain regions involved in MDD pathophysiology include the prefrontal cortex (PFC), hippocampus, nucleus accumbens (NAc), and amygdala (Fig. 2A). The PFC and hippocampus are intimately involved in executive functioning as well as learning and memory. The NAc, part of the striatum, is critical for motivation and reward processing. The amygdala is necessary for appraising emotional stimuli for relevance and meaning and for processing threats. Broadly speaking, the activity of these brain regions and their connectivity with each other are primarily dampened in MDD, impairing one’s ability to manage cognitive and emotional information (reviewed in Thompson [44]). However, MDD cannot be characterized by a single signature of network dysfunction; rather, the symptoms of MDD, such as negativity bias, anhedonia, and cognitive dysfunction, are each associated with distinct yet overlapping alterations in activity and connectivity [45, 46].
The PFC and hippocampus are smaller in volume in people with MDD [47]. Similar decreases in PFC and hippocampal volume have been found in rodent models of chronic stress that mirror some symptoms of human MDD [44]. The activity of these regions is primarily decreased in MDD, but details vary with symptom variability across MDD patients, as reviewed in syntheses of circuit dysfunction in MDD [44, 46]. For instance, some behavioral states like rumination may involve heightened activity in the PFC, while decreased activity in the NAc is associated with anhedonia. Additionally, people with MDD have reduced amygdala responses to positive emotional stimuli, while the negativity bias is associated with heightened amygdala activity (Fig. 2B) [46, 48].
Cortico-mesolimbic circuitry, which includes connections between the PFC, hippocampus, and NAc, is weakened in MDD, especially in contexts involving cognition, attention, and motivation [44,45,46]. The salience network, which shares many brain regions and pathways with the cortico-mesolimbic reward circuitry and includes the amygdala, also suffers from disordered connectivity in MDD, with decreased communication between the amygdala and PFC according to fMRI studies [45, 46, 49]. Weakened connectivity from the amygdala to the striatum (including the NAc) and between the amygdala and temporal lobe (including the hippocampus) is also evident in MDD (Fig. 2B) [50, 51]. These networks rely heavily on monoaminergic transmission, particularly dopamine in cortico-mesolimbic reward circuitry and prefrontal cognitive function and serotonin and norepinephrine in emotional processing within the amygdala and hippocampus, as previously reviewed [52,53,54].
Weakened connectivity among these networks in MDD suggests an impaired ability for the nodes—the individual brain regions—to regulate and communicate with one another, leaving them stuck in their dysfunctional activity patterns. Other studies, outside of the context of MDD, support the idea that inflexible activity patterns relate to poorer psychological wellbeing. In a random sampling of adults in the United States, persistently elevated amygdala activation after viewing negative stimuli is correlated with greater negative affect and reduced psychological wellbeing [55]. Conversely, amygdala activation that returns more quickly to baseline is associated with positive affect and greater psychological wellbeing. Similarly, high moment-to-moment variability in neuronal activity in regions of the cortex while viewing both positive and negative stimuli predicts greater efficacy of cognitive behavior therapy (CBT) among those with social anxiety [56]. These observations suggest that neural variability allows the brain to be flexible in the face of changing circumstances [57], akin to how higher heart rate variability (HRV) indicates adaptive stress and arousal responses and the ability to transition between parasympathetic and sympathetic nervous system activation as necessary [58]. Interestingly, people with MDD show reduced HRV [59], and reduced HRV in MDD may be associated with cognitive impairments and PFC dysfunction [60].
Cellular level: synaptic dysfunction and reduced neurotrophic factor signaling
What cellular-level changes in the brain might account for changes in brain regions and networks observed in MDD? Though MDD cannot accurately be attributed to a deficit in serotonin, this neurotransmitter still plays a role in its pathophysiology, as recently reviewed by Jauhar et al. [61]. This narrative review covers, for instance, evidence that depleting the serotonin precursor tryptophan is not sufficient to induce depressive symptoms in healthy individuals but does worsen mood in remitted patients, suggesting that reduced serotonin interacts with pre-existing neurobiological vulnerabilities. The therapeutic efficacy of serotonergic drugs such as SSRIs and psychedelics, which we will discuss in the following section, further suggests that serotonin is likely to still be relevant to MDD pathophysiology beyond a simple deficit.
Beyond serotonin, studies in people with MDD and rodent stress models of depression-like behaviors show evidence of dysfunction in synapses: the junctions between neurons where electrical signals and chemical neurotransmitters pass from the axon of a sending (presynaptic) neuron to the dendrites of a receiving (postsynaptic) neuron (Fig. 3A). Synaptic dysfunction is most evident in pyramidal neurons in the PFC and hippocampus, a prevalent class of neurons that release glutamate, the brain’s major excitatory neurotransmitter, thereby activating target neurons that release other neurotransmitters, such as serotonin, norepinephrine, dopamine, and acetylcholine. In rodent models, chronic stress reduces the number of synapses that pyramidal neurons receive, which results in decreased excitation of target neurons (reviewed in McEwen et al. [62]). The reduced number of synapses comes from less dendritic branching of pyramidal neurons, as well as fewer and smaller dendritic spines, which are protrusions from dendritic branches that are points of synaptic contact between neurons (Fig. 3B). When a pyramidal neuron receives fewer excitatory synaptic contacts, it is impaired in its ability to pass along a signal to receiving neurons. Fewer dendritic branches and spines are hypothesized to underlie the reduced volume and activity seen in brain regions involved in MDD, such as within the PFC and hippocampus, as previously reviewed [63, 64]. Fewer synapses in these regions correlate with more severe depression symptoms in MDD [65].
A In the prefrontal cortex and hippocampus of a healthy brain, a presynaptic neuron (neuron 1) releases glutamate to send excitatory stimulation to a postsynaptic terminal of a pyramidal neuron (neuron 2), located on a dendritic spine. A healthy pyramidal neuron has numerous dendritic spines and branches, allowing for many points of synaptic contact with other neurons. When activated, pyramidal neuron 2 fires frequent action potentials that travel to its presynaptic terminal to release glutamate and excite a target postsynaptic neuron (neuron 3). B In a brain with MDD, neuron 1 is still communicating with neuron 2, but neuron 2 has smaller and fewer dendritic spines, as well as fewer dendritic branches. Therefore, neuron 2 is less active, so fewer action potentials travel to its presynaptic terminal, resulting in less excitation of a target postsynaptic neuron (neuron 3). Factors related to MDD, such as genetic vulnerabilities or stress exposure, can contribute to the healthy pyramidal neuron in (A) becoming structurally and functionally like the pathological pyramidal neuron in (B). By contrast, effective treatments for MDD can restore the structure and function of the pathological pyramidal neuron in (B) to that of the healthy pyramidal neuron in (A). MDD major depressive disorder. Created with BioRender.com.
Decreased synaptic density in MDD is likely related to reduced support from neurotrophic factors, which are proteins that support the growth and survival of neurons and synapses. A key source of neurotrophic support is expression of brain-derived neurotrophic factor (BDNF) and its binding to its receptor, tropomyosin receptor kinase B (TrkB). BDNF-TrkB signaling is necessary for the formation, maintenance, and plasticity of synapses, as previously reviewed [66]. MDD is associated with decreased BDNF-TrkB signaling [67, 68]. Heightened neuroinflammation, a contributing factor for depressive symptoms, decreases BDNF gene expression and disrupts neuroplasticity in preclinical models, including decreased dendritic branching and spine density in emotional and cognitive brain regions including the PFC and hippocampus [69,70,71]. The BDNF gene also has a common polymorphism (Val66Met), which leads to reduced BDNF-TrkB signaling and, in humans that carry it, is associated with reduced hippocampal volume, MDD, and suicidality [68, 72, 73]. All of these pathological changes to synaptic density and neurotrophic signaling result in impaired functioning of the brain’s cells and circuits. When this dysfunction occurs within the cells and circuits underlying cognition, reward, and emotion, then symptoms like negative affective bias, anhedonia, and depressed mood arise.
Treatment for MDD understood as enhancing mechanisms of neuroplasticity
What is neuroplasticity?
A growing body of recent evidence suggests that treatments for MDD work, at least in part, by enhancing neuroplasticity, rewiring dysfunctional brain circuits and synapses in adaptive ways that allow patients to become “unstuck” from negative thoughts, emotions, and behaviors. The broadest definition of neuroplasticity is “the brain’s ability to change.” In the context of treating MDD, we define neuroplasticity at the cellular and molecular level as changes in the connections between neurons (e.g., synapse number, the electrophysiological firing properties of neurons), whether resulting from increased receptor-mediated signaling, neurotrophic factor signaling, or other mechanisms. At the network level, we interpret changes in the activity, connectivity, and/or volume of brain regions as large-scale indications that neuroplasticity has occurred. Evidence that neuroplasticity has taken place can also be seen through lasting changes in cognitive and emotional behaviors. Collectively, neuroplasticity represents a category of mechanisms that treat MDD by restoring synaptic strength and functional connectivity in dysfunctional brain circuits. We now know that both pharmacological and non-pharmacological treatments for MDD engage multiple mechanisms of neuroplasticity, which drives their therapeutic efficacy. These mechanisms of neuroplasticity underly the sustained effects of pharmacological and non-pharmacological treatments; they also treat MDD through acute changes in emotional and cognitive information processing, as we will discuss.
Neuroplastic effects of typical monoaminergic antidepressants
Chronic administration of typical monoaminergic antidepressants repairs dysfunction at the behavioral, network, and synaptic levels. At the behavioral level, chronic SSRI administration improves the negativity bias in MDD and helps restore healthy emotional and cognitive information processing [74]. These cognitive and emotional changes are evidence of underlying changes in the brain. At the network level, SSRIs repair dysfunction in reward and executive functioning circuits, such as increasing NAc activity and NAc-PFC connectivity [75,76,77]. At the synaptic level, typical antidepressants restore synaptic functioning and neurotropic signaling. In rodent chronic stress models, typical antidepressants restored hippocampal synaptic structure (i.e., synapse number) and function (i.e., electrophysiological properties) [78, 79] (Fig. 3). In people without depression, SSRI treatment increases synaptic density in the PFC and hippocampus after 5 weeks [80]. In MDD, treatment with typical antidepressants increases synthesis of BDNF [81] and increases activation of its receptor TrkB [82], enhancing downstream synapse formation and maintenance. In rodent models, the behavioral benefits of antidepressants are abolished when BDNF-TrkB signaling is impaired, likely because this prohibits antidepressants from activating the molecular mechanisms of synaptic formation and maintenance that occur downstream of BDNF-TrkB [83].
Typical antidepressants may promote a neuroplastic state similar to the highly malleable brain in early development, possibly allowing for changes in brain structure and function that are otherwise limited in the adult brain, which is relatively more stable, as previously reviewed [84,85,86]. In rodent studies, this has been demonstrated in the cortex, hippocampus, and amygdala [87,88,89,90], in which environmental interventions were insufficient to induce functional changes on their own; only the combination of antidepressants and the intervention resulted in functional change. For example, rats raised in isolation develop aggressive behavior that persists in adulthood despite resocialization interventions, likely due in part to decreased BDNF signaling. However, when adult resocialization is combined with SSRI administration, BDNF signaling is restored in the PFC and the aggressive behavior remits [90]. Thus, antidepressant-induced neuroplasticity could sensitize an organism to environmental conditions.
The neuroplastic benefits of antidepressants are likely enhanced when they occur in the context of positive experiences in the patient’s everyday environment. People living in more favorable conditions (e.g., employed, college educated, high income) may be more likely to benefit from an antidepressant alone than those not living in favorable conditions [91]. Similarly, psychotherapy, which provides a supportive environment to facilitate positive changes in thinking, feeling, and behaving, enhances the therapeutic potential of antidepressants [92]. In this way, antidepressants can be thought of as opening a window of plasticity that allows the benefits of a positive environment to take hold in neural circuits. By contrast, if experiences in the environment are negative, rodent studies suggest that antidepressant treatment may even be detrimental because enhanced neuroplasticity in an adverse situation facilitates learning that the environment is harmful [93, 94]. However, not all findings support the notion that antidepressants simply make people more susceptible to their environments: rodent studies also show that antidepressant administration prior to stress exposure can buffer against the effects of adversity [95, 96]. Therefore, antidepressants may confer resilience in the face of negative experiences in addition to their ability to enhance the benefits of a positive environment.
If it’s all about neuroplasticity, how do antidepressant-induced changes in serotonergic transmission treat MDD?
Viewing the treatment effects of typical antidepressants through a neuroplasticity lens, in which the benefits of antidepressants are dependent upon new learning opportunities, offers an alternative to the outdated (although still frequently referenced) idea that antidepressants are correcting a monoaminergic deficit to restore monoamine neurotransmission. Monoamines are still essential for antidepressant effects, but rather than correcting a deficit, the serotoninergic, dopaminergic, and noradrenergic activity of typical antidepressants likely play two major roles in facilitating the therapeutic response: (1) triggering downstream molecular cascades that result in neuroplasticity more chronically, and (2) changing emotional processing and behavior more acutely.
The first major role of typical antidepressants is supported by rodent studies suggesting that activation of multiple types of serotonin receptors results in downstream effects that enhance neuroplasticity through many of the mechanisms previously discussed, such as increased expression of growth factors. For example, knocking out the 5-HT1A receptor on granule cells in the dentate gyrus decreases fluoxetine-induced expression of BDNF and blocks proliferation of new hippocampal neurons in mice [97]. The 5-HT4 receptor also appears to be necessary for inducing de-maturation of granule cells in the dentate gyrus, which plays a role in the overall neuroplastic effects of antidepressants in mice [98]. Additionally, there are antidepressants other than SSRIs and SNRIs that are still thought to work through serotonergic mechanisms. For instance, vortioxetine has multimodal actions on serotonin receptors, including antagonism, agonism, or partial agonism, depending on receptor subtype, and has been shown to increase neurotransmission of serotonin, dopamine, norepinephrine, and glutamate in emotional and cognitive brain regions (reviewed in Alvarez et al. [99]). Vortioxetine appears especially effective for treating cognitive impairment in clinical and preclinical studies (reviewed in Sanchez et al. [100]). In rodents, vortioxetine increases hippocampal synaptic plasticity and dendritic spine maturation [101, 102]. Though vortioxetine’s precise mechanisms of action remain unknown, its multimodal serotonergic actions highlight the complexity of the serotonergic system in the pathophysiology and treatment of MDD. Finally, signaling cascades that facilitate neuroplasticity, such as through gene expression changes and increased expression of BNDF, can occur downstream of dopamine and norepinephrine receptors as well as serotonin receptors, suggesting that enhanced signaling of these monoamines is relevant to the neuroplastic effects of antidepressants as well (reviewed in Pittenger and Duman [21]).
The second major role of typical antidepressants is supported by evidence of acute changes in cognitive and emotional processing following administration. For instance, serotonergic antidepressants result in acute changes in emotion processing within a week (and even after one dose) in humans, such that individuals are less likely to interpret ambiguous faces as negative and demonstrate increased attentional and memory bias for positive versus negative stimuli (reviewed by Godlewska and Harmer [36]). Thus, before the medication has time to enact its chronic effects (i.e., enhancing mechanisms of neuroplasticity), it acutely changes the way individuals process the world around them. This cognitive neuropsychological model of antidepressant action suggests that acute effects of antidepressants on emotional processing promote more positive social interactions [36, 103]. Eventual increases in neuroplasticity enhance the impact that these new biases in emotion processing and social experiences have on shaping patterns of beliefs and behaviors. For example, people struggling with depression are likely to have negative emotional biases, which might make them think that others dislike them. This might cause people with depression to avoid interacting with others. After taking an antidepressant, reduced negativity bias would promote more positive social experiences, and eventually enhance motivation to engage with others.
An important consideration in the serotonergic treatment of MDD is evidence that, among children, teenagers, and young adults before age 25, serotonergic antidepressant treatment is associated with a small but significantly increased risk of suicide [104]. This seemingly contradictory observation calls into question the hypothesis that serotonergic antidepressants facilitate a more positive outlook on life, and also highlights the complexity of MDD, the pathophysiology of which is also impacted by age. For instance, MDD in young people may be more likely to involve impulsivity and/or symptoms of mania due to differences in brain maturation, which increase the risk for suicide [104]. Serotonin has also been implicated in impulsivity [105]. The developing brain is highly plastic relative to the adult brain at baseline, and the effects of serotonergic antidepressants may differ against the labile background of development versus the stable background of adulthood [106]. For instance, a meta-analysis showed that antidepressants have a similar efficacy between adolescents and adults, but with adverse events being common among adolescents [107]. Despite greater neuroplasticity of the developing brain compared to the aging brain, age-dependent effects of antidepressants on neuroplasticity remain largely unstudied.
Novel antidepressants: ketamine and psychedelics
In contrast to the slow therapeutic actions of typical antidepressants, a single subanesthetic dose of ketamine produces rapid (within 24 h of administration) symptomatic relief in MDD and treatment resistant depression in clinical trials, though the effects are transient [108]. Repeated dosing of ketamine is more efficacious in clinical trials than typical antidepressants alone [109, 110]. In 2019, the FDA approved an intranasal formulation of ketamine, specifically esketamine (Spravato®), the first truly new antidepressant in 50 years. Ketamine produces rapid changes in structural and functional connectivity within many regions associated with reward, emotion, and cognitive function, likely by acting on glutamate and increasing excitation in hypoactive regions of the brain, as previously reviewed [44, 111]. Neuroimaging research shows that ketamine also rescues anhedonia and enhances sensitivity to reward in patients with MDD, which is accompanied by increased activity in relevant mesolimbic brain networks [112, 113]. Preclinical studies suggest that the beneficial actions of ketamine are mediated by activation of both BDNF-TrkB signaling and endogenous synaptic strengthening mechanisms [114]. Distinct mechanisms of action likely underlie ketamine’s rapid versus sustained antidepressant effects, with acute increases in glutamatergic transmission triggering sustained increases in downstream processes underlying neuroplasticity, as reviewed by Kim et al. [115]. For a comprehensive synthesis of the evidence for ketamine’s efficacy and mechanisms of action, we refer readers to the review by McIntyre et al. [116], which also discusses tolerability and safety concerns that remain under investigation. For instance, preclinical and clinical studies suggest that the opioid system is necessary (although not sufficient) for ketamine’s antidepressant effects, raising possible concerns about addiction and abuse potential [117, 118]. Ketamine abuse has been reported in the literature, as previously reviewed [119] and was the topic of a 2020 special issue of Behavioural Brain Research [120], emphasizing the need for caution. However, for esketamine in the treatment of depression, the current FDA approved use is once to twice per week [121], lowering risks of abuse liability in clinical settings. Editorial discussions on the effects of ketamine on the opioid system encourage continued investigation into the mechanisms of ketamine’s antidepressant effects alongside safety monitoring of its clinical use [122, 123].
Psychedelics such as psilocybin and lysergic acid diethylamide (LSD) are also novel therapeutics for MDD that show promise in clinical trials [124,125,126]. However, these trials remain preliminary, and one showed that psilocybin was not significantly different from the SSRI escitalopram in antidepressant efficacy [127]. Psilocybin and LSD are serotonergic psychedelics that act as agonists of essentially all serotonin receptors and produce their psychedelic response by activating 5-HT2A receptors. As with SSRIs, their enhancement of serotonergic transmission is likely relevant to their antidepressant effects through downstream changes in gene expression, indirect effects on glutamatergic and dopaminergic systems, and changes in emotional and cognitive brain networks, summarized in recent reviews [128, 129]. Syntheses of the evidence for psychedelics’ antidepressant mechanisms of action propose that, like typical antidepressants and ketamine, psychedelics facilitate neuroplasticity at the behavioral, network, and synaptic levels [130, 131]. Indeed, in an open-label clinical trial, psilocybin improved cognitive flexibility in patients with MDD [132]. In healthy human participants, psilocybin induces rapid yet persistent changes in functional connectivity between brain regions including the PFC and amygdala [133, 134], consistent with findings in chronically stressed mice showing that acute administration of psilocybin restores excitatory synaptic function and healthy reward behaviors [135]. In cultured neurons and in mice, psilocybin promotes structural changes in dendrites and synapses, increasing and amplifying the synaptic connections between neurons [136, 137]. According to rodent research, psychedelics may promote synaptic connections by enhancing BDNF-TrkB signaling similar to typical antidepressants [138]. However, similar to the cognitive neuropsychological model of typical antidepressant action described above, acute changes in cognitive and emotional processing following administration of ketamine or psychedelics may also be therapeutic, though this remains to be investigated alongside continued research into their efficacy relative to typical antidepressants.
Non-pharmacological approaches: psychotherapy and neurostimulation
The mechanisms by which non-pharmacological treatments for depression work may be the same as or similar to those acted on by typical antidepressants and psychedelics. Non-pharmacological treatments include psychotherapeutic techniques, like cognitive behavioral therapy (CBT) and mindfulness, as well as neurostimulation approaches, such as electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and deep brain stimulation (DBS). Each of these treatment approaches can be used individually or in conjunction with each other, including alongside pharmacological agents.
According to human neuroimaging studies, neurostimulation therapies enhance activity in cortical and mesolimbic circuits involved in cognition, reward, and motivation [139,140,141]. TMS for MDD increases activation in the dorsolateral PFC, connectivity in cortico-mesolimbic circuitry, and extracellular glutamate and dopamine in the NAc [140, 142]. The Stanford Neuromodulation Therapy (SNT) TMS protocol uses intermittent theta burst stimulation (iTBS) targeting the left dorsolateral PFC and was shown in a 2021 clinical trial to be highly efficacious for MDD [143]. A follow-up trial showed that the SNT TMS protocol changed functional connectivity patterns between the salience network, the amygdala, and the striatum such that treated MDD patients now had functional connectivity patterns that were comparable to control patients without MDD [144]. DBS for MDD targets the cingulate cortex or the striatum, which includes the NAc, and has been shown to enhance activity in these regions and connected areas involved in anhedonia and motivation [145, 146], as well as activity in the PFC and amygdala [139]. In contrast, ECT causes more global excitation throughout the brain as opposed to initially targeting a single brain region. Some of the network-level effects of ECT are shared with the effects of “treatment as usual” (combined medication, psychotherapy, and case management), while other effects differ: both modalities increase cortical thickness, while only ECT appears to increase hippocampus and amygdala volume in patients with MDD [141].
As with pharmacological antidepressants, neurostimulation therapies likely also have acute effects on cognitive and emotional processing that are part of their therapeutic efficacy. A single ECT session decreased neural reactivity in the PFC in response to unpleasant stimuli in patients with MDD, even before improvement in mood was evident [147]. According to sham-controlled fMRI studies, changes in brain activity patterns in the PFC after a single ECT session may reflect early signs of sustained therapeutic changes in emotional and cognitive circuits [148, 149].
Brain imaging and EEG recording studies show that psychotherapeutic techniques also work to change activity and connectivity patterns in brain regions involved in emotion regulation, such as the salience network [150,151,152]. For instance, CBT helps patients process information in positive or productive ways that change the cognitive and emotional schemas with which they understand their past and contextualize their future [153]. Activities like physical exercise and meditation also have antidepressant effects, likely in part through enhancing signaling of neurotrophic factors like BDNF, summarized in previous reviews [154, 155]. Fundamentally, these non-pharmacological treatments for MDD induce neuroplasticity in much the same way as pharmacological approaches.
Communicating an accurate and accessible neuroplasticity framework of MDD
Summary
Here, we describe how MDD arises from cognitive and emotional circuits that are “stuck” in a state characterized by negativity bias and impaired flexibility. Effective treatments—from typical antidepressants to psychedelics, psychotherapy, and brain stimulation—work through multiple mechanisms of neuroplasticity to allow these circuits to adaptively change and restore healthy functioning. Though MDD cannot accurately be considered a deficit in serotonin, serotonin still has a role to play through its interactions with mechanisms of neuroplasticity. We believe that this model, which is based on a solid foundation of clinical and preclinical research, is understandable, accurate, and flexible enough to be refined by future research. Another advantage of this model of MDD is that it provides a framework for describing the potential interactions between pharmacological treatments and the environment, and it also emphasizes the importance of prescribing antidepressants alongside concurrent opportunities for positive learning experiences, such as psychotherapy [156].
The importance of science communication
Given rising mental health crises alongside public mistrust in science and medicine, we believe that effective science communication is of critical importance. Specifically, an accessible and accurate framework of MDD is necessary to replace the serotonin deficit hypothesis in the public’s understanding of depression. We offer two metaphors to help researchers and clinicians communicate this neuroplasticity framework of MDD with general and patient populations (Fig. 4).
Multiple factors can act independently or interact with each other to contribute to MDD. The pathophysiology of MDD can be understood as the brain being “stuck” in a state of maladaptive cognitive and emotional processing related to dysfunction in brain circuits and synapses that regulate cognition and reward processing. At the behavioral level, this dysfunction results in negativity bias, decreased cognitive and emotional flexibility, and decreased responses to and motivation for rewards. Treatments for MDD, both pharmacological (SSRIs, SNRIs, MAOIs, psychedelics) and non-pharmacological (TMS, DBS, ECT, psychotherapies), work by enhancing neuroplasticity to release brain circuits from their stuck state, enable adaptive rewiring, and restore healthy functioning. This model of MDD is like (A) a car running off the road and into a ditch, requiring the help of a tow truck to get back out, or (B) a broken leg requiring physical therapy and anti-inflammatory medication to heal. MDD major depressive disorder, SSRIs selective serotonin reuptake inhibitors, SNRIs serotonin and norepinephrine reuptake inhibitors, MAOIs monoamine oxidase inhibitors, TMS transcranial magnetic stimulation, DBS deep brain stimulation, ECT electroconvulsive therapy. Created with Canva.com and BioRender.com.
The first metaphor is that of a car driving along a road, but then getting stuck in a ditch and needing help to get out. In this metaphor, the driver and the vehicle can be thought of as predisposing factors, such as genetics and a stressful upbringing, that influence one’s risk for getting stuck in the ditch that is MDD. Some drivers have a new four-wheel drive car capable of navigating rugged terrain or slick roads, akin to people having a supportive upbringing or genes associated with resilience. Other drivers have an old clunker with threadbare tires that represent vulnerability genes or high exposure to adverse childhood experiences (ACEs). The road is the current experiences of life, such as stress exposure. On a rainy or snowy day, when visibility is poor and the pavement is slick, a car is more likely to run off the road, especially if its tires are worn out. Once stuck in the ditch of MDD, spinning the wheels can get the car even more stuck, like the dysfunctional cognitive and emotional circuits that reinforce MDD. In this sense, the dysfunctional cognitive and emotional circuits that characterize MDD are the ruts on the side of the road. Treatments are the tow trucks that pull the car out of the ditch and the mechanics that get it back on the road (Fig. 4A).
In the second metaphor, MDD and its treatments are likened to physical rehabilitation after an injury. Similar to a broken leg that lacks the full range of motion, the brain with MDD lacks flexibility and the capacity for the full range of emotion. For the injured leg, regaining function and mobility requires physical therapy, just as the brain in MDD benefits from psychotherapy. In some cases, exercise must be combined with medication to manage pain and inflammation, so that the individual is able to initiate and benefit from the prescribed physical therapy. In the same way, psychotherapy for MDD can be enhanced by pharmacological treatments like typical antidepressants or esketamine. The result is recovery of the brain’s capacity to perceive and interact with the environment in a flexible way that was possible prior to the injury of MDD, ideally combined with the development of new coping strategies, like rehabilitated muscles that are even stronger and more flexible than they were before (Fig. 4B).
Conveying to a general audience the nuances and caveats inherent in a complex disorder and its treatment mechanisms may seem like a daunting task. However, as researchers and clinicians, we have a responsibility to explain to patients and the public how MDD and its treatments are currently conceptualized as simply as possible, yet without sacrificing accuracy. If we fail to do so, the risks are great: the void will inevitably be filled by unintentional or even intentional distortions that have the potential to increase misinformation and stigma while eroding public trust in science and medicine. In the end, communicating research is just as important as the research itself.
References
Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–22.
Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–64.
France CM, Lysaker PH, Robinson RP. The ‘Chemical Imbalance’ explanation for depression: origins, lay endorsement, and clinical implications. Prof Psychol Res Pr. 2007;38:411–20.
Owens MJ. Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry. 2004;65:5–10.
Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–28.
Lacasse JR, Leo J. Serotonin and depression: a disconnect between the advertisements and the scientific literature. PLoS Med. 2005;2:1211–6.
Schroder HS, Duda JM, Christensen K, Beard C, Björgvinsson T. Stressors and chemical imbalances: beliefs about the causes of depression in an acute psychiatric treatment sample. J Affect Disord. 2020;276:537–45.
Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2022;28:3243–3256.
Gregory A. Little evidence that chemical imbalance causes depression, UCL scientists find | Depression | The Guardian. The Guardian. 2022.
A popular medical explanation for depression is rebuffed. The Economist. 2022.
Bakar F. Your depression might not be due to a chemical imbalance after all. The Huffington Post. 2022.
Delaney M. New study challenges value of antidepressants. The Washington Times. 2022.
Schraer R. Did we all believe a myth about depression? BBC News. 2022.
Guzman J. Depression is likely not caused by a chemical imbalance in the brain, study says. The Hill. 2022.
Jauhar S, Arnone D, Baldwin DS, Bloomfield M, Browning M, Cleare AJ, et al. A leaky umbrella has little value: evidence clearly indicates the serotonin system is implicated in depression. Mol Psychiatry. 2023. https://doi.org/10.1038/S41380-023-02095-Y.
Bartova L, Lanzenberger R, Rujescu D, Kasper S. Reply to: ‘The serotonin theory of depression: a systematic umbrella review of the evidence’ published by Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA in Molecular Psychiatry (2022 Jul 20. https://doi.org/10.1038/s41380-022-01661-0). Mol Psychiatry. 2023;28:3153–4.
El-Mallakh RS, Doroodgar M, Elsayed OH, Kidambi N. The serotonin theory of depression. Mol Psychiatry. 2023;28:3157.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Ruhe HG, Mocking RJT, Figueroa CA, Seeverens PWJ, Ikani N, Tyborowska A, et al. Emotional biases and recurrence in major depressive disorder. Results of 2.5 years follow-up of drug-free cohort vulnerable for recurrence. Front Psychiatry. 2019;10:145.
Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25:530–43.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109.
Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–94.
Castrén E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013;36:259–67.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Sharma S, Powers A, Bradley B, Ressler KJ. Gene × environment determinants of stress- and anxiety-related disorders. Annu Rev Psychol. 2016;67:261.
Whitaker RC, Dearth-Wesley T, Herman AN, Block AE, Holderness MH, Waring NA, et al. The interaction of adverse childhood experiences and gender as risk factors for depression and anxiety disorders in US adults: a cross-sectional study. BMC Public Health. 2021;21:2078.
Hammen C, Kim EY, Eberhart NK, Brennan PA. Chronic and acute stress and the prediction of major depression in women. Depress Anxiety. 2009;26:718–23.
Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. 2021;53:151–71.
Shorter E. The 25th anniversary of the launch of Prozac gives pause for thought: where did we go wrong? Br J Psychiatry. 2014;204:331–2.
Caldieraro MAK, Baeza FLC, Pinheiro DO, Ribeiro MR, Parker G, Fleck MP. Clinical differences between melancholic and nonmelancholic depression as defined by the CORE system. Compr Psychiatry. 2013;54:11–15.
Catenaccio E, Mu W, Lipton ML. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct. 2016;221:3845–67.
Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:475.
Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment introduction-developments in defining PMDD. Curr Psychiatry Rep. 2015;17:87.
Batt MM, Duffy KA, Novick AM, Metcalf CA, Epperson CN. Is postpartum depression different from depression occurring outside of the perinatal period? A review of the evidence. Focus. 2020;18:119.
Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology. 2021;238:1265–78.
Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55.
Reinen JM, Whitton AE, Pizzagalli DA, Slifstein M, Abi-Dargham A, McGrath PJ, et al. Differential reinforcement learning responses to positive and negative information in unmedicated individuals with depression. Eur Neuropsychopharmacol. 2021;53:89–100.
Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017;78:1383–94.
Dajani DR, Uddin LQ. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 2015;38:571–8.
Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin card sorting test performance from the neurobehavioral studies program. Am J Psychiatry. 1999;156:780–2.
Wen A, Yoon KL. Depression and affective flexibility: a valence-specific bias. Behav Res Ther. 2019;123:103502.
Yasinski C, Hayes AM, Ready CB, Abel A, Görg N, Kuyken W. Processes of change in cognitive behavioral therapy for treatment-resistant depression: psychological flexibility, rumination, avoidance, and emotional processing. Psychother Res. 2020;30:983–97.
Thompson SM. Plasticity of synapses and reward circuit function in the genesis and treatment of depression. Neuropsychopharmacology. 2023;48:90–103.
Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91:561–71.
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:427–80.
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53.
Young KD, Siegle GJ, Bodurka J, Drevets WC. Amygdala activity during autobiographical memory recall in depressed and vulnerable individuals: association with symptom severity and autobiographical overgenerality. Am J Psychiatry. 2016;173:78–89.
Shao J, Meng C, Tahmasian M, Brandl F, Yang Q, Luo G, et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 2018;12:1708–19.
Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17.
Cheng W, Rolls ET, Qiu J, Xie X, Lyu W, Li Y, et al. Functional connectivity of the human amygdala in health and in depression. Soc Cogn Affect Neurosci. 2018;13:557–68.
Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67:3–8.
Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–33.
Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Puccetti NA, Schaefer SM, van Reekum CM, Ong AD, Almeida DM, Ryff CD, et al. Linking amygdala persistence to real-world emotional experience and psychological well-being. J Neurosci. 2021;41:3721–30.
Månsson KNT, Waschke L, Manzouri A, Furmark T, Fischer H, Garrett DD. Moment-to-moment brain signal variability reliably predicts psychiatric treatment outcome. Biol Psychiatry. 2022;91:658–66.
Waschke L, Kloosterman NA, Obleser J, Garrett DD. Behavior needs neural variability. Neuron. 2021;109:751–66.
Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–40.
Hartmann R, Schmidt FM, Sander C, Hegerl U. Heart rate variability as indicator of clinical state in depression. Front Psychiatry. 2019;9:735.
Zhou H, Dai Z, Hua L, Jiang H, Tian S, Han Y, et al. Decreased task-related HRV is associated with inhibitory dysfunction through functional inter-region connectivity of PFC in major depressive disorder. Front Psychiatry. 2020;10:989.
Jauhar S, Cowen PJ, Browning M. Fifty years on: serotonin and depression. J Psychopharmacol. 2023;37:237–41.
McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23.
Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50.
Tata DA, Anderson BJ. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav. 2010;99:186–93.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529.
Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27.
Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–8.
Youssef MM, Underwood MD, Huang YY, Hsiung SC, Liu Y, Simpson NR, et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol.2018;21:538.
Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. 2014;8:430.
Guo X, Rao Y, Mao R, Cui L, Fang Y. Common cellular and molecular mechanisms and interactions between microglial activation and aberrant neuroplasticity in depression. Neuropharmacology. 2020;181:108336.
Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–416.
Schenkel LC, Segal J, Becker JA, Manfro GG, Bianchin MM, Leistner-Segal S. The BDNF Val66Met polymorphism is an independent risk factor for high lethality in suicide attempts of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:940–4.
Hajek T, Kopecek M, Höschl C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: meta-analysis. World J Biol Psychiatry. 2012;13:178–87.
Warren MB, Pringle A, Harmer CJ. A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc B Biol Sci. 2015;370:20140213.
Heller AS, Tom Johnstone M, Light SN, Michael Peterson MJ, Kolden GG, Kalin NH, et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry. 2013;170:197–206.
Dunlop K, Rizvi SJ, Kennedy SH, Hassel S, Strother SC, Harris JK, et al. Clinical, behavioral, and neural measures of reward processing correlate with escitalopram response in depression: a Canadian Biomarker Integration Network in Depression (CAN-BIND-1) Report. Neuropsychopharmacology. 2020;45:1390–7.
Fischer AS, Holt-Gosselin B, Fleming SL, Hack LM, Ball TM, Schatzberg AF, et al. Intrinsic reward circuit connectivity profiles underlying symptom and quality of life outcomes following antidepressant medication: a report from the iSPOT-D trial. Neuropsychopharmacology. 2021;46:809–19.
Magariños AM, Deslandes A, Mcewen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–22.
Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013;33:15669–74.
Johansen A, Armand S, Plavén-Sigray P, Nasser A, Ozenne B, Petersen IN, et al. Effects of escitalopram on synaptic density in the healthy human brain: a randomized controlled trial. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02285-8.
Molendijk ML, Bus BAA, Spinhoven P, Penninx BWJH, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry.2011;16:1088–95.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.e19.
Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–57.
Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51.
Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–71.
Hensch TK, Bilimoria PM. Re-opening windows: manipulating critical periods for brain development. Cerebrum. 2012;2012:11.
Umemori J, Winkel F, Castrén E, Karpova NN. Distinct effects of perinatal exposure to fluoxetine or methylmercury on parvalbumin and perineuronal nets, the markers of critical periods in brain development. Int J Dev Neurosci. 2015;44:55–64.
Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale F, O’Leary O, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8.
Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Ágústsdóttir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–4.
Mikics É, Guirado R, Umemori J, Tóth M, Biró L, Miskolczi C, et al. Social learning requires plasticity enhanced by fluoxetine through prefrontal Bdnf-TrkB signaling to limit aggression induced by post-weaning social isolation. Neuropsychopharmacology. 2018;43:245.
Chiarotti F, Viglione A, Giuliani A, Branchi I. Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR*D study. Transl Psychiatry. 2017;7:e1066.
Cuijpers P, Noma H, Karyotaki E, Vinkers CH, Cipriani A, Furukawa TA. A network meta-analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry.2020;19:92–107.
Alboni S, Van DIjk RM, Poggini S, Milior G, Perrotta M, Drenth T, et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry. 2017;22:552–61.
Branchi I, Santarelli S, Capoccia S, D’Andrea I, Cirulli F, Alleva E. Antidepressant treatment outcome depends on the quality of the living environment: a pre-clinical investigation in mice. PLoS ONE. 2013;8:e62226.
Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31.
Petty F, Kramer G, Wilson L. Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacol Biochem Behav. 1992;43:361–7.
Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18:1616.
Umemori J, Winkel F, Didio G, Llach Pou M, Castrén E. iPlasticity: induced juvenile‐like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin Neurosci. 2018;72:653.
Alvarez E, Perez V, Artigas F. Pharmacology and clinical potential of vortioxetine in the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:1297–307.
Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57.
Waller JA, Chen F, Sánchez C. Vortioxetine promotes maturation of dendritic spines in vitro: a comparative study in hippocampal cultures. Neuropharmacology. 2016;103:143–54.
Dale E, Zhang H, Leiser SC, Xiao Y, Lu D, Yang CR, et al. Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. J Psychopharmacol. 2014;28:891–902.
Novick AM, Ross DA. Changing the way we think about (and with) antidepressants. Biol Psychiatry. 2018;84:e28.
Brent DA. Antidepressants and suicidality. Psychiatr Clin North Am. 2016;39:503–12.
Desrochers SS, Spring MG, Nautiyal KM. A role for serotonin in modulating opposing drive and brake circuits of impulsivity. Front Behav Neurosci. 2022;16:791749.
Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34.
Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46:735–54.
Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–6.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:903.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–38.
Zavaliangos-Petropulu A, Al-Sharif NB, Taraku B, Leaver AM, Sahib AK, Espinoza RT, et al. Neuroimaging-derived biomarkers of the antidepressant effects of ketamine. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:361–86.
Sterpenich V, Vidal S, Hofmeister J, Michalopoulos G, Bancila V, Warrot D, et al. Increased reactivity of the mesolimbic reward system after ketamine injection in patients with treatment-resistant major depressive disorder. Anesthesiology. 2019;130:923–35.
Pulcu E, Guinea C, Cowen PJ, Murphy SE, Harmer CJ. A translational perspective on the anti-anhedonic effect of ketamine and its neural underpinnings. Mol Psychiatry. 2022;27:87.
Gould TD, Zarate CA, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–36.
Kim JW, Suzuki K, Kavalali ET, Monteggia LM. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol Med. 2023;29:P364.
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178:383–99.
Klein ME, Chandra J, Sheriff S, Malinow R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci USA. 2020;117:2656–62.
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15.
Liu Y, Lin D, Wu B, Zhou W. Ketamine abuse potential and use disorder. Brain Res Bull. 2016;126:68–73.
Trujillo KA, Iñiguez SD. Ketamine beyond anesthesia: antidepressant effects and abuse potential. Behav Brain Res. 2020;394:112841.
Janssen. SPRAVATO ® (esketamine) nasal spray, CIII Highlights of Prescribing Information. AccessdataFdaGov. 2023:1–15.
Heifets BD, Williams NR, Blasey C, Sudheimer K, Rodriguez CI, Schatzberg AF. Interpreting ketamine’s opioid receptor dependent effect: response to sanacora. Am J Psychiatry. 2019;176:249–50.
Sanacora G. Caution against overinterpreting opiate receptor stimulation as mediating antidepressant effects of ketamine. Am J Psychiatry. 2019;176:249.
Gukasyan N, Davis AK, Barrett FS, Cosimano MP, Sepeda ND, Johnson MW, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J Psychopharmacol. 2022;36:151–8.
Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387:1637–48.
Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177:391–410.
Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384:1402–11.
Husain MI, Ledwos N, Fellows E, Baer J, Rosenblat JD, Blumberger DM, et al. Serotonergic psychedelics for depression: what do we know about neurobiological mechanisms of action? Front Psychiatry. 2023;13:1076459.
Ling S, Ceban F, Lui LMW, Lee Y, Teopiz KM, Rodrigues NB, et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. 2022;36:17–30.
de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. 2021;12:724606.
Kadriu B, Greenwald M, Henter ID, Gilbert JR, Kraus C, Park LT, et al. Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. Int J Neuropsychopharmacol. 2021;24:8–21.
Doss MK, Považan M, Rosenberg MD, Sepeda ND, Davis AK, Finan PH, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. 2021;11:574.
Barrett FS, Doss MK, Sepeda ND, Pekar JJ, Griffiths RR. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep. 2020;10:2214.
Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL. Therapeutic mechanisms of psilocybin: changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol. 2020;34:167–80.
Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. PNAS. 2021;118:e2022489118.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–44.e4.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023;26:1032–41.
Zhu Z, Hubbard E, Guo X, Barbosa DAN, Popal AM, Cai C, et al. A connectomic analysis of deep brain stimulation for treatment-resistant depression. Brain Stimul. 2021;14:1226–33.
Tik M, Hoffmann A, Sladky R, Tomova L, Hummer A, Navarro de Lara L, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289–96.
Bracht T, Walther S, Breit S, Mertse N, Federspiel A, Meyer A, et al. Distinct and shared patterns of brain plasticity during electroconvulsive therapy and treatment as usual in depression: an observational multimodal MRI-study. Transl Psychiatry. 2023;13:6.
Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–5.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41.
Batail JM, Xiao X, Azeez A, Tischler C, Kratter IH, Bishop JH, et al. Network effects of Stanford Neuromodulation Therapy (SNT) in treatment-resistant major depressive disorder: a randomized, controlled trial. Transl Psychiatry. 2023;13:240.
Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75.
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23:843–9.
Miskowiak KW, Macoveanu J, Jørgensen MB, Ott CV, Støttrup MM, Jensen HM, et al. Effect of electroconvulsive therapy on neural response to affective pictures: a randomized, sham-controlled fMRI study. Eur Neuropsychopharmacol. 2018;28:915–24.
Miskowiak KW, Kessing LV, Ott CV, Macoveanu J, Harmer CJ, Jørgensen A, et al. Does a single session of electroconvulsive therapy alter the neural response to emotional faces in depression? A randomised sham-controlled functional magnetic resonance imaging study. J Psychopharmacol. 2017;31:1215–24.
Miskowiak KW, Macoveanu J, Jørgensen MB, Støttrup MM, Ott CV, Jensen HM, et al. Neural response after a single ECT session during retrieval of emotional self-referent words in depression: a randomized, sham-controlled fMRI study. Int J Neuropsychopharmacol. 2018;21:226–35.
Pagani M, Di Lorenzo G, Verardo AR, Nicolais G, Monaco L, Lauretti G, et al. Neurobiological correlates of EMDR monitoring—an EEG study. PLoS ONE. 2012;7:e45753.
Maj van der Velden AM, Scholl J, Elmholdt EM, Fjorback LO, Harmer CJ, Lazar SW, et al. Mindfulness training changes brain dynamics during depressive rumination: a randomized controlled trial. Biol Psychiatry. 2023;93:233–42.
Zhou W, Yuan Z, Yingliang D, Chaoyong X, Ning Z, Chun W. Differential patterns of dynamic functional connectivity variability in major depressive disorder treated with cognitive behavioral therapy. J Affect Disord. 2021;291:322–8.
Brewin CR. Understanding cognitive behaviour therapy: a retrieval competition account. Behav Res Ther. 2006;44:765–84.
Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–41.
You T, Ogawa EF. Effects of meditation and mind-body exercise on brain-derived neurotrophic factor: a literature review of human experimental studies. Sports Med Health Sci. 2020;2:7–9.
Novick AM. What to say: changing the way we think about (and with) antidepressants. National Neuroscience Curriculum Initiative (NNCI); 2019. https://nncionline.org/course/what-to-say-changing-the-way-we-think-about-and-with-antidepressants/.
Acknowledgements
We thank Clint Carlson, MS, Director of Education Technology Innovations at the University of Colorado Anschutz Medical Campus, Department of Psychiatry, for creating the brain image used in Fig. 2. We appreciate the careful reading of a previous draft of this manuscript and comments given by Robert Freedman, MD.
Author information
Authors and Affiliations
Contributions
CEP, CNE, AMN, KAD, and SMT all conceptualized the manuscript. CEP primarily wrote the manuscript with contributions and feedback from all authors. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
CNE receives consulting feeds from Sage Therapeutics and Health Rhythms for Research unrelated to this manuscript. CNE participates on scientific advisory boards for Embark/Neuro and BabyScripts, and has unpaid roles with the National Network of Depression Centers (Member at Large), The Society of Biological Psychiatry (Council Member), and the American College of Neuropsychopharmacology (Executive Council). SMT is listed as an inventor on and receives royalties from patents related to treating depression that are held by the University of Maryland, Baltimore. SMT has received honoraria from several non-profit or academic institutions and from companies developing psychiatric drugs for general lectures and advice about depression treatments. SMT has patents issued and pending from the University of Maryland, Baltimore, for various means for treating depression. SMT serves as an unpaid member of several committees for the American College of Neuropsychopharmacology. AMN is supported by the National Institute of Child Health and Development K23HD110435. CEP and KAD have no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Page, C.E., Epperson, C.N., Novick, A.M. et al. Beyond the serotonin deficit hypothesis: communicating a neuroplasticity framework of major depressive disorder. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02625-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02625-2
- Springer Nature Limited